Abstract
Background
Critically ill coronavirus disease 2019 (COVID-19) patients present with a hypercoagulable state with high rates of macrovascular and microvascular thrombosis, for which hypofibrinolysis might be an important contributing factor.
Methods
We retrospectively analysed 20 critically ill COVID-19 patients at Innsbruck Medical University Hospital whose coagulation function was tested with ClotPro® and compared with that of 60 healthy individuals at Augsburg University Clinic. ClotPro is a viscoelastic whole blood coagulation testing device. It includes the TPA test, which uses tissue factor (TF)-activated whole blood with added recombinant tissue-derived plasminogen activator (r-tPA) to induce fibrinolysis. For this purpose, the lysis time (LT) is measured as the time from when maximum clot firmness (MCF) is reached until MCF falls by 50%. We compared COVID-19 patients with prolonged LT in the TPA test and those with normal LT.
Results
Critically ill COVID-19 patients showed hypercoagulability in ClotPro assays. MCF was higher in the EX test (TF-activated assay), IN test (ellagic acid-activated assay), and FIB test (functional fibrinogen assay) with decreased maximum lysis (ML) in the EX test (hypofibrinolysis) and highly prolonged TPA test LT (decreased fibrinolytic response), as compared with healthy persons. COVID-19 patients with decreased fibrinolytic response showed higher fibrinogen levels, higher thrombocyte count, higher C-reactive protein levels, and decreased ML in the EX test and IN test.
Conclusion
Critically ill COVID-19 patients have impaired fibrinolysis. This hypofibrinolytic state could be at least partially dependent on a decreased fibrinolytic response.
Keywords: coagulation, COVID-19, critically ill, D-dimer, fibrinogen, fibrinolysis, tissue plasminogen activator, viscoelastic test
Editor's key points.
-
•
COVID-19 patients are in a hypercoagulable state with high rates of macrovascular and microvascular thrombosis, which might involve hypofibrinolysis.
-
•
The authors retrospectively analysed fibrinolysis in 20 critically ill COVID-19 patients compared with healthy controls ex vivo using ClotPro® thromoboelastometry.
-
•
Critically ill COVID-19 patients were hypercoagulable with impaired fibrinolysis evident in prolonged clot lysis times.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak and its illness coronavirus disease 2019 (COVID-19) brought on a pandemic with high death tolls around the globe.1 , 2 Coagulation has been acknowledged as an important contributor to disease severity, as critically ill COVID-19 patients show distinct hypercoagulability3 with high D-dimers and fibrin degradation product (FDP) levels associated with poor outcome.4 , 5 This hypercoagulability is also seen in viscoelastic tests such as rotational thromboelastography (TEG®)6 and rotational thromboelastometry (ROTEM®)3 and is clinically reflected in a high rate of thromboembolic events,7 especially fatal pulmonary embolism.8 Increased alveolar capillary microthrombi are also characteristic for patients with COVID-19 than for patients with other severe respiratory viral diseases such as influenza.9
A further aggravating factor contributing to the thrombotic complications and thus to the progression and severe course of COVID-19 could be impaired fibrinolysis. In sepsis impaired fibrinolysis is associated with disease severity, markers of cellular damage, and subsequent mortality.10 , 11 In critically ill COVID-19 patients, impaired fibrinolysis, measured as elevated D-dimer and clot lysis at 30 min with TEG®, is associated with thrombosis and the need for haemodialysis.12 Venous thromboembolic events are associated with reduced clot lysis in ROTEM® as well.13 One factor, potentially leading to such a hypofibrinolytic state, could be elevated plasminogen activator inhibitor 1 (PAI-1) and thrombin activatable fibrinolysis inhibitor (TAFI) found in COVID-19 patients.14 Reduced fibrinolytic response also seems to participate in the hypofibrinolytic state of COVID-19 patients, as a self-validated tissue-derived plasminogen activator (tPA)-modified ROTEM test showed.14 However, in this research-based, modified ROTEM assay tPA had to be added manually and therefore has no CE (Conformité Européenne; European Conformity) certification. This makes comparison between centres problematic and the ROTEM assay could be run only on non-cartridge-based ROTEM systems,14 which may not be available at every ICU.
To assess the coagulation status and fibrinolytic situation in our COVID-19 patients, we used ClotPro®, which, including the commercially available assays, is CE-marked. To measure the fibrinolytic reaction to tPA, we used the ClotPro TPA test, a recombinant tissue-derived plasminogen activator (r-tPA) challenge assay. We studied the occurrence of reduced fibrinolytic response and which routinely measured inflammatory and coagulatory parameters are associated with fibrinolysis in critically ill COVID-19 patients.
Methods
This retrospective study includes 20 critically ill COVID-19 patients (confirmed by polymerase chain reaction [PCR]) and 60 healthy persons. Remainder samples from routine blood donations made by healthy persons were analysed. All critically ill COVID-19 patients treated between the start of the COVID-19 pandemic and April 17, 2020 with at least one ClotPro measurement (first on April 2, 2020) during their stay in an ICU of Innsbruck Medical University Hospital were included in the analysis. ClotPro measurements were done when the physician felt the need for rapid insight into blood coagulation because extensive hypercoagulability was suspected. Hypercoagulability was defined either by difficulties in reaching the 0.3–0.5 IU ml−1 anti-Xa target range despite high doses of low molecular weight heparin (LMWH) or elevated D-dimer levels of >2000 μg L−1. Only one patient did not fulfil one of these criteria, but his coagulation status was analysed because of developing thrombocytopaenia.
This study was approved by the institutional review board of the Medical University of Innsbruck (Vote #1139/2020). There was no need to obtain oral and written informed consent from the study participants because the data were retrospectively collected and anonymously processed. In order to have normal values for ClotPro analysis, leftover whole blood samples from healthy blood donors were obtained for ClotPro measurements. The institutional review board of Augsburg University Clinic gave its permission for this analysis without the need for obtaining oral or written informed consent from the blood donors (Vote #2018-13).
