Abstract
The coronavirus disease-2019 (COVID-19) pandemic has overwhelmed healthcare systems around the world, resulting in morbidity, mortality, and a dramatic economic downturn In the United States. Urgent responses to the pandemic halted routine hospital workflow in an effort to increase hospital capacity, maintain staffing, and ration protective gear. Most notably, New York saw the largest surge of COVID-19 cases nationwide. Healthcare personnel and physician leaders at Northwell Health, the largest healthcare system in New York, have worked together to successfully implement operational changes resulting in a paradigm shift in cardiac care delivery. In this manuscript, we detail specific protocol adjustments made in our cardiology department, cardiology service line, and healthcare system in the face of the COVID-19 pandemic. We discuss the sustainability of this shift moving forward and the opportunity to optimize care for cardiovascular patients in the post COVID-19 era.
Introduction
The coronavirus disease-2019 (COVID-19) pandemic has overwhelmed healthcare systems around the world. In the United States (US), urgent responses to the pandemic altered routine hospital work flow with rapid adjustments to expand inpatient beds and intensive care unit (ICU) capacity. Staffing models and redeployment strategies were designed to deal with the projected surge of disease and its burden on healthcare systems. Specifically, down state New York has been the epicenter of this pandemic in the United States, and during its height, had one-third of total US cases.1 During this time, bed capacity at Northwell Health's tertiary centers was increased by approximately 50% including makeshift tents for inpatient care, converting conference rooms and auditoriums into COVID wards, and repurposing of multiple procedural or surgical recovery areas into ICUs. As an example, Northwell Health's Long Island Jewish Medical Center had a peak census of 903 inpatients, a 50% increase from their normal census of 583 beds.
In this document we share our extensive COVID experience with a focus on the following aspects of cardiovascular2 care: (1) ambulatory access, (2) hospital care, (3) postsurge transition to the new normal, and (4) lessons learned during this crisis to better prepare for any subsequent pandemics or crises. Without an effective targeted treatment, vaccine or sufficient herd immunity for COVID-19, future waves of disease are likely to continue as economic activity resumes and travel restrictions are ease.3 We discuss the sustainability of these measures in an effort to adapt to a more efficient way of cardiac healthcare delivery across Northwell Health system, which comprises of 23 hospitals with over 6500 long-term beds and 700 outpatient centers. Crises such as this provide an opportunity to gather important knowledge, develop new strategies, and implement change.4
Ambulatory Care
Minimizing exposure to cardiovascular patients requires social distancing and increasing levels of remote cardiovascular monitoring.5 At the beginning of the COVID-19 pandemic, we immediately stopped nonurgent, in-person office visits (Fig 1 ). To address urgent needs, 1 physician was available to see ambulatory patients deemed to be urgent to avoid unnecessary visits to an already overwhelmed emergency room. Telemedicine was actively utilized and allowed patients to be remotely monitored and assessed from the safety of their homes and only patients who required advanced in-hospital cardiovascular management were seen in-person.
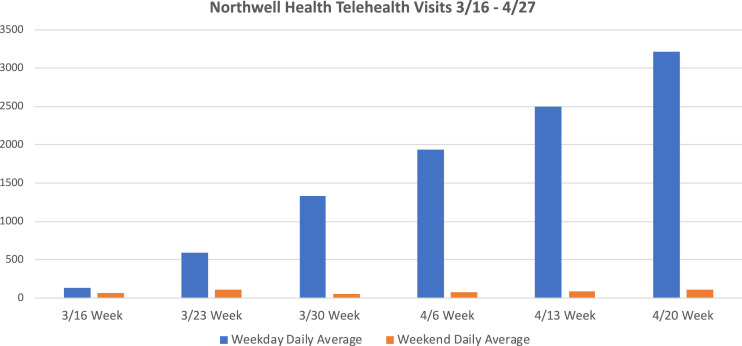
FIG. 1.
Daily telehealth visits. 3/16/2020 indicating beginning of lock down within health system.
