Abstract
The recent pandemic of COVID-19 caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) presents an extraordinary challenge to identify effective drugs for prevention and treatment. The pathogenesis implicate acute respiratory disorder (ARD) which is attributed to significantly triggered “cytokine storm” and compromised immune system. This article summarizes the likely benefits of roflumilast, a Phosphodiesterase-4 (PDE-4) inhibitor as a comprehensive support COVID-19 pathogenesis. Roflumilast, a well-known anti-inflammatory and immunomodulatory drug, is protective against respiratory models of chemical and smoke induced lung damage. There is significant data which demonstrate the protective effect of PDE-4 inhibitor in respiratory viral models and is likely to be beneficial in combating COVID-19 pathogenesis. Roflumilast is effective in patients with severe COPD by reducing the rate of exacerbations with the improvement of the lung function, which might further be beneficial for better clinical outcomes in COVID-19 patients. However, further clinical trials are warranted to examine this conjecture.
Keywords: COVID-19, Phosphodiesterase‐4 inhibitor, Roflumilast, Airway inflammation, Immunomodulation
1. Introduction
Corona virus disease 2019 (COVID-19) is a viral pandemic, originated in Wuhan, China and is considered to be one of the most fatal pandemics which dramatically spread all over the world [1] and escalated into an international medical emergency. COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had infected almost 16 million people across 216 countries/regions and caused 640,000 deaths [2]. Besides the tragic consequence caused by COVID- 19 on human health, the pandemic is likely to cost the global economy approximately $2 trillion [3].
COVD-19 is primarily a droplet-spread infection and patients exhibit various symptoms of which fever, dry cough, and fatigue is predominant. In some cases, the symptoms had rapidly developed to acute respiratory distress syndrome, metabolic acidosis, septic shock, coagulation dysfunction eventually leading to multiple organ failure [[4], [5], [6]]. However, the mild and/or asymptomatic type of COVID-19 patients can recover shortly subsequent to isolation and healthy lifestyle and food habits [7,8]. There is no effective treatment recommended for COVID-19 except for comprehensive support. Evidences from in-vitro studies suggested that anti-malarial drugs such as chloroquine and hydroxychloroquine effectively blocked viral entry through inhibition of host receptor glycolysation, proteolytic processing and endosomal acidification [[9], [10], [11]]. Ramsedivir, a broad spectrum anti-viral drug is now in clinical trial and had demonstrated in-vitro and in-vivo efficacy against SARS-CoV-2 [12]. Combination of Lopinavir/ritonavir used for treating HIV demonstrated efficacious against other novel corona viruses via inhibition of 3-chymotrypsin-likeprotease, in-vitro [13]. In addition, other potential drugs such as corticosteroids, IL-6 antagonist and granulocyte–macrophage colony-stimulating factor are proposed in the treatment of COVID-19 and their clinical investigations are underway.
2. Pathogenesis of COVID-19
Although the pathophysiological implications of SARS-CoV-2 infection are not well elucidated, it closely resembles that of SARS-CoV infection characterized by fatal acute respiratory disease (ARD) [14]. SARS CoV mediated destructive inflammation exhibited increased release of pro inflammatory cytokines. Similarly, plasma analysis of SARS-CoV-2 patients showed marked elevation of pro-inflammatory cytokines like IL2, IL7, IL10 and GCSF, IP10, MCP1, MIP1α, and TNF-α suggesting the presence of cytokine storm mediated disease progression and severity [6]. The chaotic inflammatory status in severely affected COVID-19 patients also lead to lymphocyte apoptosis and depletion thereby resulting in a drastic down regulation of CD4+, CD8+, B and Natural Killer (NK) cells [5,[15], [16], [17]]. The massive release of inflammatory cytokines damages the lung epithelium and also triggers rigorous co morbidities in cardiac, kidney tissue and other body tissues as well resulting in respiratory blockade and multiple organ failure [18]. Recent reports also suggest massive infiltration of large number of macrophages, neutrophils, diffuse alveolar wall thickening and alveolar damage in severely infected COVID-19 patients [[19], [20], [21]]. Further, autopsy finding of COVID-19 cases also revealed the bilateral diffuse alveolar damage linked with pulmonary edema, pro-inflammatory concentrates, pneumocyte desquamation and indications of early-phase acute respiratory distress syndrome [19].