Data collection
We collected the patient characteristics age, sex, and diagnosed underlying diseases, if applicable. Blood gas and routine laboratory test results (complete blood count, blood chemistry, organ function biomarkers, inflammation and plasma coagulation parameters) at ICU admission and closest to ClotPro measurements were included. ICU charts were used to collect data on organ function, the Simplified Acute Physiology Score 3 (SAPS 3; a prediction model applied at ICU admission to determine probability of death at hospital discharge)15 was calculated for the ICU admission day, and Sequential Organ Failure Assessment (SOFA) scores were calculated for the day of ICU admission and ClotPro assay.
ClotPro
ClotPro measurements were routinely carried out in COVID-19 patients in order to gain rapid insight into blood coagulation properties when extensive hypercoagulability was suspected and to assess their fibrinolytic response. ClotPro is a commercially available (enicor GmbH, Munich, Germany), CE-marked viscoelastic in vitro coagulation analyser used mainly in Central Europe as a point-of-care test.16 , 17 It uses pipettes prefilled with starting and modifying agents and 340 μl of citrated whole blood for initiating measurement. A stationary pin is placed in a clockwise and counter-clockwise moving cup, from which the reduction of movement is detected and charted as the amplitude resulting in thromboelastometry curves known from other viscoelastic test methods such as ROTEM. The samples from healthy blood donors and COVID-19 patients were run within 4 h of blood draw.
Standard tests used in COVID-19 patients were the EX test (tissue factor (TF)-activated assay), IN test (ellagic acid-activated assay), FIB test (functional fibrinogen assay), and TPA test (r-tPA within an extrinsic pathway-based assay), and the RVV test (Russel viper venom-based assay) if the patient received heparin, or the ECA test (ecarin-based assay) if the patient received argatroban, a direct thrombin inhibitor, as anticoagulant. The RVV test is sensitive to coagulation factor X inhibitors, whereas the ECA test is sensitive to direct thrombin inhibitors. The TPA test measures the fibrinolytic response, which might result in impaired fibrinolysis. The TPA test measures fibrinolysis by adding 650 ng ml−1 r-tPA to TF-activated whole blood. Lysis time (LT) is the time to dissolution of 50% of the clot (defined as maximum clot firmness [MCF]) by r-tPA once MCF is reached. Although the TPA test is currently available exclusively for research use by the manufacturer, this assay is a standardised assay with the reagents already placed in fixed doses in the pipettes. In the context of the COVID-19 pandemic, we use this assay routinely in critically ill COVID-19 patients to evaluate their fibrinolytic response.
Statistical analysis
We compared the LT of COVID-19 patients with that of 60 healthy persons who were analysed at Augsburg University Clinic, for whom the 95% confidence interval (CI) of the TPA test LT was 157–393 s. Therefore, decreased fibrinolytic response is defined as a LT >393 s. The critically ill COVID-19 patients were further divided into two groups to compare patients with normal fibrinolytic response (LT within reference range) and those with decreased fibrinolytic response (LT > 393 s).
Differences between groups, that is either healthy volunteers vs COVID-19 patients or COVID-19 patients with normal vs decreased fibrinolytic response, were assessed using Fisher's exact test for binary variables and the Wilcoxon rank-sum test for continuous variables. We provide effect sizes as odds ratios or estimated median differences, with corresponding 95% CIs.
Results
Patient characteristics
The healthy volunteers were 38 (28–46.25) yr, and critically ill COVID-19 patients were 61.5 (56.25–68) yr old (median [range]; P<0.01). Sex distribution did not differ between healthy persons and COVID-19 patients (P=0.08). All patients in both study populations were Caucasian. The mean ICU length of stay of the COVID-19 patients was 26.5 (10) days with ICU mortality of 20% (4 out of 20). In median, ClotPro was performed on day 8.5 (4.5–15) of ICU stay, and blood gas analyses (BGA) were made within 63.5 (48.5–98.5) min and routine laboratory measures within 8.4 (7.9–10.3) h of ClotPro assays.
Hypercoagulability in critically ill COVID-19 patients
A hypercoagulable state was seen in TF-activated ClotPro assays in COVID-19 patients as compared with healthy individuals with an EX test MCF of 68 (63–71) mm vs 61 (58–64) mm (P<0.01) and a FIB test MCF of 34 (28–39) mm vs 17 (13–20) mm (P<0.01). Although there was no significant difference in EX test clotting time (CT) of 52 (47–76) s vs 48.5 (45–54) s (P=0.08), IN test CT was longer in COVID-19 patients, namely 188 (167–215) s vs 159 (153–166) s (P<0.01), although still within the reference range (Table 1 ).
Table 1.
ClotPro analysis in healthy individuals compared with critically ill COVID-19 patients.