Telemedicine and Triaging Cardiovascular patients
Early experience with COVID-19 has shown that patients with underlying cardiovascular disease are at higher risk for morbidity and mortality.6 , 7 Cardiovascular risk factors such as hypertension, diabetes, obesity, prior coronary artery disease are associated with increased severity of COVID-19 infection and worse prognosis.8 Telemedicine allows for continued care of this vulnerable population, while minimizing the risk of exposure associated with a visit to a medical facility. Previous work has highlighted the potential for using telemedicine in public health crises and disasters.9 In the United States, telehealth had been hampered by variable insurance coverage which Centers of Medicare and Medicaid Services (CMS) approved during the pandemic. The development of a robust telemedicine program requires resources by patients and the healthcare delivery office. Cardiovascular patients are often elderly and may be technologically limited, so simpler, more “user friendly” monitoring tools with education must be available to maximize use.10 Traditionally, community and hospital-based practices have focused on in person visits and have not promoted virtual provider-patient interactions.11 , 12
A central strategy to limiting ambulatory patient visits during the pandemic was “forward triaging” – screening patients by outpatient providers through telemedicine visits and making decisions based on clinical acuity, subsequently creating low- and high-risk categories. Established cardiovascular patients, with otherwise stable chronic conditions, were converted from scheduled office visits to telemedicine visits, resulting in telemedicine growth from 60 visits to 3348 visits across the health system (Fig 1). This substantially limited travel and exposure. In the future, some routine follow-up visits will be done through telemedicine, alternating between virtual and in office visits. Appropriate reimbursement for these services will also be paramount, as in many cases they take more preparation and visit time for the provider.
eHealth
eHealth encompasses the use of information and communication technologies in the support of health.13 Direct capture of biometric data such as heart rate, blood pressure (BP), electrocardiograms (ECG), physical activity, and body weight through digital medical tools and smart phones provides objective data useful in the management of cardiovascular patients.14 Recently, 200 patients were investigated for BP control after myocardial infarction (MI), using electronic visit with 4 smart phone-compatible devices: wireless BP monitor, step counter with pulse oximetry, weight scale, and single-lead ECG device.15 The key findings were similarly regulated BP, hospitalizations, and patient satisfaction between the digital intervention and usual care groups. Similarly, noninvasive digital analytics have been utilized in telemonitoring of stable heart failure populations to inform medication adherence and guide treatment with promising results.16, 17, 18 However, these digital tools need more effective integration into the electronic health record rather than only being accessible through external systems, which has been a limitation.19
Telemedicine Post-COVID
With our recent experience, the classic paradigm of cardiology ambulatory visits every 6 months or 12 months can easily be transitioned into telemedicine. Multiple barriers exist in maintaining usual care by telemedicine in the future, including changes in commercial and government reimbursement, ensuring quality of care and determining optimal frequency of visits.20 Simpler platforms for patient access along with an increase use of patient portals to communicate with healthcare staff will be required.21 Health Insurance Portability and Accountability Act (HIPPA) regulations will need to be readdressed in the post-COVID era, and simpler, more secure devices will allow more patients to utilize these options. Although user-friendly, secure telemedicine interfaces with seamless electronic health record integration and reimbursement parity is a daunting goal, perhaps the COVID-19 pandemic will be the impetus required for much needed enhancement and upgrades.
Future Ambulatory Direction
This COVID pandemic gives us an opportunity to reconfigure our ambulatory cardiovascular services. The incorporation of telehealth visits with telemonitoring allows physicians to take care of patients without clearly defined hours. Future innovation should attempt to ease the transition from inpatient to outpatient care, especially in vulnerable populations such as those with advanced heart failure.22 Outpatient management of these patients is an opportunity to standardize the use of telemedicine to trigger outpatient intravenous diuretic therapy protocols, which has been shown to be safe and effective.23 , 24
The impact of the delay in scheduling of outpatient visits, testing and procedures during the COVID-19 pandemic on clinical outcomes remains unknown. Provisional data from the National Center for Health Statistics indicate that all-cause mortality, apart from COVID-19, in New York City is 5000 greater than expected for March 2020.30 It is critically important that routine cardiovasuclar (CV) care continue during pandemics to minimize morbidity and mortality due to heart disease and patient fears of coming to the office or hospital. We should encourage these changes in the paradigm of our delivery of ambulatory cardiac care.
