Abstract
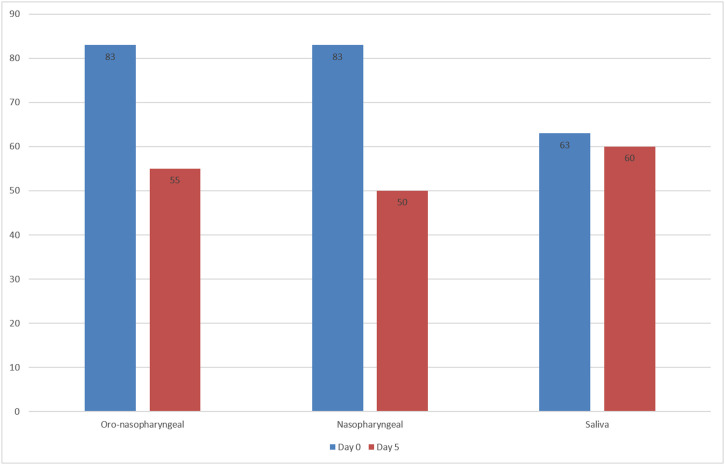
The gold standard method in the diagnosis of SARS-CoV-2 infection is the detection of viral RNA in the nasopharyngeal sample by RT-PCR. Recently, saliva samples have been suggested as an alternative sample. In the present study, we aimed to compare RT-PCR results in nasopharyngeal, oro-nasopharyngeal and saliva samples of COVID-19 patients. 98 of 200 patients were positive in RT-PCR analysis performed before the hospitalization. On day 0, at least one sample was positive in 67 % of 98 patients. The positivity rate was 83 % for both oro-nasopharyngeal and nasopharyngeal samples, while it was 63 % for saliva samples (p < 0.001). On day 5, RT-PCR was performed in 59 patients, 34 % had at least one positive result. The positivity rate was 55 % for both saliva and nasopharyngeal samples, while it was 60 % for oro-nasopharyngeal samples. Our study shows that the sampling saliva does not increase the sensitivity of RT-PCR tests at the early stages of infection. However, on the 5th day, viral RNA detection rates in saliva were similar to nasopharyngeal and oro-nasopharyngeal samples. In conclusion, we suggest that, in patients receiving treatment, RT-PCR in saliva, in addition to the standard samples, is important to determine the isolation period and control transmission.
Keywords: SARS-CoV-2, Coronavirus, RT-PCR, saliva
1. Introduction
The SARS CoV-2 infection, which has influenced the world since the end of 2009, can cause serious lower respiratory tract infections that may be fatal in some patients (Bedford et al., 2020; Rothan and Byrareddy, 2020). However, many individuals remain asymptomatic during the infection but have been a major factor in increasing the transmission rate of the disease and evolving it into a pandemic (Huang et al., 2020; Guan et al., 2020). Although the diagnosis is based on clinical findings, the detection of the virus in patients' specimens is of great importance in detecting asymptomatic individuals and monitoring the contagious period in symptomatic individuals. This situation reveals the need for a fast, reliable, easily applicable, and non-invasive test. Currently, the gold standard method in the diagnosis of SARS-CoV-2 infection is the detection of viral RNA in the nasopharyngeal swab sample by Real-Time Polymerase Chain Reaction (RT-PCR) analysis (Lippi et al., 2020). The most important disadvantage of this method is the presence of limited trained personnel available in sampling and the high risk of nosocomial infections that such personnel is exposed to. As the main source of transmission of SARS-CoV-2 infection is salivary droplets, viral RNA RT-PCR in saliva samples have been suggested as possible alternative testing (Khurshid et al., 2020). Here, we compared the viral RNA RT-PCR results in nasopharyngeal, oro-nasopharyngeal, and saliva samples in patients diagnosed with COVID-19 to investigate their possible relationships with clinical findings.