| Total (n=80) | Healthy (n=60) | COVID-19 (n=20) | Estimate with 95% CIa | P-valueb | Not known | |
|---|---|---|---|---|---|---|
| TPA test | ||||||
| CT (s) | 43 (37–51) | 42 (36–46) | 50 (42–90) | –12 (–38 to –3) | <0.01 | 0/0 |
| LT (s) | 238 (198–324) | 210 (186–261) | 508 (365–827) | –265 (–358 to –186) | <0.01 | 0/0 |
| MCF (mm) | 34 (29–41) | 32 (28–36) | 55 (38.75–64) | –21 (–27 to –13) | <0.01 | 0/0 |
| ML (%) | 95 (94–96) | 95 (94–96) | 97 (95–98) | –2 (–2 to –1) | <0.01 | 0/0 |
| EX test | ||||||
| CT (s) | 49 (45–55) | 48.5 (45–54) | 51.5 (47–76) | –5 (–16 to 1) | 0.08 | 0/0 |
| A5 (mm) | 49.5 (46–55) | 48 (44–52) | 58 (50–61) | –9 (–12 to –5) | <0.01 | 0/0 |
| A10 (mm) | 57 (54–62) | 56 (53–59) | 65.5 (58–68) | –8 (–11 to –4) | <0.01 | 0/0 |
| A20 (mm) | 61 (58–65) | 60 (58–63) | 68 (62–70) | –6.39 (–9 to –4) | <0.01 | 0/0 |
| MCF (mm | 62 (59–65) | 61 (58–64) | 68.5 (63–71) | –6 (–9 to –3) | <0.01 | 0/0 |
| ML (%) | 5 (3–8) | 6 (4–8) | 3 (2–7) | 2 (1–4) | <0.01 | 0/1 |
| IN test | ||||||
| CT (s) | 162 (154–176) | 159 (153–166) | 188 (168–215) | –30 (–47 to –17) | <0.01 | 0/0 |
| A5 (mm) | 45 (42–48) | 44 (41–47) | 52 (44–57) | –8 (–11 to –4) | <0.01 | 0/0 |
| A10 (mm) | 54 (50–57) | 53 (50–55) | 60 (53–64) | –7 (–10 to –4) | <0.01 | 0/0 |
| A20 (mm) | 59 (55–61) | 58 (55–60) | 64.5 (58–68) | –6 (–9 to –3) | <0.01 | 0/0 |
| MCF (mm) | 59 (56–62) | 59 (56–61) | 64 (59–69) | –5 (–8 to –2) | <0.01 | 0/0 |
| ML (%) | 5 (3–7) | 5 (3.5–7) | 4 (3–8) | 1 (–1 to 2) | 0.50 | 1/3 |
| FIB test | ||||||
| CT (s) | 68 (62–77) | 68 (63–75) | 72 (53–108) | –4 (–25 to 9) | 0.57 | 0/0 |
| A5 (mm) | 14 (10–20) | 12 (9–15) | 29 (23–32) | –16 (–19 to –14) | <0.01 | 0/0 |
| A10 (mm) | 16 (11–22) | 14 (10–17) | 31 (25–35) | –17 (–20 to –14) | <0.01 | 0/0 |
| A20 (mm) | 17 (12–24) | 15 (12–18) | 33 (27–37) | –17 (–21 to –14) | <0.01 | 0/0 |
| MCF (mm) | 19 (14–26) | 17 (13–20) | 34 (28–39) | –17 (–21 to –14) | <0.01 | 0/0 |
Continuous data are presented as medians (25th to 75th percentile).
A5, amplitude after 5 min; A10, amplitude after 10 min; A20, amplitude after 20 min; CI, confidence interval; CT, clotting time; LT, lysis time; MCF, maximal clot firmness; ML, maximum lysis.
Odds ratios for binary variables and estimated median difference for continuous variables (CI).
Assessed with Fisher's exact test for categorical variables and the Wilcoxon rank sum test for continuous variables.
COVID-19 patients had a median BMI of 28.8 (24.3–31.0). Their medical history showed that 50% had hypertension, 40% cardiovascular diseases, 25% diabetes mellitus, 10% solid oncologic diseases, and 5% immunologic diseases. Two patients (10%) also had a history of thromboembolic events before contracting SARS-CoV-2 (Table 2 ). Median SAPS 3 (on ICU admission) for our critically ill COVID-19 patients was 56 (53–64) points; SOFA score was 6.5 (3–8.2) at ICU admission and 6.5 (6–8.2) on the day of ClotPro assay (Supplementary file 1, Table S3).
Table 2.
Baseline characteristics of COVID-19 patients.
| Total (n=20) | TPA LT ≤393 s (n=6) | TPA LT >393 s (n=14) | Estimate with 95% CIa | P-valueb | Not known | |
|---|---|---|---|---|---|---|
| Age (yr) | 61.5 (56.25–68) | 66 (61–70.25) | 61 (54.75–65.25) | 6 (–4 to 15) | 0.27 | 0/0 |
| Female sex | 6/20 (30%) | 2/6 (33.3%) | 4/14 (28.6%) | 0.81 (0.07–12.38) | 1 | 0/0 |
| Height (cm) | 174 (170–176) | 166 (163–169) | 174 (170–177) | –10 (–25 to 4) | 0.19 | 4/5 |
| Weight (kg) | 82 (74.2–97) | 82 (80–98) | 84 (73.9–95) | 4.5 (–18 to 23) | 0.71 | 1/4 |
| BMI (kg m−2) | 28.8 (24.3–31.0) | 28.3 (25.9–30.7) | 28.8 (25.6–30.8) | 2.0 (–9.2 to 13.3) | 0.71 | 4/6 |
| History of thromboembolic events | 2/20 (10%) | 0/6 (0%) | 2/14 (14.3%) | Inf (0.08 to Inf) | 1 | 0/0 |
| Medical history | ||||||
| Cardiovascular | 8/20 (40%) | 4/6 (66.7%) | 4/14 (28.6%) | 0.22 (0.01–2.24) | 0.16 | 0/0 |
| Central nervous system | 4/20 (20%) | 1/6 (16.7%) | 3/14 (21.4%) | 1.34 (0.08–85.67) | 1 | 0/0 |
| Coagulation | 2/20 (10%) | 0/6 (0%) | 2/14 (14.3%) | Inf (0.08 to Inf) | 1 | 0/0 |
| Diabetes mellitus | 5/20 (25%) | 0/6 (0%) | 5/14 (35.7%) | Inf (0.42 to Inf) | 0.26 | 0/0 |
| Gastrointestinal | 3/20 (15%) | 0/6 (0%) | 3/14 (21.4%) | Inf (0.17 to Inf) | 0.52 | 0/0 |
| Haematologic | 1/20 (5%) | 0/6 (0%) | 1/14 (7.1%) | Inf (0.01 to Inf) | 1 | 0/0 |
| Hepatologic | 3/20 (15%) | 2/6 (33.3%) | 1/14 (7.1%) | 0.17 (0–4.11) | 0.20 | 0/0 |
| Hypertension | 10/20 (50%) | 2/6 (33.3%) | 8/14 (57.1%) | 2.54 (0.26–37.18) | 0.63 | 0/0 |
| Immune | 1/20 (5%) | 1/6 (16.7%) | 0/14 (0%) | 0 (0–16.71) | 0.3 | 0/0 |
| Scores on ICU admission | ||||||
| SAPS 3 (points) | 56 (53–64) | 56 (55–66) | 55 (53–61.5) | 3 (–6 to 14) | 0.38 | 1/0 |
| SOFA Respiratory system (points) | 3 (3–4) | 3 (3–3) | 3 (3–4) | 0 (–1 to 1) | 0.79 | 0/0 |
| SOFA Respiratory system >2 | 17/20 (85%) | 6/6 (100%) | 11/14 (78.6%) | 0 (0–5.81) | 0.52 | 0/0 |
| SOFA Coagulation (points) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.26 | 0/0 |
| SOFA Liver (points) | 0 (0–0) | 0 (0–0.75) | 0 (0–0) | 0 (0–1) | 0.44 | 0/0 |
| SOFA Cardiovascular system (points) | 3 (0–3.25) | 0 (0–2.25) | 3 (0.75–3.75) | –1 (–3 to 0) | 0.20 | 0/0 |
| SOFA Cardiovascular system >2 | 12/20 (60%) | 2/6 (33.3%) | 10/14 (71.4%) | 4.57 (0.45–70.73) | 0.16 | 0/0 |
| SOFA Nervous system (points) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.59 | 0/0 |
| SOFA Renal (points) | 0 (0–1) | 0 (0–0) | 0 (0–1) | 0 (–1 to 0) | 0.36 | 0/0 |
| SOFA (points) | 6.5 (3–8.25) | 3.5 (3–6.25) | 7 (3.5–8.75) | –2 (–5 to 1) | 0.30 | 0/0 |
Binary data are presented as no./total no. (%), continuous data as medians (25th to 75th percentile).