Hospital Care
Delivering high quality cardiovascular care to the patients while maintaining a safe work environment for the staff is paramount. Adequate testing was a challenge from the onset of the COVID-19 pandemic. Swab testing was not widely available; it was reserved for patients with symptoms, despite a sizeable asymptomatic COVID-19 patient population, and had delays in test results.25 , 26 Atypical presentation of cardiovascular diseases, such as MI and heart failure, along with the above issues made for increased staff exposure.
Northwell Health was one of the first healthcare systems to mandate the universal use of surgical masks in the hospital, including patients and healthcare workers (HCW), N95 (3M, Minnesota) masks were reserved for staff members with direct contact with suspected or confirmed COVID-19 patients in the units, floors, or procedural areas. Italy and Spain had a surge of HCWs infected with COVID-19, with HCWs comprising 10% and 14% of all confirmed cases, respectively.27 Given that hospitals are the local epicenter of the disease in this and future pandemics, aggressive action is required early in future surges. As we will discuss later, prioritizing personal protective equipment (PPE) stock piles will be imperative after COVID-19.
Routine ECG, Physical Examination, Testing
Novel approaches for managing cardiovascular inpatients are necessary not only for conserving limited resources but also reducing in-hospital exposure. We experienced an increased need for telemetry monitoring for COVID-19 positive patients related to the use of proarrhythmic medications, including hydroxychloroquine and azithromycin.28 We incorporated mobile continuous telemetry monitors to maximize and streamline our monitoring capabilities Mobile continuous telemetry monitors allowed for continuous near real time monitoring on nontelemetry floors. This, along with utilizing telemetry to monitor the corrected QT interval when available, eliminated the need for serial ECGs, thus reducing potential exposure of staff and helped conserve PPE.29 All requisitions for other tests, such as an ECG, echocardiography, blood draws, and x-rays were reviewed by cardiologists prior to approval. There is evidence that provider education can lead to a decrease in the frequency of testing, decreasing healthcare costs, and most importantly, result in improved HCW safety during a pandemic without compromising patient care.30
Cardiovascular point of care ultrasound by trained providers was utilized for the critical care management of patients whenever possible.31 This was shown to be effective in Wuhan in patients with severe COVID-19 infection and associated CV disease.32 This approach conserved PPE and limited transmission through providers or stethoscope. These measures are crucial in these unprecedented times, and can be protocolized for future surges to conserve scant resources and avoid exposure to HCWs.
Cardiovascular Procedures
Most institutions placed a moratorium on elective invasive catheterization and electrophysiology (EP) laboratory (EP) procedures early in the COVID-19 pandemic.33 , 34 Northwell stopped elective procedures even before the state mandate given the dramatic increase of COVID-19 patients in our health system, resulting in a decrease in 83% of cardiac catheterization procedures and 82% of EP procedures, respectively (Table 1 ). The goal was to reduce unchecked community spread, conserve equipment, and re-purpose staff and space to address COVID-19 patients. Despite this moratorium, the surge in COVID patients in New York quickly overwhelmed the available resources. Most recovery rooms and short stay units were utilized as ICUs. Some staff in the catheterization and EP laboratories were kept out of the hospital to minimize exposure in the event of a surge in HCW infections.
TABLE 1.
Changes in cardiology department volume
CT, computed tomography; LIJMC, Long Island Jewish Medical Center; MR, magnetic resonance; NSUH, North Shore University Hospital; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
 |
Patients were risk stratified into tiers (0-4), with tier 1 for elective, nonurgent cases, tier 4 for emergency cases, and tiers 2 and 3 in between (TABLE 2, TABLE 3, TABLE 4 ). Tier 4 patients underwent procedures throughout the pandemic; no other patients underwent cardiology procedures during this time. Patients with rescheduled elective procedures were followed closely as outpatients through the use of telemedicine and these patients were educated to report any change in their clinical status. Simultaneously, there was a dramatic and surprising drop in procedural volume, including urgent cases, likely due to patient fear of contracting COVID-19 in the hospital. We saw a 69% drop in acute MI patients as compared to the same time period in 2019.35 In an effort to conserve resources and improve staff resource utilization for COVID-19 related needs, Northwell Health regionalized cardiac catheterization care to 1 cardiac catheterization and EP laboratory per geographical region.
TABLE 2.