2. Methods
2.1. Patients
A cross-sectional study was conducted in repurposed Genomic Laboratory (GLAB), Umraniye Teaching and Research Hospital, in Istanbul, with a total of 200 consecutive patients who met the possible case definition for COVID-19 and were hospitalized with moderate-severe disease (Doganay et al., 2020). According to the diagnostic algorithm provided by The Turkish Ministry of Health, the possible cases were defined as those who presented with;
*History of fever or acute respiratory symptoms,
and
*Travel history from an endemic area of COVID-19 within 14 days,
or
*History of contact with an individual who was confirmed or suspected having COVID-19,
or
*Presence of hospitalization requirement due to respiratory tract infection.
Within the scope of the present study, after hospitalization saliva, oro-nasopharyngeal, and nasopharyngeal samples were taken from all 200 patients within the first 24 h, and it was defined as day 0 sample. On day 5, patients were resampled.
Demographic characteristics, symptoms at presentation, comorbid diseases, and clinical findings during hospitalization were collected for each patient. All subjects provided informed consent, and the study was approved by the ethics committee of Umraniye Teaching and Research Hospital (B.10.1.THK.4.34.H.GP.0.01/167)
Patients admitted to ICU, not giving consent to study, incapable of providing saliva sample and patients under the age of 18 were excluded.
2.2. Sample collection
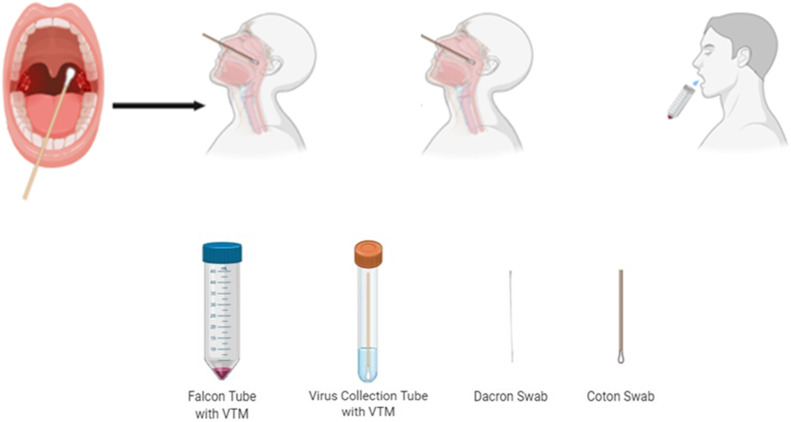
In all patients, an oro-nasopharyngeal sample was taken with a cotton swab used for the viral RT-PCR test before hospitalization as a standard diagnostic approach. In the scope of our study, oro-nasopharyngeal samples were taken with a cotton swab and nasopharyngeal samples with dacron swab. Details of the sampling processes are given in Supplementary Fig. 1. Before the saliva collection, participants were given brief explanations about the difference between saliva and sputum, then they were asked to give saliva samples prior to other samples by a drooling technique. They spit approximately 1 mL into the falcon tubes containing the viral transport medium (VTM, Innomed VTM001) used in standard sampling. Single trained healthcare professional took samples to avoid possible variations in the collection technique. All samples were transferred to our laboratory within 1 h of sampling stored in the refrigerator, and RT-PCR was performed on the day they were collected.