CI, confidence interval; TPA LT, lysis time in TPA test; SAPS 3, Simplified Acute Physiology Score 3; SOFA, Sequential Organ Failure Assessment.
Odds ratios for binary variables and estimated median difference for continuous variables (CI).
Assessed with Fisher's exact test for categorical variables and the Wilcoxon rank sum test for continuous variables.
Hypofibrinolysis
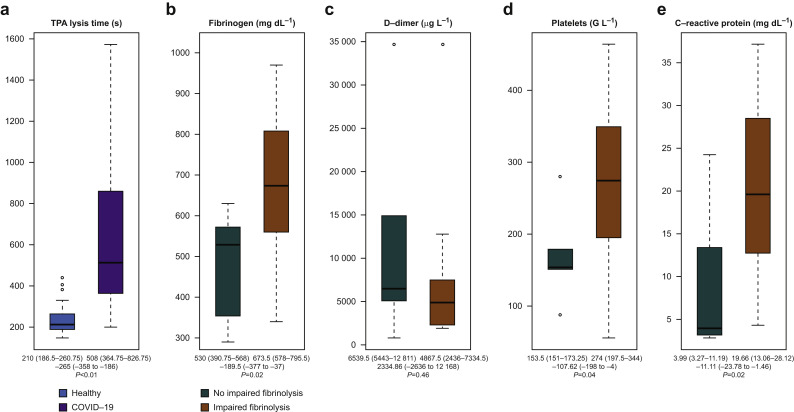
Critically ill COVID-19 patients presented with impaired fibrinolysis compared with healthy individuals (Table 1). This was indicated by longer fibrinolytic response reflected by the LT in the TPA test than in healthy persons: 508 (365–827) s vs 210 (186–261) s (P<0.01), respectively (Fig. 1 a). Less lysis was also seen in the EX test, where maximum lysis (ML) was significantly lower in the COVID-19 population than in healthy persons: 3% (2–7%) vs 6% (4–8%) (P<0.01).
Fig 1.
Decreased fibrinolytic response in critically ill COVID-19 patients. The numbers below the box plots depict median (25th to 75th percentile) and estimated median differences with corresponding 95% confidence intervals (CIs) and P-value. (a) Prolonged lysis time (LT) in the TPA test assay in critically ill COVID-19 patients as compared with healthy persons. Fibrinogen levels (b), platelet counts (d), and levels of C-reactive protein (e) were significantly elevated in patients with decreased fibrinolytic response, whereas D-dimers did not differ between patients with and without impaired fibrinolysis (c).
Of the 20 critically ill COVID-19 patients with suspected extensive hypercoagulability, 14 (70%) showed decreased fibrinolytic response as shown in the r-tPA challenge test (LT >393 s), whereas only six patients had a normal response to fibrinolysis activation (LT ≤393 s). Decreased fibrinolytic response (LT >393 s in the TPA test) was also associated with hypofibrinolysis as shown by a significantly lower EX test ML of 2% (1–3%) and IN test ML of 3% (2–4%) compared with 8% (5–9%) and 8% (5–10%) in critically ill COVID-19 patients with normal fibrinolytic response (P<0.01 and P=0.04), respectively. COVID-19 patients with decreased fibrinolytic response (prolonged TPA LT) also showed increased clot strength as reflected by a higher EX test MCF than did those within normal fibrinolytic response (70 [68 to 72] mm vs 60 [57–63] mm; P<0.01) (Table 3 ).
Table 3.
ClotPro analysis of critically ill COVID-19 patients with decreased fibrinolytic response compared with critically ill COVID-19 patients with normal fibrinolytic response.
