Abstract
Introduction
In patients with severe coronavirus disease 2019 (COVID-19), respiratory failure is a major complication and its symptoms occur around one week after onset. The CURB-65, A-DROP and expanded CURB-65 tools are known to predict the risk of mortality in patients with community-acquired pneumonia. In this retrospective single-center retrospective study, we aimed to assess the correlations of the A-DROP, CURB-65, and expanded CURB-65 scores on admission with an increase in oxygen requirement in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia.
Methods
We retrospectively analyzed 207 patients who were hospitalized with SARS-CoV-2 pneumonia at the Self-Defense Forces Central Hospital in Tokyo, Japan. Performance of A-DROP, CURB-65, and the expanded CURB-65 scores were validated. In addition, we assessed whether there were any associations between an increase in oxygen requirement and known risk factors for critical illness in COVID-19, including elevation of liver enzymes and C-reactive protein (CRP), lymphocytopenia, high D-dimer levels and the chest computed tomography (CT) score.
Results
The areas under the curve for the ability of CURB-65, A-DROP, and the expanded CURB-65 scores to predict an increase in oxygen requirement were 0.6961, 0.6980 and 0.8327, respectively, and the differences between the three groups were statistically significant (p < 0.001). Comorbid cardiovascular disease, lymphocytopenia, elevated CRP, liver enzyme and D-dimer levels, and higher chest CT score were significantly associated with an increase in oxygen requirement
Conclusions
The expanded CURB-65 score can be a better predictor of an increase in oxygen requirement in patients with SARS-CoV-2 pneumonia.
Keywords: COVID-19, CURB-65, A-DROP, Expanded CURB-65, SARS-CoV-2 pneumonia
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is spreading worldwide and poses a serious threat to public health.
In Japan, a polymerase chain reaction (PCR) and antigen test are used to confirm the diagnosis in suspected patients of coronavirus disease 2019 (COVID-19) and patients have ready access to primary health and hospital care. Given that there is no other way of differentiating between community-acquired pneumonia (CAP) and SARS-CoV-2 pneumonia, Japanese patients with pneumonia must be examined by a doctor at the beginning of or prior to the onset of symptoms. Although COVID-19 is likely to be mild in about 80% of the patients, the remaining 20% can progress to severe disease [1]. In critically patients with COVID-19, respiratory failure is a major complication and its symptoms, including dyspnea and tachypnea, occur around one week after onset [1,2].
During the present SARS-CoV-2 epidemic, medical resources including hospital beds and health care workers must be used as effectively as possible to prevent the collapse of healthcare systems. Therefore, it is important for clinicians to assess early in the disease course whether a patient's symptoms will deteriorate. For this purpose, a versatile, easy-to-use tool is needed for predicting an increase in disease severity.
CURB-65 and A-DROP have been widely used to predict the mortality risk in patients with CAP [3,4], and these tools have also been reported to be useful for predicting a disease severity including mortality in patients with COVID-19 [5,6]. Furthermore, a number of clinical and laboratory parameters, including comorbidities, lymphopenia, thrombocytopenia, elevation of liver enzyme, high D-dimer levels and lactate dehydrogenase (LDH), and hypoalbuminemia, are thought to be helpful for predicting mortality in patients with COVID-19 [[7], [8], [9], [10], [11], [12]]. Liu et al. reported that the expanded CURB-65 score, which includes hypoalbuminemia, LDH, and thrombocytopenia in addition to the items of CURB-65, was more accurate for evaluation of the mortality risk in patients with CAP [13]. We speculated that this score could also be useful for predicting an increase in disease severity in patients with SARS-CoV-2 pneumonia.
In this retrospective study, we set an increase in disease severity as the increase in oxygen requirement which is the main outcome considering the characteristic of severe COVID-19 mentioned above. The aim of this study was to compare the accuracy of the three scoring systems, namely, A-DROP, CURB-65, and expanded CURB-65, which include vital signs and common blood analysis parameters that are easily accessible by clinicians, for predicting the increase in oxygen requirement of SARS-CoV-2 pneumonia in hospitalized patients. We also assessed whether there were any associations between an increase in oxygen requirement and known risk factors for critical illness in COVID-19 or mortality, including elevation of liver enzymes and C-reactive protein (CRP), lymphocytopenia, and the chest computed tomography (CT) score.