2.3. RT-PCR workflow
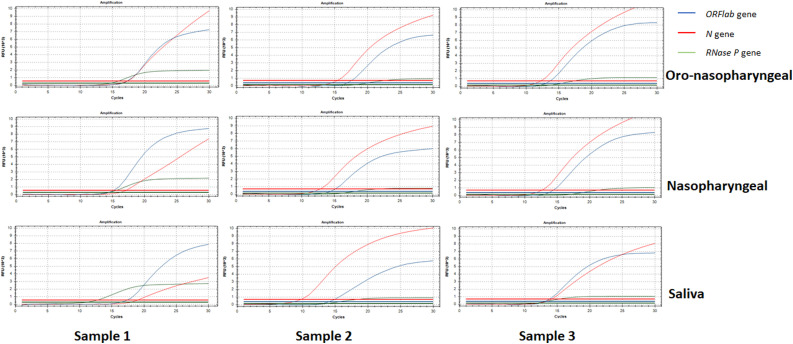
Orflab and N gene of SARS-COV-2 were targeted for the diagnosis of the SARS-COV-2 infected patients. For this purpose, The Direct Detection of SARS-COV-2 Detection Kit was used (Coyote Bioscience Co., Ltd). The kit procedure was based on the detection of the protected region of ORFlab coated with a pair of specific primers and a fluorescently labeled probe, and the N gene of SARS-COV-2 by the RT-PCR method. Since this kit did not need a separate RNA extraction, the samples in the VTM medium had briefly vortexed and taken directly into the study. Biorad CFX 96 Real-Time PCR systems were used. FAM channel for ORF lab gen, ROX channel for N gene, and HEX channel for internal RNase P gene of human control was selected. These channels should have the logarithmic growth period with the Ct value ≤29, for a positive result.
2.4. Interpretation of results
In the RT-PCR results of the samples, the internal RNase P gene was positive for all samples. If both the ORFlab and the N gene were positive, the result was considered as presumptive positive. If both the ORFlab and the N gene were negative, the result was considered as presumptive negative. If one of them was positive and the other was negative, the test for this sample was repeated. If the same result was achieved again, a new nasopharyngeal and oropharyngeal swab was requested.
2.5. Statistical analysis
SPSS.22 package program (IBM Company, Armonk, NY, USA) was used for statistical analysis. Mean, median, and standard deviation were used for descriptive statistical information. Categorical variables were analyzed with chi-square test. The results of the tests performed in three different samples on the 0th and 5th days were compared among themselves using the Cohran Q test. All calculated P values are double-sided and for significant statistical results, P < 0·05 was accepted.
3. Results
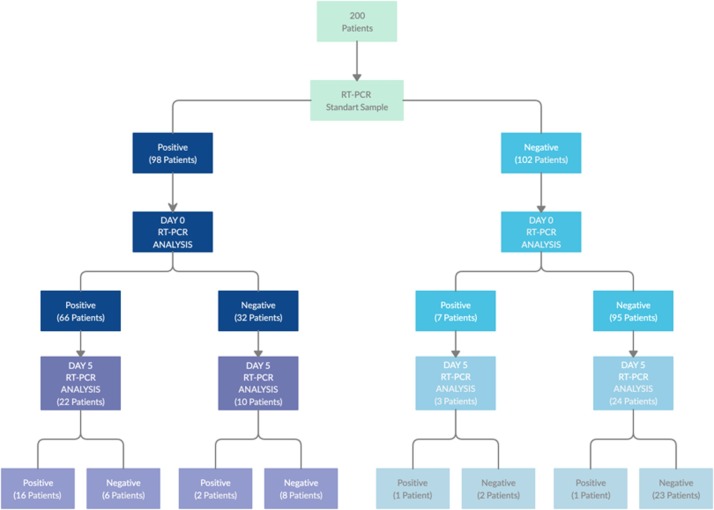
Ninety-eight of 200 (49 %) patients in the study group were positive in RT-PCR analysis performed on the samples taken as a standard diagnostic procedure before the hospitalization. The clinical and demographic characteristics of the patients with positive results are presented in supplementary Table 1. Although 102 patients met the possible case definition, the viral RT_PCR tests on admission were negative (Fig. 1 ).
Fig. 1.
Study workflow diagram.