| Total (n=20) | TPA LT ≤393 s (n=6) | TPA LT >393 s (n=14) | Estimate with 95% CIa | P-valueb | Not known | |
|---|---|---|---|---|---|---|
| TPA test | ||||||
| CT (s) | 49.5 (41.5–90.2) | 73.5 (46.5–109.5) | 47.5 (40.5–84.25) | 12 (–19 to 66) | 0.27 | 0/0 |
| LT (s) | 508 (364.75–826.75) | 321.5 (278.75–347) | 573 (503–982) | –296.5 (–688 to –160) | <0.01 | 0/0 |
| MCF (mm) | 55 (38.75–64) | 34 (27.5–43.5) | 62 (55–64.75) | –22.98 (–36 to –10) | <0.01 | 0/0 |
| ML (%) | 97 (95–98) | 95.5 (95–96.75) | 97 (96.25–98) | –1 (–3 to 0) | <0.01 | 0/0 |
| EX test | ||||||
| CT (s) | 51.5 (46.75–76.25) | 72 (46.25–106) | 51.5 (47–65.5) | 13.49 (–15 to 62) | 0.59 | 0/0 |
| A5 (mm) | 58 (50–61) | 49 (47.25–52.25) | 59.5 (57.25–64) | –10 (–15 to –3) | 0.01 | 0/0 |
| A10 (mm) | 65.5 (58–67.5) | 56.5 (55–59.5) | 66 (64.5–69) | –9 (–14 to –3) | <0.01 | 0/0 |
| A20 (mm) | 67.5 (62–70.25) | 60 (57.25–62.75) | 69.5 (67.25–71.75) | –8.9 (–13 to –3) | <0.01 | 0/0 |
| MCF (mm) | 68.5 (62.75–71.25) | 60 (57.25–62.75) | 70.5 (68.25–72) | –9 (–14 to –4) | <0.01 | 0/0 |
| ML (%) | 3 (2–7) | 7.5 (4.75–8.75) | 2 (1–3) | 4.6 (1–7) | <0.01 | 0/1 |
| IN test | ||||||
| CT (s) | 188 (167.75–214.75) | 203 (183.75–250) | 180 (164–208.25) | 23 (–18 to 71) | 0.11 | 0/0 |
| A5 (mm) | 52.5 (44–56.75) | 44 (41.75–45.5) | 54.5 (51.5–59) | –10.75 (–15 to –3) | 0.01 | 0/0 |
| A10 (mm) | 60.5 (53–64.5) | 53 (50–54.5) | 62 (60.25–66) | –9 (–14 to –3) | 0.01 | 0/0 |
| A20 (mm) | 64.5 (58–68.25) | 57.5 (54–58.75) | 66.5 (64.25–69) | –9 (–13 to –4) | <0.01 | 0/0 |
| MCF (mm) | 64.5 (59–69) | 58 (54–59) | 66.5 (64.25–69) | –9.99 (–14 to –3) | <0.01 | 0/0 |
| ML (%) | 4 (3–8) | 7.5 (4.75–9.5) | 3 (2–4) | 4 (0–7) | 0.04 | 0/3 |
| FIB test | ||||||
| CT (s) | 72 (53–108) | 94 (64–119) | 66 (52–104) | 15 (–18 to 61) | 0.28 | 0/0 |
| A5 (mm) | 29 (23–32) | 22 (18–25) | 32 (28–34) | –10 (–15 to –3) | <0.01 | 0/0 |
| A10 (mm) | 31 (25–35) | 24 (20–27.25) | 34 (31–36.5) | –10 (–16 to –4) | <0.01 | 0/0 |
| A20 (mm) | 33 (27–37) | 25 (22–28) | 36 (32–38) | –11 (–16 to –4) | <0.01 | 0/0 |
| MCF (mm) | 34 (28–39) | 26 (24–30) | 38 (34–40) | –11 (–17 to –4) | <0.01 | 0/0 |
Continuous data as medians (25th to 75th percentile).
A5, amplitude after 5 min; A10, amplitude after 10 min; A20, amplitude after 20 min; CI, confidence interval; CT, clotting time; LT, lysis time; MCF, maximal clot firmness; ML, maximum lysis; TPA LT, lysis time in TPA test.
Odds ratios for binary variables and estimated median difference for continuous variables (CI).
Assessed with Fisher's exact test for categorical variables and the Wilcoxon rank sum test for continuous variables.
BMI and sex distribution were not significantly different between the groups with normal fibrinolytic response and decreased fibrinolytic response in the TPA test (P=0.71 and P=1). Also, medical history showed no significant difference between the groups for normal and decreased fibrinolytic response (Table 2). There were no significant differences in SAPS 3 and SOFA scores between patients with normal and prolonged LT in the TPA test (P=0.38 and P=0.30, respectively).
Critically ill COVID-19 patients with decreased fibrinolytic response had significantly higher fibrinogen measurements. Fibrinogen was 674 (578–796) mg dl−1 vs 530 (390–568) mg dl−1 (P=0.02), and FIB test MCF was 38 (34–40) mm vs 26 (24–30) mm (P<0.01) in the groups with prolonged vs normal LT, respectively (Fig 1b). There was no difference in D-dimers between the groups. Patients with impaired fibrinolysis had D-dimer levels of 4860 (2440–7330) μg L−1 and patients with normal fibrinolysis time 6540 (5440–12 810) μg L−1 (P=0.46; Fig. 1c).
Platelet number was higher in patients with decreased fibrinolytic response: 274 (198–344) G L−1 compared with 154 (151–173) G L−1 (P=0.04) (Fig. 1d). Furthermore, patients with impaired fibrinolysis showed higher levels of C-reactive protein (CRP) at ICU admission (23 [17–29] mg dl−1 vs 10 [5–13] mg dl−1, P<0.01) and at time of ClotPro assay (20 [13–28] mg dl−1 vs 4 [3–11] mg dl−1, P=0.02), as depicted in Fig. 1e.
Systemic hypoperfusion parameters did not differ between those with normal and impaired fibrinolytic response as shown by lactate 8 (8–14) vs 9 (8–11) mg dl−1 (P=1) and base excess (BE) 6.4 (4.4–7.2) vs 6.25 (2.2–8.4) mmol L−1 (P=0.90), respectively (Supplementary file, Table S4).
Three patients had a Horovitz Index <100 mm Hg at ClotPro analysis, all of whom showed impaired fibrinolysis (LT >393 s). In addition, although no significant difference in death rate was seen between the two COVID-19 groups (P=0.27), all four patients who died had impaired fibrinolysis.