3. Phosphodiesterase-4 inhibitors
PDE-4 is the main selective cyclic adenosine monophosphate (cAMP) metabolizing enzyme in inflammatory cells as well as immune cells. PDE-4 is predominantly expressed in a range of cells including, lymphocytes, monocyte/macrophages, granulocytes, fibroblasts and epithelial cells [[22], [23], [24]]. Inhibition of PDE-4 enhances intracellular cAMP accumulation which subsequently activates protein kinase A (PKA). PKA activation further triggers anti-inflammatory cascade of events including decreased release of inflammatory mediators thereby suppressing neutrophil elastase and MMP-9 expression in neutrophils, eventually leading to reduced reactive oxygen species generation. PKA also augment IL-10 levels in macrophage and monocyte and inhibits eosinophil chemotaxis and degranulation and lymphocyte proliferation as well. This enhances relaxation of airway smooth muscle leading to bronchodilation [[25], [26], [27], [28], [29]]. Besides, cAMP inhibits NF-kβ pathway which also contribute to slowing down of immunosuppressive and anti-inflammatory events [30]. A wide range of studies have demonstrated the anti-inflammatory effects of PDE-4 inhibitors in in-vivo respiratory models of chemical, bacterial and viral infections mediated lung injury [[31], [32], [33], [34], [35], [36], [37]].
Several PDE-4 inhibitors have been identified such as Cilomilast, Piclamilast, Oglemilast, Tetomilast, Tofimilast, ronomilast, Revamilast, UK-500,001, AWD 12–281, CDP840, CI-1018, GSK256066, YM976, GS-5759 to treat chronic obstructive pulmonary disease (COPD) and asthma. CHF 6001, is an inhaled PDE-4 inhibitor currently undergoing phase II clinical trials for COPD. Also, two orally administered PDE-4 inhibitors such as roflumilast and apremilast have been approved in a row as treatments against inflammatory diseases including COPD, psoriasis and psoriatic arthritis. Furthermore, Crisaborole is another example of PDE-4 inhibitor was approved for the topical treatment of mild to-moderate atopic dermatitis [38,39]. Recently, Mugheddu et al. reported that a 45‐year‐old obese male patient receiving apremilast for erythrodermic psoriasis recovered from COVID-19 without any adverse effects, suggesting that the anti-inflammatory properties of apremilast might have contributed to its preferential effect against SARS-CoV-2 infection [40]. Dalamaga et al., suggested that the selective inhibition of PDE-4 showed significant anti-inflammatory effects in a wide range of inflammatory cells, which may in fact imply a promising treatment option for treatment of COVID-19 at the initial phase [38].