3.1. Day 0 analysis results
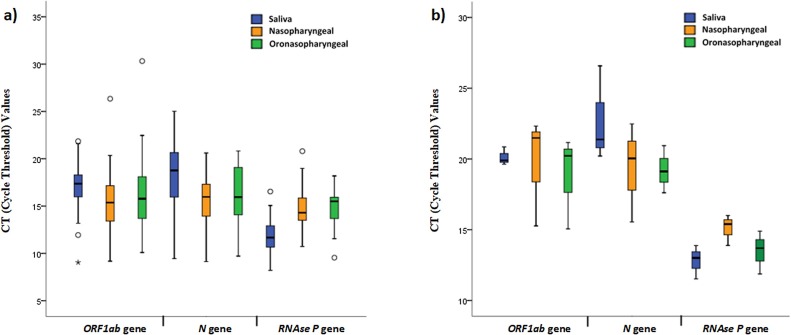
At day 0 RT-PCR analysis, at least one sampling method showed positivity in 66 of the above mentioned 98 patients. Six (9 %) patients had positive results only in nasopharyngeal swab sample, 5 (8 %) were only positive in oro-nasopharyngeal sample and 3 (5 %) patients had positive test results only in saliva samples. The sensitivity rates were presented in Table 1 . The difference in the sensitivity rates among all sampling methods was statistically significant (P < 0·001). The mean Ct values determined for FAM and ROX were shown in supplementary Fig. 2. Those three patients who had only saliva positivity had a history of COVID-19 positive household contact and had mild involvement in thorax CT. Of 102 patients with a previous negative result, only 7 (6·9 %) had positive RT-PCR results obtained in at least one sample type (Fig. 1). While the oro-nasopharyngeal samples were positive in all 7 patients, nasopharyngeal samples were positive in 3. There was only one patient whose saliva sample was positive. Oro-nasopharyngeal and nasopharyngeal samples in this patient were also positive (Fig. 2 ).
Table 1.
RT-PCR study results of saliva, oronasopharyngeal and nasopharyngeal samples on day 0 in 98 patients who were found positive in RT-PCR analysis before hospitalization. We would like to remind that of 98 patients, 66 (67 %) had at least one positive result in saliva or swab samples.
Fig. 2.
Sensitivity rate of each sampling methods in day 0 and 5 RT-PCR analysis.
3.2. Day 5 analysis results
On day 5, we were able to collect samples from 59 (30 %) of 98 patients since 39 patients did not come for follow-up. Of 59 patients, 20 (34 %) had at least one positive result in saliva or in one of the swab samples. The sensitivity rates were presented in Table 2 and in the statistical analysis, there was no difference between the sample types in terms of the sensitivity (P = 0·368). The mean Ct values determined for FAM and ROX were shown in Supplementary Fig. 2. There were 5 patients who showed RT-PCR positivity only on the saliva sample. These patients had a longer prior history with positive RT-PCR results in oro-nasopharyngeal samples, home-treated with hydroxychloroquine for five days, clinically deteriorated with radiological findings, tested positive again in oro-nasopharyngeal samples, and hospitalized.
Table 2.
RT-PCR study results of saliva, oro-nasopharyngeal and nasopharyngeal samples on day 5 in 59 patients. We would like to remind that of 59 patients, 20 (34 %) had at least one positive result in saliva or swab sample.