Case prioritization: catheterization
CCS, Canadian Cardiovascular Society; LV, left ventricle; NSTEMI, non-ST elevation myocardial infarction; STEMI, ST elevation myocardial infarction; VT, ventricular tachycardia.
 |
TABLE 3.
Case prioritization: EPS and ablation
AF, atrial flutter; EPS, electrophysiology service; LBBB: left bundle branch block; SVT, supraventricular tachycardia; VT, ventricular tachycardia; WPW, Wolf Parkinson Syndrome.
 |
TABLE 4.
Case prioritization: CIED, TEE, and cardioversion
AVB, atrioventricular block; CIED, cardiovascular implantable electronic device; CRT, cardiac resynchronization therapy; ERI, elective replacement indicator; ICD, implantable defibrillator device; ILR, implantable loop recorder; MCOT, mobile cardiac output telemetry; PM, pacemaker; SND, sinus node dysfunction; TEE, transesophageal echocardiography.
 |
Procedural Best Practice
The COVID-19 pandemic caused us to re-examine how we provided care. Established practice was rethought and changed. Same day discharge after percutaneous coronary intervention (PCI), pacemakers, implantable cardioverter defibrillator (ICDs), and routine ablation was encouraged. These have been shown to be safe,43, 44, 45 preferred by patients,46 and cost saving. Adoption of 7-day a week model facilitated capability to perform all necessary procedures even on the weekend and reduce length of stay. Meticulous PPE donning and doffing protocols were universally employed.36 , 42
Radial access was encouraged to allow for earlier ambulation and discharge after PCI.37, 38, 39 Lastly, given the short- and intermediate-term equipoise between PCI and coronary artery bypass surgery for multivessel disease, and the desire to reserve ICU capacity and ventilators for COVID patients, PCI was chosen over coronary artery bypass surgery when reasonable.40 , 41
Similar to catheterization procedures, EP procedures were limited to urgent/emergent cases, eg, for permanent pacing for symptomatic heart block, pacer-dependent patients with device nearing end of life, extraction for infected or failed leads or ventricular tachycardia ablation in unstable/hospitalized patients. Cardioversions for uncontrolled atrial fibrillation were also facilitated utilizing CT scan to clear the left atrium (LA) appendage, and avoid the exposure of transesophageal echocardiogram (TEE) when possible.42 , 43 If anesthesia deemed a patient to be high risk for respiratory compromise requiring intubation, then patients underwent elective controlled endotracheal intubation prior to the procedure.44
In collaboration with the Heart Rhythm Society, guidance to help clinicians limit consultation and manage cardiovascular implantable electronic device patients was developed (Table 5 ). Specific cardiovascular implantable electronic device programmers were designated for use with COVID-19 and each was extensively decontaminated after every use. In addition, we made pacemaker magnets widely available on COVID-19 ICUs and wards. In patients being transitioned to do not resuscitate (DNR)/palliative care status a magnet could be taped over the device to turn-off device therapies.
TABLE 5.
Indications for CIED evaluation
CIED, cardiovascular implantable electronic device; CRT, cardiac resynchronization therapy; ICD, implantable defibrillator device; MRI, magnetic resonance imaging.
 |
Post Peak-COVID-19 Procedures
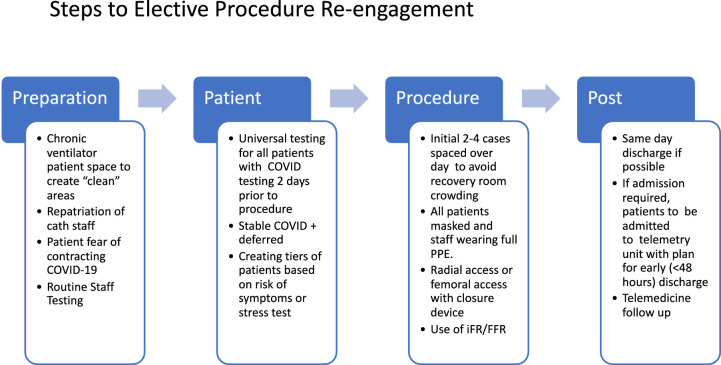
We decided that reintegration of elective procedures in the cardiac catheterization and EP laboratories will need to be done in a phased and systematic fashion. Patient apprehension of coming into the hospital will need to be addressed. The assurance of a “clean” space will be necessary to assuage patient fears. Addressing testing needs of both the patient and staff as well as creating “COVID-free” space is necessary. The foundation of any attempt at complete re-engagement is expanded testing to truly understand the disease burden. A proposed algorithm (Fig 2 ). would incorporate the following key elements:
-
1.