2. Materials and methods
2.1. Study population
We retrospectively reviewed patients with a diagnosis of SARS-CoV-2 pneumonia, confirmed by polymerase chain reaction (PCR) testing of pharyngeal or nasopharyngeal swabs or sputum specimens and chest CT findings, who were hospitalized at the Self-Defense Forces Central Hospital, Tokyo, Japan, between February 2 and August 31, 2020. Patients who declined to participate in the study, who participated in clinical trials of drugs taken before oxygen requirement increased, and those under the age of 18 years were excluded. The study population included the passengers and crew members who were aboard the Diamond Princess cruise ship in February 2020. Therefore, asymptomatic patients who were diagnosed on the cruise ship before referral to our hospital were included as previously reported [14]. Most patients with mild or moderate COVID-19 without a need for oxygen therapy were accepted on request from the district public healthcare center. The study protocol was approved by our hospital ethics committee (approval number, 01–011; approval date, March 5, 2020). Informed consent was obtained by the opt-out method.
The severity of SARS-CoV-2 pneumonia on admission was assessed using CURB-65, A-DROP, and expanded CURB-65 scores. All components of each scoring system were clearly recorded in the medical charts on admission.
The CURB-65 scoring system assesses the following parameters: advanced age (≥65 years), dehydration (blood urea nitrogen > 19 mg/dL), respiratory failure (respiratory rate ≥30), hypotension (systolic blood pressure ≤ 90 mmHg or diastolic blood pressure ≤60 mmHg), and confusion. One point is assigned for each of the CURB-65 components. The total score ranges from 0 to 5, with a score of 5 suggesting the poorest prognosis [3].
The A-DROP scoring system predicts severe respiratory illness using the following parameters: advanced age (≥70 years in men, ≥ 75 years in women), dehydration (blood urea nitrogen ≥ 21 mg/dL), respiratory failure (arterial oxygen saturation ≤ 90% or arterial oxygen pressure ≤ 60 mmHg), hypotension (systolic blood pressure ≤90 mmHg), and confusion. One point is assigned to each of the A-DROP components. The total score ranges from 0 to 5, with a score of 5 suggesting the poorest prognosis [4].
The expanded CURB-65 score includes the original five parameters in CURB-65 plus elevated serum LDH (≥230 IU/L), hypoalbuminemia (albumin <3.5 g/dL), and thrombocytopenia (platelet count < 105/mL). One point is assigned to each of the expanded CURB-65 components. The total score ranges from 0 to 8 [13]. Participant information was retrospectively obtained from the hospital medical records, laboratory findings, and chest CT images. Medical history taking, physical examination, blood tests and CT scan were performed within 24 h of admission.
2.2. Evaluation of chest CT images
All CT images were reviewed independently by two radiologists with 6 and 11 years of experience (W.M. and Y.S.) who were blinded to the patients’ clinical information, and all final decisions were reached by consensus. The CT scans were evaluated semi-quantitatively using the scoring system for COVID-19-related findings in all cases. The axial images were visually scored using previous studies for reference [15,16]. Each of the 5 lung lobes was visually scored from 0 to 5 as 0 (0%), 1 (1–5%), 2 (6–25%), 3 (25–50%), 4 (51–75%), and 5 (75–100%). The final score was the sum of the individual lobar scores and ranged from 0 (no involvement) to 25 (maximum involvement).
2.3. Definitions
We defined an increase in disease severity as an increase in oxygen requirement. Within the first 10 days of admission, we evaluated the status of patients with increased oxygen administration by 1 L/min (equivalent to 4% of increased fraction of inspired oxygen) or more compared to admission. Patients who experienced an increase in severity were classified as severe patients.