4. Discussion
Our study demonstrates that saliva sampling did not improve diagnostic sensitivity in patients who had a negative result in initial testing before hospitalization. Our results also revealed that at the early stages of the infection, saliva sampling had a lower sensitivity to detect viral RNA compared to other sampling methods. Although some recent studies have shown similar results to ours, there are also other studies suggesting that saliva samples are compatible with the results of nasopharyngeal samples starting from the early period of the disease (Azzi et al., 2020, To et al., 2020, Pasomsub et al., 2020, Kam et al., 2020; Chong et al., 2020, Jamal et al., 2020; Kim et al., 2020; Kojima et al., 2020; Yokota et al., 2020, Yee et al., 2020; Santos et al., 2020). Some researchers suggest that saliva sampling can allow for an efficient, relatively inexpensive surveillance system however, they also point to the need for pilot studies (Fogarty et al., 2020, Medeiros da Silva et al., 2020). In this study, we consecutively recruited 200 inpatients who presented with clinical signs compatible with Covid-19 and at initial testing 98 of them revealed SARS-COV-2 RT-PCR positivity. The sensitivity rate for saliva samples was 63 % and this was significantly lower than nasopharyngeal and oro-nasopharyngeal swabs. However, our data on the 5th day showed that the viral RNA detection rates in saliva samples were similar to those of nasopharyngeal and oro-nasopharyngeal samples. Two similar studies supported our results and showed that SARS-CoV-2 detection from saliva was more consistent during extended hospitalization and recovery (Azzi et al., 2020; To et al., 2020). A recent study also reported less variation in levels of SARS-CoV-2 RNA in the saliva specimens than in the nasopharyngeal swab specimens in inpatients during the clinical course (Wyllie et al., 2020). Thus, we suggest that RT-PCR analysis in saliva samples may be beneficial when a follow-up test beyond day 5 is needed especially in hospitalized patients (e.g. before discharge). Compared to nasopharyngeal swabs, sampling saliva causes less discomfort in patients and reduces the risk for nosocomial infection among healthcare workers as patients can give saliva samples by themselves.
On day five, out of 59 patients, 5 (8 %) had detectable viral RNA only in the saliva sample. The fact that all of these patients were using hydroxychloroquine prior to hospitalization, may have hindered the detection of the virus in nasopharyngeal and oro-nasopharyngeal samples. However, it was not possible to suggest by what mechanism hydroxychloroquine caused such variation and novel studies are needed on this subject. With this result, we suggest that taking saliva samples along with the standard method before ending the isolation in individuals treated with hydroxychloroquine can be effective in minimizing contamination in the community by reducing the false negativity rate. In addition, we recommend saliva sampling in patients who show progression despite at-home treatment. Such an approach would increase the detection of positive cases, thereby enabling more accurate planning of treatment, follow-up, and subsequent discharge.
One of the most important issues affecting false negative rates in standard nasopharyngeal sampling is the use of inappropriate techniques. In our study, taking all samples by single trained healthcare staff is the strength of our study and enabled us to achieve a safer result. The most important limitation of our study is that no study has been performed in asymptomatic individuals and mild cases that do not require hospitalization. Therefore, it was not possible to make a comment about whether saliva samples can be used in screening.
In conclusion, our study shows that the saliva sample is not as sensitive as standard nasopharyngeal swabs in determining viral RNA and it does not improve the detection rate in PCR negative patient group. However, in the later stage of the disease, the RT-PCR test in saliva sample might help to detect deteriorating patients or determine the isolation period more effectively after treatment.
Funding
None.
CRediT authorship contribution statement
Ozlem Akgun Dogan: Formal analysis, Investigation, Methodology, Writing - original draft. Betsi Kose: Formal analysis, Investigation, Methodology, Writing - original draft. Nihat Bugra Agaoglu: Formal analysis, Investigation, Methodology, Supervision, Writing - review & editing. Jale Yildiz: Investigation. Gizem Alkurt: Investigation. Yasemin Kendir Demirkol: Investigation. Arzu Irvem: Investigation. Gizem Dinler Doganay: Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing - original draft, Writing - review & editing. Levent Doganay: Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
None.