There was no difference in number of thromboses between patients with impaired fibrinolysis and those without. In each group, one patient experienced thrombotic events. One patient with normal fibrinolytic response to r-tPA in vitro suffered from pulmonary embolism, which was the reason for ICU admission. The ClotPro assessment was performed 4 days after the event with successful lysis therapy with tenecteplase, and therefore correlation between this thromboembolic event and ClotPro assay results is limited. The other patient experienced three thrombotic events, a spleen infarction, and bilateral jugular vein thrombosis diagnosed on day 7 after ClotPro analysis. The TPA test showed a decreased fibrinolytic response to r-tPA in this patient.
Anticoagulation
On the day of ClotPro analysis, 16 patients received anticoagulation with enoxaparin, an LMWH, at a median dose of 80 (60–100) mg day−1 with corresponding peak plasma levels of 0.30 (0.23–0.32) IU ml−1. Target anti-Xa levels were set at 0.3–0.5 IU ml−1, and patients who reached these levels received a medium LMWH dose of 100 (80–100) mg day−1, whereas patients who did not reach this target level also received 100 (80–120) mg day−1. There was no statistical difference in any of the routinely measured laboratory parameters between patients who reached the target level and those who did not.
The other four critically ill COVID-19 patients received argatroban, with three of them having an available argatroban plasma concentration measurement (anti-IIa measured via diluted thrombin time) on the day of ClotPro assay. Two patients did not reach the argatroban target level of 0.3–0.6 μg ml−1 at that time. They received 0.1 and 0.56 μg kg−1 min−1 argatroban with corresponding plasma levels of 0.29 and 0.13 μg ml−1, respectively. The patient who reached the target range received 0.42 μg kg−1 min−1 with a corresponding argatroban level of 0.50 μg ml−1. The reason for argatroban administration was that these patients did not respond to LWMH well enough as seen from the fact that the anti-Xa target range could not be reached despite high doses of LMWH. These patients did not suffer from suspected or proven heparin-induced thrombocytopenia (HIT).
Discussion
We analysed the coagulation properties, especially fibrinolysis, of critically ill COVID-19 patients with suspected extensive hypercoagulability admitted to the ICU of Innsbruck Medical University Hospital. We found that, in addition to a hypercoagulable state, 70% of these patients suffered from impaired fibrinolysis accompanied by a decreased response to r-tPA-induced clot lysis as measured with viscoelastic whole blood coagulation assays (ClotPro).
In critically ill COVID-19 patients, a prothrombotic coagulopathy is commonly found.18 In addition to increased factor levels18 and endotheliopathy,19 impaired fibrinolysis (lysis at 30 min in TEG) was observed in this particular patient group, and this was associated with increased thrombosis risk and need for dialysis.18 , 20 , 21 The problem with impaired fibrinolysis is that already formed thromboses and microthromboses cannot be completely resolved and therefore might contribute to the high rate of thrombotic complications, such as pulmonary embolism, in COVID-19 patients.18
Our ICU patients with decreased fibrinolytic response also showed hypofibrinolysis in the EX test. Therefore, it can be concluded that this hypofibrinolytic state might be at least partially caused by a decreased response to the pro-fibrinolysis factor r-tPA and not merely by less lysis activation inside the clot. This finding is supported by a recent study by Nougier and colleagues,14 who report that the hypofibrinolytic state in critically ill COVID-19 patients was mainly attributable to increased fibrinolysis inhibitor levels of PAI-1 and TAFI.14 Inflammation itself promotes local release of tPA and PAI-1 from endothelial cells.22 This increase in PAI-1 levels might affect fibrinolysis more than the simultaneous increase in tPA arising in COVID-19 patients.10 , 14 Also, platelets provide a major source of PAI-1, which can be released by various triggers such as hypoxaemia23 or activation via thrombin.24 Our patients with hypofibrinolysis had a higher platelet count and therefore potentially higher releasable amounts of PAI-1. Although platelet count does not indicate the activity state of platelets, platelet inhibition leads to improved lung function in COVID-19 patients.25
Regardless of their source, PAI-1 and TAFI were identified as predictive biomarkers for decreased fibrinolytic response in non-COVID-19 patients suffering from pulmonary embolism, whereas fibrinogen, α2-antiplasmin, plasminogen, thrombin time, and D-dimer were not.26 Although D-dimer is formed during fibrinolysis, it gives no information about the state of fibrinolysis (hypo-, normo- or hyper-fibrinolysis) as it is not known how much fibrin is formed in vivo and the fibrinolytic system might fail to clear the huge amount of fibrin that has already formed.27 This might be the reason why we could not find a difference in D-dimers between patients with and without impaired fibrinolytic response, although fibrinogen levels were significantly higher in patients with impaired fibrinolysis. Another explanation as to why D-dimers were not lower in patients with impaired fibrinolysis might be that plasminogen not only cleaves fibrinogen, but also misfolded proteins and necrotic tissue,27 which could be present in a larger amount in patients with a higher state of inflammation, as shown in our patients with impaired fibrinolysis.
Thrombus formation with impaired fibrinolysis not only leads to thrombosis and microthrombosis in the vascular system, but also to fibrin deposition in the alveoli. Increased levels of PAI-1 in bronchoalveolar lavage fluid are found in multiple pulmonary disorders,28 and in patients with pneumonia intra-alveolar fibrin deposition was associated with high levels of CRP.29 Our COVID-19 patients showed an association between higher levels of CRP and impaired fibrinolysis, which might contribute to intra-alveolar fibrin deposition.
Inflammation and subsequent fibrin deposition within the alveolar space might impair gas exchange.30 In our patients, it is notable that three patients had a Horovitz index <100 mm Hg at ClotPro analysis, all of whom showed impaired fibrinolysis. As fibrin deposition in the alveolar space appears to be part of the COVID-19 pathomechanism, fibrinolytic therapy was applied in only few patients, either with intravenous tPA31 32 or nebulised plasminogen.33 These studies showed transient or permanent improvement in lung function and thus oxygenation.33
Other reasons for fibrin deposition and the high rate of thrombosis during COVID-19 should not be ignored. Endothelial dysfunction contributes to the formation of microthrombi, tissue oedema, and tissue hypoxia.34 Endothelial activation with TF expression promotes plasma coagulation and, if the endothelial line is disrupted, exposure to subendothelial structures such as collagen activates platelets. The endothelial dysfunction in COVID-19 is caused, on the one hand, directly by SARS-CoV-2 when it enters the cell19 and also by the inflammatory response and hypoperfusion due to thrombosis and microthrombosis formation. Furthermore, systemic hypoperfusion and capillary leakage and shock were reported to be present in COVID-19 patients.35 At any rate, in our critically ill COVID-19 patients, hypoperfusion caused by circulatory impairment seemed not to play a role as systemic hypoperfusion markers did not indicate such a state, but local hypoperfusion and tissue hypoxia might still be an issue.