2.4. Statistical analysis
The Shapiro-Wilk test was used to assess the distribution of data. For non-normally distributed data, continuous variables are presented as the median (interquartile range). The Wilcoxon rank sum test was used to compare two groups. Kruskal-Wallis test was used to compare three or more groups. The chi-squared test was used to compare clinical characteristics among different groups. Receiver-operating characteristic (ROC) curve analysis and the area under the ROC curve were used to evaluate the ability of each scoring system to predict an increase in disease severity.
Correlations between two continuous variables were assessed using the Spearman's rank correlation coefficient. Corresponding 95% confidence interval (CI) were calculated for each variable. All statistical analyses were performed using STATA version 16.1 (StataCorp LLC, College Station, TX). A p-value <0.05 was considered statistically significant.
3. Results
A total of 214 patients were enrolled in the study. All patients were discharged from our hospital or died. Seven patients who were under 18 years of age were excluded, leaving data for 207 patients for inclusion in the analysis.
3.1. Patient characteristics
Patient characteristics are shown in Table 1 . Median age was 50 (interquartile range [IQR] 40–71) years, and 59% were men. The most common symptom on admission was cough. Forty patients (19%) were asymptomatic on admission. The median time from symptom onset to presentation was 5 (IQR 4–8) days. On admission, there were eight patients who required oxygen therapy via a nasal cannula or mask except for those who performed home oxygen therapy. All eight patients increased in oxygen requirement during hospitalization and required high-flow nasal therapy or mechanical ventilation therapy. They also met our definition of severe patients. Forty patients (19%) required supplemental oxygen, which was administered via a nasal cannula or mask in 23 patients (11%) and by non-invasive positive pressure ventilation or high-flow nasal cannula in 11 (5%); 6 patients (3%) were intubated. Three patients died (1%).
Table 1.
Characteristics of the patients.
| All patients (N = 207) | |
|---|---|
| Basic information | |
| Age, years [median (25%, 75%)] | 50 (40, 71) |
| Male gender, n (%) | 121 (59) |
| Nationality | |
| Asia including Japan, n (%) | 185 (89) |
| Japan, n (%) | 158 (76) |
| Europe, n (%) | 5 (2) |
| North or South America | 12 (6) |
| Oceania, n (%) | 5 (2) |
| Smoking status | |
| Current or ex-smoker, n (%) | 66 (32) |
| Never smoker, n (%) | 141 (68) |
| Comorbidities | |
| Any, n (%) | 101 (49) |
| Cardiovascular disease, n (%) | 12 (6) |
| Diabetes, n (%) | 12 (6) |
| Chronic respiratory disorder, n (%) Requirement of HOT, n (%) |
20 (10) 2 (1) |
| Malignancy, n (%) | 6 (3) |
| Asymptomatic cases, n (%) | 40 (19) |
| Symptomatic cases, n (%) | 167 (81) |
| Patients with respiratory failureexcept for those who required HOT, n (%) | 8 (4) |
| Nasal canula | 5 (2) |
| Oxygen mask | 3 (1) |
| Time from symptom onset to presentation, days median[median (25%, 75%)] | 5 (4, 8) |
| Symptoms on admission | |
| Fever, n (%) | 63 (30) |
| Cough, n (%) | 79 (38) |
| Headache, n (%) | 26 (13) |
| Fatigue, n (%) | 44 (21 |
| Sore throat, n (%) | 21 (10) |
| Diarrhea, n (%) | 17 (8) |
| Nasal discharge, n (%) | 26 (13) |
| Dyspnea, n (%) | 24 (12) |
| Laboratory findings on admission | |
| White blood cell, 104/μL [median (25%,75%)] |
5290 (4060, 6530) |
| Lymphocyte count, 104/μL [median (25%,75%)] |
1252 (860, 1694) |
| Platelet, 104/μL[median (25%,75%)] | 21.2 (17.5, 26.6) |
| Albumin, g/dL | 4.1 (3.8, 4.3) |
| CRP, mg/dL[median (25%,75%)] | 0.73 (0.13, 2.79) |
| BUN, mg/dL[median (25%,75%)] | 13 (11, 17) |
| LDH (mg/L), IU/L [median (25%,75%)] |
211 (177, 266) |
| AST, IU/L[median (25%,75%)] | 27 (21, 37) |
| ALT, IU/L[median (25%,75%)] | 27 (17, 42) |
| Outcome | |
| Severe patients, n (%) | 40 (19) |
| Nasal canula or oxygen mask, n (%) | 23 (11) |
| Non-invasive positive pressure ventilationor high flow nasal, n (%) | 11 (5) |
| Intubation, n (%) | 6 (3) |
| Death, n (%) | 3 (1) |
HOT = home oxygen therapy; CRP= C-reactive protein; BUN = blood urea nitrogen; LDH = lactate dehydrogenase; AST = aspartate aminotransferase; ALT = alanine aminotransferase.