Preparation: Creating “COVID-19-free” space by moving chronic ventilator patients and emphasizing testing of patients and staff.
-
2.
Patient: Start with 2-4 elective cases per day to avoid recovery room crowding using the tier system previously mentioned while also allowing for strict implementation of screening practices.
-
3.
Procedure: All patients should be universally tested for COVID-19 prior to arriving to the hospital. Telemedicine will be utilized to presurgically evaluate patients and get their consent as well as provide sites for COVID-19 testing.
-
4.
Post: Limiting procedures to same day discharge as stated above, followed by early discharge with telehealth follow-up.
FIG. 2.
Steps to elective procedure re-engagement.
Redeployment of Staff and Fellows
As discussed above, many COVID-19 related changes were designed to limit exposure and preserve scarce resources. An essential resource was the HCW. The creation of new “wards” and ICUs required “repurposed” staff including nurses, advanced clinical practioner (ACPs), and physicians. All attendings and trainees within our Northwell Health were pooled together for the relief effort and placed into 3 tiers based on their skill set, age, medical conditions, and needs in their subdivision. The scheduled assignments originated from a centralized command to ensure equitable staff allocation from all divisions. A total of 72 physicians from our cardiology department, made up of 77% of total fellows and 80% of hospital full time faculty, were deployed to either a medical COVID floor or a repurposed COVID-19 ICU (Table 6 ). In a concerted effort to avoid staff burn out,45 all shifts were limited to 12 hours with rest days in between for a maximum of 5 nonconsecutive days in a 2-week period. A recent study showed that limiting shifts to <16 hours yielded 18% reduction in attention failures in trainees.46 Every effort was taken to reduce these attention failures, minimize occupational exposure, and avoid physician burn out. Staff were repurposed back into the cardiovascular space after 2-3 weeks and another set of physicians redeployed to provide some reprieve from the stresses of the “COVID” floors.
TABLE 6.
Cardiology staff deployment
 |
Lessons Learned for the Next Pandemic
How to Handle the Next One
Perhaps the only silver lining from the tragic COVID-19 pandemic is that it may serve as a wakeup call to prepare for future pandemics. To be effective for the next pandemic, cardiac care delivery protocols must be in place to respond more effectively and quickly to crises situation. In this paper we have presented some of the new protocols that were developed to deal with this crisis and this can be used as a template for unforeseen calamities such as the COVID-19 pandemic.
Clean Hospitals
Some have proposed the concept of “clean” hospitals, which has been done in parts of Canada such as Quebec. However, the term is a misnomer since only patients are tested, and if positive, are promptly transferred to COVID-19 designated hospitals. To be completely clean and COVID-19 free facilities will require universal testing for the HCW and staff to identify and exclude all COVID-19 positive personnel. This may be resource intensive upfront, requiring expanded testing, but would improve safety of staff and prevent further transmission within and outside of the hospital.
A feeling of safety would allow patients to seek care, in a timely manner, in “non-COVID-19” facilities. Non-COVID-19 deaths related to other medical illness may be as high or higher than COVID-19 related deaths during this pandemic. On par with this vision, Northwell Health's Syosset Hospital has been cleared and cleaned to re-open as a designated “clean” facility and restart semielective procedures. However, due to financial pressures on individual hospitals and hospitals systems, the concept of a clean hospital is not generalizable and needs to be discussed in the context of care regionalization and load balancing.
Load Balancing and PPE
Load balancing applied to a pandemic would be a situation where hospital systems would share patients, beds, and equipment to preferentially target the hardest hit regions and maintain resources throughout a given region as opposed to a single hospital or private network. Hospitals operate on very slim margins requiring near 100% utilization of facilities. This leaves no capacity for a sudden outbreak. Creation of this capacity comes at a cost. To enact these changes on a grand scale will require federal and state involvement to maintain hospital margins and the ability to resume normal revenue-generating procedures once the pandemic subsides. Additionally, given PPE shortages during the pandemic, a regional and federal stockpile of PPE should exist at all times. PPE is low cost, lifesaving, and these supplies have a long shelf life. COVID-19 crisis has clearly uncovered lack of preparedness at both federal and state levels, which will need to be addressed moving forward.