Acknowledgements
We thank to Murat Kaya for technical assistance
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jviromet.2020.114049.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Azzi L., Carcano G., Gianfagna F., et al. Saliva is a reliable tool to detect SARS-CoV-2. J. Infect. 2020 doi: 10.1016/j.jinf.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford J., Enria D., Giesecke J., et al. COVID-19: towards controlling of a pandemic. Lancet. 2020;395(10229):1015–1018. doi: 10.1016/S0140-6736(20)30673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong C.Y., Kam K.Q., Li J., et al. Saliva is not a useful diagnostic specimen in children with Coronavirus Disease 2019 [published online ahead of print, 2020 Sep 14] Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1376. ciaa1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doganay L., Agaoglu N.B., Irvem A. et al., Responding to COVID-19 in Istanbul: Perspective from genomic laboratory. North Clin Istanb. 2020;7(3):311–312. doi: 10.14744/nci.2020.30075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty A., Joseph A., Shaw D. Pooled saliva samples for COVID-19 surveillance programme. Lancet Respir. Med. 2020;8(11):1078–1080. doi: 10.1016/S2213-2600(20)30444-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal A.J., Mozafarihashjin M., Coomes E., et al. Sensitivity of nasopharyngeal swabs and saliva for the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [published online ahead of print, 2020 Jun 25] Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa848. ciaa848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam K.Q., Yung C.F., Maiwald M., et al. Clinical utility of buccal swabs for severe acute respiratory syndrome coronavirus 2 detection in coronavirus disease 2019-Infected children. J. Pediatric Infect. Dis. Soc. 2020;9(3):370–372. doi: 10.1093/jpids/piaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurshid Z., Asiri F.Y.I., Al Wadaani H. Human saliva: non-invasive fluid for detecting novel coronavirus (2019-nCoV) Int. J. Environ. Res. Public Health. 2020;17(7) doi: 10.3390/ijerph17072225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.E., Lee J.Y., Lee A., et al. Viral load kinetics of SARS-CoV-2 infection in saliva in korean patients: a prospective multi-center comparative study. J. Korean Med. Sci. 2020;35(31):e287. doi: 10.3346/jkms.2020.35.e287. Published 2020 Aug 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N., Turner F., Slepnev V., et al. Self-collected oral fluid and nasal swab specimens demonstrate comparable sensitivity to clinician-collected nasopharyngeal swab specimens for the detection of SARS-CoV-2 [published online ahead of print, 2020 oct 19] Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1589. ciaa1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Simundic A.M., Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19) Clin. Chem. Lab. Med. 2020 doi: 10.1515/cclm-2020-0285. [DOI] [PubMed] [Google Scholar]
- Medeiros da Silva R.C., Nogueira Marinho L.C., de Araújo Silva D.N., Costa de Lima K., Pirih F.Q., Luz de Aquino Martins A.R. Saliva as a possible tool for the SARS-CoV-2 detection: a review [published online ahead of print, 2020 Nov19] Travel Med. Infect. Dis. 2020;38 doi: 10.1016/j.tmaid.2020.101920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasomsub E., Watcharananan S.P., Boonyawat K., et al. Saliva sample as a non-invasive specimen for the diagnosis of coronavirus disease 2019: a cross-sectional study. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos C.N., Rezende K.M., Oliveira Neto N.F., Okay T.S., Braz-Silva P.H., Bönecker M. Saliva: an important alternative for screening and monitoring of COVID-19 in children. Braz. Oral Res. 2020;34:e0125. doi: 10.1590/1807-3107bor-2020.vol34.0125. Published 2020 Nov 20. [DOI] [PubMed] [Google Scholar]
- To K.K., Tsang O.T., Chik-Yan Yip C., et al. Consistent detection of 2019 novel coronavirus in saliva. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A.L., Fournier J., Casanovas-Massana A., et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2 [published online ahead of print, 2020 Aug 28] N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2016359. doi:10.1056/NEJMc2016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee R., Truong T., Pannaraj P.S., et al. Saliva is a promising alternative specimen for the detection of SARS-CoV-2 in children and adults [published online ahead of print, 2020 Nov 25] J. Clin. Microbiol. 2020 doi: 10.1128/JCM.02686-20. JCM.02686-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota I., Shane P.Y., Okada K., et al. Mass screening of asymptomatic persons for SARS-CoV-2 using saliva [published online ahead of print, 2020 Sep 25] Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1388. ciaa1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.