3.2. Correlations between each scoring system and an increase in oxygen requirement
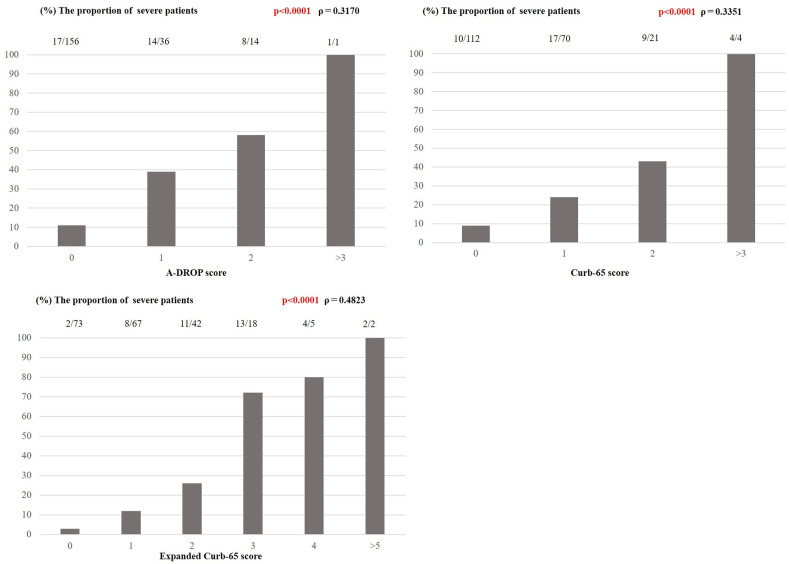
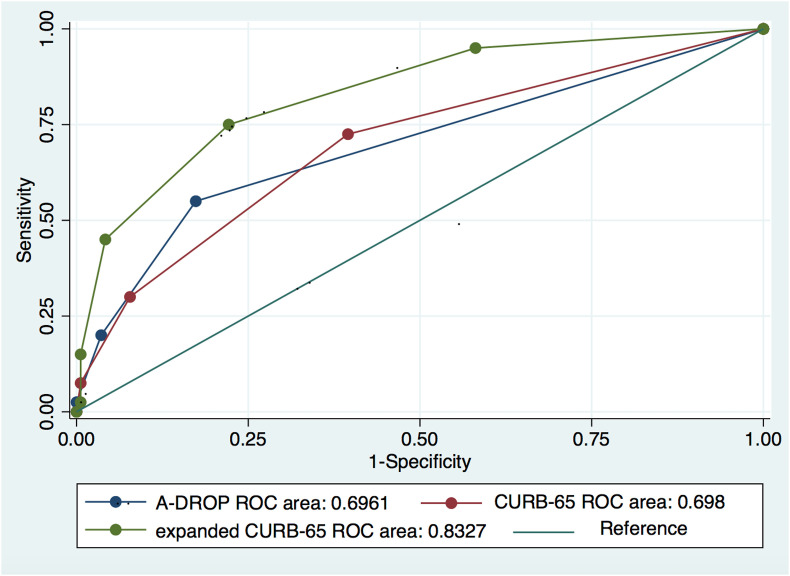
Fig. 1 shows the outcome according to A-DROP, CURB-65, and expanded CURB-65 score subgroup and the correlation between each of the three scoring systems and disease severity. Scores obtained by the three methods had a significant positive correlation with disease severity. The expanded CURB-65 score showed the strongest correlation (Spearman's coefficient, ρ = 0.48; p < 0.0001) with an area under the curve (AUC) of 0.8327 (95% CI, 0.7636–0.9018). The AUCs for A-DROP and CURB-65 were 0.6961 (95% CI, 0.6108–0.7814) and 0.6980 (95% CI, 0.61021–0.7858), respectively (Fig. 2 ). The difference in AUCs between the groups was statistically significant (p < 0.001), suggesting that the expanded CURB-65 score had the best predictive ability for identifying patients with SARS CoV-2 pneumonia who would have an increase in oxygen requirement. The optimal cutoff for the expanded CURB-65 total score was 2; using this cutoff, sensitivity was 75% and specificity was 78%.