Beyond COVID: Lessons Learned
-
1.
Telemedicine should be integrated into mainstream ambulatory practices.
-
2.
HCW safety requires preparedness and strong advocacy. Central to this is having adequate PPE stockpiles in every region.
-
3.
The foundation of re-engagement of procedures is universal testing and creating a tier system of patients based on status.
-
4.
Dyad leadership of physicians and administrators is quintessential to plan, adapt and implement new protocols, and to maintain an open communication with all aspects of work force.
-
5.
This will not be the last pandemic. Novel ways of rapidly reorganizing healthcare delivery across a region instead of within hospitals will be necessary temporarily during pandemics and must be explored.
Conclusion
In conclusion, Northwell Health has been at the epicenter of the COVID-19 crisis in the United States and several lessons have been learned. This manuscript provides practical guidance for healthcare systems along with HCWs delivering cardiovascular care in the face of the COVID-19 pandemic and future inevitable surges. The anticipation and preparedness of institutions to disease outbreaks is crucial to providing the best quality of cardiovascular care while maintaining a safe work place for healthcare professionals and patients.
Footnotes
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.John Hopkins Coronavirus Research Center 2020 [cited 2020 April 18]. Available from: https://coronavirus.jhu.edu/us-map.
- 2.Giaccone G., Dalesio O., McVie G.J., Kirkpatrick A., Postmus P.E., Burghouts J.T., et al. Maintenance chemotherapy in small-cell lung cancer: long-term results of a randomized trial. European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol. 1993;11(7):1230–1240. doi: 10.1200/JCO.1993.11.7.1230. [DOI] [PubMed] [Google Scholar]
- 3.Leung K., Wu J.T., Liu D., Leung G.M. First-wave COVID-19 transmissibility and severity in China outside Hubei after control measures, and second-wave scenario planning: a modelling impact assessment. Lancet. 2020;395(10233):1382–1393. doi: 10.1016/S0140-6736(20)30746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fauci A.S., Lane H.C., Redfield R.R. Covid-19 - Navigating the Uncharted. N Engl J Med. 2020;382(13):1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):1–8. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonow R.O., Fonarow G.C., O'Gara P.T., Yancy C.W. Association of Coronavirus Disease 2019 (COVID-19) With Myocardial Injury and Mortality. JAMA Cardiol. 2020;5(7):751–753. doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lurie N., Carr B.G. The Role of Telehealth in the Medical Response to Disasters. JAMA Intern Med. 2018;178(6):745–746. doi: 10.1001/jamainternmed.2018.1314. [DOI] [PubMed] [Google Scholar]
- 10.Kim E.H., Gellis Z.D., Bradway C.K., Kenaley B. Depression care services and telehealth technology use for homebound elderly in the United States. Aging Ment Health. 2019;23(9):1164–1173. doi: 10.1080/13607863.2018.1481925. [DOI] [PubMed] [Google Scholar]
- 11.Tanne J.H., Hayasaki E., Zastrow M., Pulla P., Smith P., Rada A.G. Covid-19: how doctors and healthcare systems are tackling coronavirus worldwide. BMJ. 2020;368:m1090. doi: 10.1136/bmj.m1090. [DOI] [PubMed] [Google Scholar]
- 12.Hollander J.E., Carr B.G. Virtually Perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382:1679–1681. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 13.Saner H., van der Velde E. eHealth in cardiovascular medicine: A clinical update. Eur J Prev Cardiol. 2016;23(2 suppl):5–12. doi: 10.1177/2047487316670256. [DOI] [PubMed] [Google Scholar]