Known difficulties encountered in administering sufficient anticoagulation are also present in COVID-19 patients and might contribute to the high rate of thrombotic complications, as these patients seem to need higher doses of, for example LMWH because of heparin resistance.36 At Innsbruck Medical University Hospital, argatroban is used as an alternative anticoagulant in critically ill patients.37 This is also done in COVID-19 patients, and in this particular patient population argatroban might have an additional benefit because this direct thrombin inhibitor seems to enhance fibrinolysis more than heparin does.38
As of now, impaired fibrinolysis seems to be only one pathophysiological aspect of COVID-19, and other drivers of the disease need to be further investigated and targeted. Nevertheless, it is a mechanism that can be treated (or counteracted), and knowing whether a patient suffers from decreased fibrinolytic response might be of greater importance when treatment with tPA is considered.31 , 32
Conclusions
We conclude that critically ill COVID-19 patients are in a hypercoagulable and hypofibrinolytic state, which is at least partly dependent on a decreased response to tPA-induced fibrinolysis. The decreased fibrinolytic response was associated with higher fibrinogen, platelet count, and CRP. Nevertheless, possible relationships of impaired fibrinolysis measured in vitro to clinical outcomes need to be further investigated.
Limitations
A major limitation is that our study was conducted retrospectively in a small number of patients. In addition, the healthy volunteers were much younger than the COVID-19 patients. Therefore, it is not clear whether the differences found were attributable to the older age or to the disease itself. Also, the healthy population could not be matched for other variables such as comorbidities, and therefore different ClotPro results might not solely attributable to COVID-19 infection. Furthermore, our findings rely on comparison of results from two different centres, which includes some risk of bias between the centres. However, from the magnitude of the difference we observed, we do not expect inter-centre variability to have a significant effect.
Another point is that it does not reflect coagulation throughout COVID-19, but presents only a momentary picture during the phase of critical illness when ClotPro was performed (median, day 8.5 of ICU stay). Furthermore, ClotPro was not performed from blood drawn at the same time as the blood draw for routine laboratory tests, although one might argue that most parameters do not zigzag to the extreme during this period (median 8.4 h). Unfortunately, given these low numbers of thrombotic events in this study population and the lack of ClotPro assays at ICU admission, it is not possible to assess whether a patient with LT >393 s is more likely to experience a thromboembolic event. The inconsistent timing of the ClotPro assay during hospital admission is a weakness of our study because it makes it difficult to compare test results between patients. Furthermore, ClotPro was not performed in all critically ill COVID-19 patients, but in those already suspected of being in a hypercoagulable state or heparin-resistant, which may cause an indication bias.
Authors' contributions
Idea development and concept of analysis: MB, DF
Statistical analysis and figure/tables: TH
ClotPro assays: MB, JB, DS
Collection of COVID patient-related data: MB, DS, JB
COVID patient data and samples: DF, JB, MS, SK, VS, GL, MJ, DF
Data from healthy persons: AG
Declarations of interest
MB has received research funding and travel grants from LFB Biomedicaments, Baxter GmbH, CSL Behring GmbH, Mitsubishi Tanabe and non-financial support from TEM International outside the submitted work. MJ reported receiving grants from Baxter; grants and personal fees from Fresenius Kabi; and speaking, consulting honoraria, or both from Sphingotec, CLS-Behring, Fresenius and Astute Medical outside the submitted work. CT reports grants and personal fees from BrainLab, grants and personal fees from DePuySynthes, grants and personal fees from Intrinsic Therapeutics, grants from TETEC AG, personal fees from Aesculap, grants and personal fees from Signus Medizintechnik, grants and personal fees from Medtronic, grants and personal fees from Icotec AG, grants and personal fees from Edge Therapeutics, grants from BIT-Pharma, outside the submitted work. DF has received study funding, honoraria for consultancy and board activity from Astra Zeneca, AOP orphan, Baxter, Bayer, BBraun, Biotest, CSL Behring, Delta Select, Dade Behring, Edwards, Fresenius, Glaxo, Haemoscope, Hemogem, Lilly, LFB, Mitsubishi Pharma, NovoNordisk, Octapharm, Pfizer, Tem-Innovation outside the submitted work. The other authors declare no conflicts of interest.