Fig. 1.
The proportions of SARS-CoV-2 pneumonia patients with an increase in severity for each subgroup of A-DROP, Curb-65 and expanded Curb-65 and the correlation between each of the three scoring systems and the increase in severity.
Fig. 2.
ROC curves for three scoring systems of SARS-CoV-2 pneumonia patients.
3.3. Factors related to an increase in oxygen requirement
We compared patient characteristics that have been reported to be risk factors for mortality in patients with COVID-19 according to whether there was an increase in oxygen requirement. Comorbid cardiovascular disease was significantly more common in severe patients than in non-severe patients (p = 0.04). Only 1 patient developed confusion and none had a low platelet count. All the items in the expanded CURB-65, except for confusion and thrombocytopenia, were assessed using the chi-squared test. Age ≥65 years, respiratory rate ≥30/min, albumin <3.5 g/dL, and serum LDH >230 μ/L were significantly associated with an increase in oxygen requirement. In addition, lymphocytopenia, elevated C-reactive protein (CRP), liver enzyme and D-dimer levels, and higher chest CT score were significantly associated with an increase in oxygen requirement (Table 2 ).
Table 2.
Statistical significance of clinical findings for severe patients who increased in oxygen requirement, and non-severe patients.
| severe n (%), N = 40 |
non-severe n (%), N = 167 |
P-value | |
|---|---|---|---|
| Nationality | 0.53 | ||
| Asia | 35 (88) | 151 (90) | |
| Europe | 0 (0) | 4 (2) | |
| North or South America | 3 (8) | 9 (5) | |
| Oceania | 2 (5) | 3 (2) | |
| Current or ex-smoker | 14 (35) | 52 (31) | 0.11 |
| Comorbidities | |||
| Cardiovascular disease | 5 (13) | 7 (4) | 0.04 |
| Diabetes | 3 (8) | 9 (5) | 0.61 |
| Hypertension | 9 (23) | 24 (14) | 0.88 |
| Chronic respiratory disorder | 5 (13) | 15 (9) | 0.5 |
| Malignancy | 1 (3) | 5 (3) | 0.87 |
| Age (≧65 years old) | 10 (25) | 70 (42) | <0.05 |
| Laboratory abnormalities | |||
| Blood pressure (sBP< 90 mmHg or dBP< 60 mmHg) | 2 (5) | 7 (4) | 0.82 |
| Respiratory rate (≧30/min) | 4 (10) | 2 (1) | <0.01 |
| BUN (>19 mg/dL) | 8 (20) | 21 (13) | 0.22 |
| Albumin (<3.5 g/dL) | 9 (23) | 12 (7) | <0.01 |
| LDH (>230 u/L) | 27 (68) | 52 (31) | <0.001 |
| Lymphopenia (<800/μL) | 14 (35) | 33 (19.7) | 0.04 |
| elevation of CRP (≧10 mg/dL) | 11 (28) | 5 (3) | <0.001 |
| elevation of liver enzyme (AST> 40 IU/L and/or ALT> 40 IU/L) | 19 (48) | 43 (26) | 0.001 |
| elevation of D-dimer (>1 mg/dL) (N = 92) | N = 39 15 (39) |
N = 53 13 (25) |
<0.001 |
| CT [score median (25%, 75%)] | 9 (4.5, 13) | 3 (2, 7) | <0.001 |
sBP = systolic blood pressure; dBP = diastolic blood pressure; BUN = blood urea nitrogen; LDH = lactate dehydrogenase; CRP= C-reactive protein; AST = aspartate aminotransferase; ALT = alanine aminotransferase.