- 14.Topol E.J., Steinhubl S.R., Torkamani A. Digital medical tools and sensors. JAMA. 2015;313(4):353–354. doi: 10.1001/jama.2014.17125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Treskes R.W., van Winden L.A.M., van Keulen N., van der Velde E.T., Beeres S., Atsma D.E., et al. Effect of Smartphone-Enabled Health Monitoring Devices vs Regular Follow-up on Blood Pressure Control Among Patients After Myocardial Infarction: A Randomized Clinical Trial. JAMA Netw Open. 2020;3(4) doi: 10.1001/jamanetworkopen.2020.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaudhry S.I., Mattera J.A., Curtis J.P., Spertus J.A., Herrin J., Lin Z., et al. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363(24):2301–2309. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koehler F., Winkler S., Schieber M., Sechtem U., Stangl K., Bohm M., et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation. 2011;123(17):1873–1880. doi: 10.1161/CIRCULATIONAHA.111.018473. [DOI] [PubMed] [Google Scholar]
- 18.Anker S.D., Koehler F., Abraham W.T. Telemedicine and remote management of patients with heart failure. Lancet. 2011;378(9792):731–739. doi: 10.1016/S0140-6736(11)61229-4. [DOI] [PubMed] [Google Scholar]
- 19.Kao D.P., Trinkley K.E., Lin C.T. Heart Failure Management Innovation Enabled by Electronic Health Records. JACC Heart Fail. 2020;8(3):223–233. doi: 10.1016/j.jchf.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeRouge C., Garfield M.J. Crossing the telemedicine chasm: have the U.S. barriers to widespread adoption of telemedicine been significantly reduced? Int J Environ Res Public Health. 2013;10(12):6472–6484. doi: 10.3390/ijerph10126472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed M.E., Huang J., Brand R., Ballard D., Yamin C., Hsu J., et al. Communicating Through a Patient Portal to Engage Family Care Partners. JAMA Intern Med. 2018;178(1):142–144. doi: 10.1001/jamainternmed.2017.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson S.L., Tong X., King R.J., Loustalot F., Hong Y., Ritchey M.D. National Burden of Heart Failure Events in the United States, 2006 to 2014. Circ Heart Fail. 2018;11(12) doi: 10.1161/CIRCHEARTFAILURE.117.004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veilleux R.P., Wight J.N., Cannon A., Whalen M., Bachman D. Home diuretic protocol for heart failure: partnering with home health to improve outcomes and reduce readmissions. Perm J. 2014;18(3):44–48. doi: 10.7812/TPP/14-013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazkani M., Ota K.S. The role of outpatient intravenous diuretic therapy in a transitional care program for patients with heart failure: a case series. J Clin Med Res. 2012;4(6):434–438. doi: 10.4021/jocmr1106w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L., et al. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W., Du R.H., Li B., Zheng X.S., Yang X.L., Hu B., et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9(1):386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raphael Minder E.P. New York Times; 2020. Vulnerable Health Care Workers Falling Ill. 2020 March 25Sect. A. [Google Scholar]
- 28.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang D.S.M., Gabriels J., Ismail H., Goldner B., Willner J., Beldner S., Mitra R., JR, Epstein L.M. Inpatient Use of Ambulatory Telemetry Monitors for COVID-19 Patients Treated with Hydroxychloroquine and/or Azithromycin. J Am Coll Cardiol. 2020 Jun 16;75(23):2992–2993. doi: 10.1016/j.jacc.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thakkar R.N., Kim D., Knight A.M., Riedel S., Vaidya D., Wright S.M. Impact of an educational intervention on the frequency of daily blood test orders for hospitalized patients. Am J Clin Pathol. 2015;143(3):393–397. doi: 10.1309/AJCPJS4EEM7UAUBV. [DOI] [PubMed] [Google Scholar]
- 31.Johri A.M., Galen B., Kirkpatrick J.N., Lanspa M., Mulvagh S., Thamman R. ASE Statement on Point-of-Care Ultrasound during the 2019 Novel Coronavirus Pandemic. J Am Soc Echocardiogr. 2020;33(6):670–673. doi: 10.1016/j.echo.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L.W.B., Zhou J., Kirkpatrick J., Xie M., Johri A.M. Bedside Focused Cardiac Ultrasound in COVID-19 Infection From the Wuhan Epicenter: The Role of Cardiac Point of Care Ultrasound (POCUS), Limited Transthoracic Echocardiography and Critical Care Echocardiography. J Am Soc Echocardiogr. 2020 Jun;33(6):676–682. doi: 10.1016/j.echo.