Handling editor: Hugh C Hemmings Jr
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bja.2020.12.010.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.World Health Organization . 7 September 2020. Epidemiological Update, Coronavirus disease 2019 (COVID-19)https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200907-weekly-epi-update-4.pdf?sfvrsn=f5f607ee_2 Available from: [Google Scholar]
- 2.Morens D.M., Daszak P., Markel H., Taubenberger J.K. Pandemic COVID-19 joins history’s pandemic legion. mBio. 2020;11:e00812–e00820. doi: 10.1128/mBio.00812-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spiezia L., Boscolo A., Poletto F. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120:998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panigada M., Bottino N., Tagliabue P. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klok F.A., Kruip M., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wichmann D., Sperhake J.P., Lütgehetmann M. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ackermann M., Verleden S.E., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitt F.C.F., Manolov V., Morgenstern J. Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study. Ann Intensive Care. 2019;9:19. doi: 10.1186/s13613-019-0499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panigada M., Zacchetti L., L'Acqua C. Assessment of fibrinolysis in sepsis patients with urokinase modified thromboelastography. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright F.L., Vogler T.O., Moore E.E. Fibrinolysis shutdown correlates to thromboembolic events in severe COVID-19 infection. J Am Coll Surg. 2020;231:193–203. doi: 10.1016/j.jamcollsurg.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Creel-Bulos C., Auld S.C., Caridi-Scheible M. Fibrinolysis shutdown and thrombosis in a COVID-19 ICU. Shock. August 4 2020 doi: 10.1097/SHK.0000000000001635. Adv Access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nougier C., Benoit R., Simon M. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-CoV2 associated thrombosis. J Thromb Haemost. 2020;18:2215–2219. doi: 10.1111/jth.15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreno R.P., Metnitz P.G., Almeida E. SAPS 3—from evaluation of the patient to evaluation of the intensive care unit: Part 2. Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31:1345–1355. doi: 10.1007/s00134-005-2763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groene P., Sappel S.R., Saller T. Functional testing of tranexamic acid effects in patients undergoing elective orthopaedic surgery. J Thromb Thrombolysis. September 12, 2020 doi: 10.1007/s11239-020-02272-8. Adv Access. [DOI] [PubMed] [Google Scholar]
- 17.Oberladstätter D., Voelckel W., Schlimp C. A prospective observational study of the rapid detection of clinically-relevant plasma direct oral anticoagulant levels following acute traumatic injury. Anaesth. September 18, 2020 doi: 10.1111/anae.15254. Adv Access. [DOI] [PubMed] [Google Scholar]
- 18.Helms J., Tacquard C., Severac F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright F.L., Vogler T.O., Moore E.E. Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J Am Coll Surg. 2020;231:193–203.e1. doi: 10.1016/j.jamcollsurg.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edler C., Schröder A.S., Aepfelbacher M. Dying with SARS-CoV-2 infection—an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Leg Med. 2020;134:1275–1284. doi: 10.1007/s00414-020-02317-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meltzer M.E., Lisman T., de Groot P.G. Venous thrombosis risk associated with plasma hypofibrinolysis is explained by elevated plasma levels of TAFI and PAI-1. Blood. 2010;116:113–121. doi: 10.1182/blood-2010-02-267740. [DOI] [PubMed] [Google Scholar]
- 23.Chaurasia S.N., Kushwaha G., Kulkarni P.P. Platelet HIF-2α promotes thrombogenicity through PAI-1 synthesis and extracellular vesicle release. Haematologica. 2019;104:2482–2492. doi: 10.3324/haematol.2019.217463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huebner B.R., Moore E.E., Moore H.B. Thrombin provokes degranulation of platelet α-granules leading to the release of active plasminogen activator inhibitor-1 (PAI-1) Shock. 2018;50:671–676. doi: 10.1097/SHK.0000000000001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viecca M., Radovanovic D., Forleo G.B., Santus P. Enhanced platelet inhibition treatment improves hypoxemia in patients with severe Covid-19 and hypercoagulability. A case control, proof of concept study. Pharmacol Res. 2020;158:104950. doi: 10.1016/j.phrs.2020.104950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stubblefield W.B., Alves N.J., Rondina M.T., Kline J.A. Variable resistance to plasminogen activator initiated fibrinolysis for intermediate-risk pulmonary embolism. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medcalf R.L., Keragala C.B., Myles P.S. Fibrinolysis and COVID-19: a plasmin paradox. J Thromb Haemost. 2020;18:2118–2122. doi: 10.1111/jth.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sisson T.H., Simon R.H. The plasminogen activation system in lung disease. Curr Drug Targets. 2007;8:1016–1029. doi: 10.2174/138945007781662319. [DOI] [PubMed] [Google Scholar]
- 29.Nagata N., Wakamatsu K., Kumazoe H. Clinical significance of intra-alveolar fibrin deposition in transbronchial lung biopsy in patients with organizing pneumonia. Lung. 2015;193:203–208. doi: 10.1007/s00408-015-9689-7. [DOI] [PubMed] [Google Scholar]
- 30.Schermuly R.T., Günther A., Ermert M. Conebulization of surfactant and urokinase restores gas exchange in perfused lungs with alveolar fibrin formation. Am J Physiol Lung Cell Mol Physiol. 2001;280:L792–L800. doi: 10.1152/ajplung.2001.280.4.L792. [DOI] [PubMed] [Google Scholar]
- 31.Wang J., Hajizadeh N., Moore E.E. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020;18:1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrett C.D., Oren-Grinberg A., Chao E. Rescue therapy for severe COVID-19 associated acute respiratory distress syndrome (ARDS) with tissue plasminogen activator (tPA): a case series. J Trauma Acute Care Surg. 2020;89:453–457. doi: 10.1097/TA.0000000000002786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y., Wang T., Guo C. Plasminogen improves lung lesions and hypoxemia in patients with COVID-19. QJM. 2020;113:539–545. doi: 10.1093/qjmed/hcaa121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paulus P., Jennewein C., Zacharowski K. Biomarkers of endothelial dysfunction: can they help us deciphering systemic inflammation and sepsis? Biomarkers. 2011;16:S11–S21. doi: 10.3109/1354750X.2011.587893. [DOI] [PubMed] [Google Scholar]
- 35.Fox S., Vashisht R., Siuba M., Dugar S. Evaluation and management of shock in patients with COVID-19. Clevel Clin J Med. July 17 2020 doi: 10.3949/ccjm.87a.ccc052. Adv Access. [DOI] [PubMed] [Google Scholar]
- 36.White D., MacDonald S., Bull T. Heparin resistance in COVID-19 patients in the intensive care unit. J Thromb Thrombolysis. 2020;50:287–291. doi: 10.1007/s11239-020-02145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bachler M., Hell T., Bösch J. A prospective pilot trial to assess the efficacy of argatroban (Argatra(®)) in critically ill patients with heparin resistance. J Clin Med. 2020;9:963. doi: 10.3390/jcm9040963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nielsen V.G., Kirklin J.K. Argatroban enhances fibrinolysis by differential inhibition of thrombin-mediated activation of thrombin activatable fibrinolysis inhibitor and factor XIII. Blood Coagul Fibrinolysis. 2008;19:793–800. doi: 10.1097/MBC.0b013e328317f5aa. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.