4. Discussion
We found that the three scoring systems evaluated in this study were useful for predicting an increase in oxygen requirement of SARS-CoV-2 pneumonia, and that the expanded CURB-65 score was the most useful.
In the current pandemic, not only clinicians specialized in infectious diseases and respiratory medicine specialists but also those specialized in other areas of internal medicine and general practitioners will encounter patients with COVID-19. Examining these patients and implementing the necessary infection control measures adds a substantial burden to their clinical workload. There is also an ever-present risk that healthcare systems could collapse due to limited healthcare facilities and personnel. Therefore, we need to focus on developing the most efficient methods for examination of patients with COVID-19. Furthermore, COVID-19 can be community-acquired, and clinicians cannot differentiate between CAP and SARS-CoV-2 pneumonia on examination when there is no clear history of exposure to COVID-19. For all these reasons, there is a need for a versatile prediction tool that can be used when the even causative pathogen is unclear early in the disease course of patients with pneumonia.
In this study, we did not include the Pneumonia Severity Index and SMART-COP score as target prediction tools because of the large number of factors involved and the relatively complicated calculations needed [17,18].
In a previous study that investigated the correlation between the expanded CURB-65 score and mortality in patients with CAP, it revealed high odds ratios for confusion and respiratory rate for 30-day mortality [13]. In our study, we found significant associations between an increase in oxygen requirement of SARS-CoV-2 pneumonia and tachypnea, hypoalbuminemia, and increased serum LDH among the components of expanded CURB-65.
Previous studies of COVID-19 have suggested that certain comorbidities, namely, cardiovascular disease, diabetes mellitus, hypertension, chronic lung disorders, and cancer, are potential risk factors for severe illness and mortality [8,9]. In our study, only cardiovascular disease was associated with an increase in oxygen requirement. We also found that several laboratory abnormalities, including lymphopenia and elevated liver enzyme, CRP, and D-dimer levels, were significantly associated. There was also a significant correlation between the chest CT score on admission, which was used to assess the extent of pneumonia, and an increase in oxygen requirement.
The results of our study have potentially important implications for clinical practice. Specifically, when two or more items from the expanded CURB-65 score are present and other risk factors are seen on examination, including comorbidities and elevated liver enzymes, inflammatory markers, and D-dimer, careful follow-up with the prediction of the onset or progression of respiratory failure is necessary. In addition to this, the extent of CT shadow on admission is also helpful for predicting the increase in oxygen requirement.
Several antiviral agents with immunomodulating effects have been proposed for use in patients with COVID-19. In this study, 18 out of 40 severe patients received antiviral agents or systemic steroid therapy. Four patients who needed oxygen therapy on admission were given antiviral and/or systemic steroid therapy before an increase in oxygen requirement was noted. The remaining 14 patients had not been given these drugs and had been provided with only conservative and supportive care before they increased oxygen requirement. Therefore, in this study, we could not see the effect of these drugs on our outcome.
To our knowledge this is the first study to investigate whether the scoring systems developed to predict mortality in patients with CAP can be used to predict an increase in oxygen requirement of SARS-CoV-2 pneumonia considering the characteristics of COVID-19 with respiratory failure.