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welt F.G.P., Shah P.B., Aronow H.D., Bortnick A.E., Henry T.D., Sherwood M.W., et al. Catheterization Laboratory Considerations During the Coronavirus (COVID-19) Pandemic: From ACC's Interventional Council and SCAI. J Am Coll Cardiol. 2020 May;75(18):2372–2375. doi: 10.1016/j.jacc.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lakkireddy D.R., Chung M.K., Gopinathannair R., Patton K.K., Gluckman T.J., Turagam M., et al. Guidance for Cardiac Electrophysiology During the Coronavirus (COVID-19) Pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.03.028. S1547-5271(20)30289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia S A.M., Meraj P.M., Schmidt C., Garberich R., Jaffer F.A., Dixon S R.J., Tannenbaum M., Chambers J., Huang P.P., Henry T.D. Reduction in ST-Segment Elevation Cardiac Catheterization Laboratory Activations in the United States during COVID-19 Pandemic. J Am Coll Cardiol. 2020;75(22):2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szerlip M., Anwaruddin S., Aronow H.D., Cohen M.G., Daniels M.J., Dehghani P., et al. Considerations for Cardiac Catheterization Laboratory Procedures During the COVID-19 Pandemic Perspectives from the Society for Cardiovascular Angiography and Interventions Emerging Leader Mentorship (SCAI ELM) Members and Graduates. Catheter Cardiovasc Interv. 2020:1–12. doi: 10.1002/ccd.28887. [DOI] [PubMed] [Google Scholar]
- 37.Ziakas A.A., Klinke B.P., Mildenberger C.R., Fretz D.E., Williams E.M., Kinloch F.R., et al. Safety of same-day-discharge radial percutaneous coronary intervention: a retrospective study. Am Heart J. 2003;146(4):699–704. doi: 10.1016/S0002-8703(03)00258-8. [DOI] [PubMed] [Google Scholar]
- 38.Bertrand O.F., De Larochelliere R., Rodes-Cabau J., Proulx G., Gleeton O., Nguyen C.M., et al. A randomized study comparing same-day home discharge and abciximab bolus only to overnight hospitalization and abciximab bolus and infusion after transradial coronary stent implantation. Circulation. 2006;114(24):2636–2643. doi: 10.1161/CIRCULATIONAHA.106.638627. [DOI] [PubMed] [Google Scholar]
- 39.Jolly S.S., Yusuf S., Cairns J., Niemela K., Xavier D., Widimsky P., et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377(9775):1409–1420. doi: 10.1016/S0140-6736(11)60404-2. [DOI] [PubMed] [Google Scholar]
- 40.Serruys P.W., Morice M.C., Kappetein A.P., Colombo A., Holmes D.R., Mack M.J., et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 41.Tonino P.A., De Bruyne B., Pijls N.H., Siebert U., Ikeno F., van' t Veer M., et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 42.Lakkireddy D.R., Chung M.K., Gopinathannair R., Patton K.K., Gluckman T.J., Turagam M., et al. Guidance for Cardiac Electrophysiology During the Coronavirus (COVID-19) Pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Circulation. 2020;141(21):e823–e831. doi: 10.1161/CIRCULATIONAHA.120.047063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guglielmo M., Baggiano A., Muscogiuri G., Fusini L., Andreini D., Mushtaq S., et al. Multimodality imaging of left atrium in patients with atrial fibrillation. J Cardiovasc Comput Tomogr. 2019;13(6):340–346. doi: 10.1016/j.jcct.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Rubin G.A., Biviano A., Dizon J., Yarmohammadi H., Ehlert F., Saluja D., et al. Performance of Electrophysiology Procedures at an Academic Medical Center Amidst the 2020 Coronavirus (COVID-19) Pandemic. J Cardiovasc Electrophysiol. 2020 Jun;31(6):1249–1254. doi: 10.1111/jce.14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.West C.P., Dyrbye L.N., Shanafelt T.D. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516–529. doi: 10.1111/joim.12752. [DOI] [PubMed] [Google Scholar]
- 46.Weaver M.D., Landrigan C.P., Sullivan J.P., O'Brien C.S., Qadri S., Viyaran N., et al. The Association Between Resident Physician Work-Hour Regulations and Physician Safety and Health. Am J Med. 2020 Jul;133(7):e343–e354. doi: 10.1016/j.amjmed.2019.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]