This study has several limitations. First, this is a small study population. Second, it had a retrospective design and was performed in a single center, so there was selection bias. Moreover, around 40% of the patients enrolled in the study were transferred from the cruise ship Diamond Princess, which resulted in a large cluster. Relatively healthy patients were included and asymptomatic ones were also hospitalized. Thus, the patient population was not general in terms of clinical background, as mentioned by another study we performed in the same hospital [14]. Therefore, the generalizability of our results is limited in terms of locality and timing. Fourth, we could not determine whether the A-DROP, CURB-65, and expanded CURB-65 scores can predict mortality because there were only three deaths during the study period. Lastly, we were unable to assess the effect of antiviral or anti-inflammatory agents as we excluded the patients who participated in clinical trials of drugs taken before oxygen requirement increased and the number of patients who had given them before they increased oxygen requirement was low.
In conclusion, this study suggests that the expanded CURB-65 score is better than the A-DROP or CURB-65 score as a predictor of an increase in oxygen requirement patients with SARS-CoV-2 pneumonia.
Declaration of competing interest
None.
Acknowledgements
We thank everyone involved in the COVID-19 management and treatment team from the Self-Defense Forces Central Hospital in Japan and members who were assembled from other institutes of Japan Self-Defense Forces.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wan D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. https://jamanetwork.com/journals/jama/fullarticle/2761044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim W.S., van der Eerden M.M., Laing R., Boersma W.G., Karalus N., Town G.I., et al. Defining community acquired pneumoniae severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyashita N., Matsushima T., Oka M. Japanese Respiratory S. The JRS guidelines for the management of community-acquired pneumonia in adults: an update and new recommendation. Intern Med. 2006;45:419–428. doi: 10.2169/internalmedicine.45.1691. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen Y., Corre F., Honsel V., Curac S., Zarrouk V., Fantin B., et al. Applicability of the CURB-65 pneumonia severity score for outpatient treatment of COVID-19. J Inf. 2020;81:e96–e98. doi: 10.1016/j.jinf.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan G., Tu C., Zhou F., Liu Z., Wang Y., Song B., et al. Comparison of severity scores for COVID-19 patients with pneumonia: a retrospective study. Eur Respir J. 2020:2002113. doi: 10.1183/13993003.02113-2020. https://erj.ersjournals.com/content/early/2020/07/06/13993003.02113-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian W., Jiang W., Yao J., Nicholson C.J., Li R.H., Sigurslid H.H., et al. Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2020 May 22 doi: 10.1002/jmv.26050. 10.1002/jmv.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrisson S.L., Fazio-Eynullayeva E., Lane D.A., Underhill P., Lip G.Y.H. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai M., Liu D., Liu M., Zhou F., Li G., Chen Z., et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Canc Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. http://cancerdiscovery.aacrjournals.org/contet/10/6/783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang J., Cheng A., Kumar R., Fang Y., Chen G., Zhu Y., et al. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol. 2020 May 14 doi: 10.1002/jmv.26003. 10.1002/jmv.26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao D., Zhou F., Luo L., Xu M., Wang H., Xia J., et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol. 2020;7:e671–e678. doi: 10.1016/s2352-3026(20)30217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F., Yu T., Du R., Fan G., Liu Y., Liy Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J.L., Xu F., Zhou H., Wu X.J., Shi L.X., Lu R.Q., et al. Expanded CURB-65: a new score system predicts severity of community-acquired pneumonia with superior efficiency. Sci Rep. 2016;6:22911. doi: 10.1038/srep22911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabata S., Imai K., Kawano S., Ikeda M., Kodama T., Miyoshi K., et al. Clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on the Diamond Princess cruise ship: a retrospective analysis. Lancet Infect Dis. 2020;20:1043–1050. doi: 10.1016/S1473-3099(20)30482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan F., Ye T., Sun P., Gui S., Liang B., Li L., et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan M., Yin W., Tao Z., Tan W., Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PloS One. 2020;15 doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fine M.J., Aubie T.E., Yealy D.M., Hanusa B.H., Weissfeld L.A., Singer D.E., et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. https://www.nejm.org/doi/full/10.1056/NEJM199701233360402 [DOI] [PubMed] [Google Scholar]
- 18.Charles P.G., Wolfe R., Whitby M., Fine M.J., Fuller A.J., Stirling R., et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47:375–384. doi: 10.1086/589754. [DOI] [PubMed] [Google Scholar]