4. Roflumilast
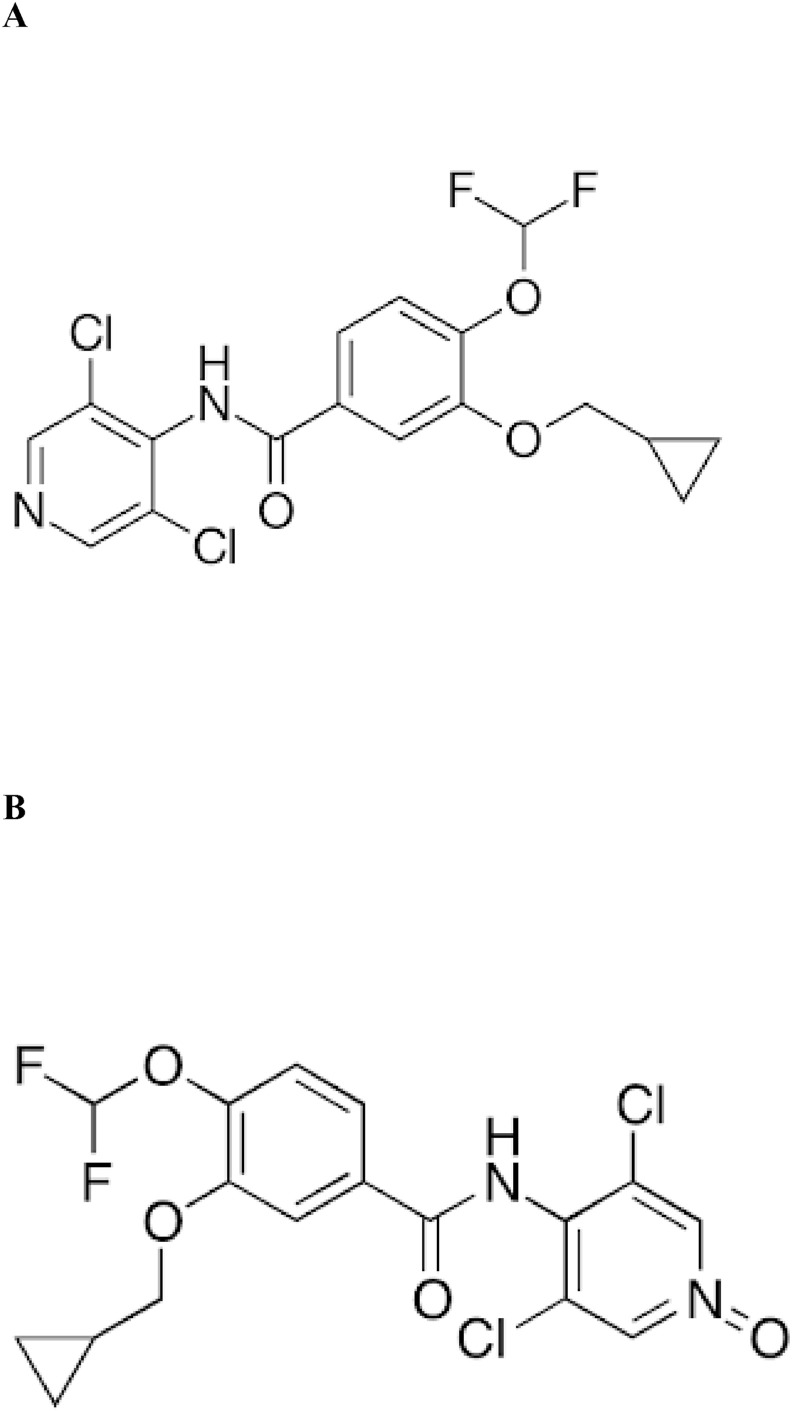
Roflumilast (3-cyclo-propylmethoxy-4-difluoromethoxy-N-[3,5-di-chloropyrid-4-yl]-benzamide; Fig. 1 A), has been identified as a potent and highly selective second generation PDE-4 inhibitor [41]. The anti-inflammatory and immunomodulatory effects of roflumilast was studied in human leukocytes using cell specific interactions such as monocyte-derived macrophages, formyl-methyl-leucyl-phenylalanine (fMLP)-induced formation of LTB4 and ROS generation, dendritic cells and anti-CD3/anti-CD28 monoclonal antibody-stimulated proliferation etc. Results from the studies suggest that roflumilast is a potent immunomodulator and effects are substantially high compared to other structurally unrelated PDE-4 inhibitors like cilomilast and rolipram [42]. An in-vitro study suggested that roflumilast inhibited fibroblast chemotaxis in TGβ1 stimulated fibroblasts through activation of cAMP, signalled by PKA [43]. Also, roflumilast suppressed leukocyte-endothelial interactions and augmented endothelial permeability, significant hallmarks of chronic inflammation in lipopolysaccharide (LPS) model of inflammation, in-vitro and in-vivo [44]. Roflumilast and its active metabolite, roflumilast-N-oxide was reported to ameliorate LPS triggered inflammatory cytokines release and TNF-α release from human lung parenchymal explants [45]. In addition, roflumilast was reported to ameliorate bleomycin-induced lung fibrosis [46] and also inhibited benzo (a) pyrene induced lung carcinogenesis in mice models [47]. Further, it also showed to stabilize LPS-induced systemic inflammation in rats [48]. Izikki et al., demonstrated that roflumilast alleviated pulmonary hypertension in chronic hypoxia and monocrotaline (MCT) rat model without affecting systemic artery pressure (SAP) in rats [49,50]. Similarly, roflumilast slowed down progression of emphysema and prevented parenchymal destruction in mice exposed to cigarette smoke [51,52]. Treatment with roflumilast in ovalbumin challenged BALB/c mice showed down regulated IL-17A, TNF-alpha, granulocyte-macrophage colony-stimulating factor and IL-6 expression and also suppressed the enhanced mRNA expression of growth factors such as TGF-beta1 and FGF-2 in airway epithelium [53]. Roflumilast also showed down regulated expression of IL-6 and TNF- α in cecal ligation and puncture (CLP)-induced sepsis in mice [54].
Fig. 1.
Structure of (A) Roflumilast and (B) Roflumilast N-Oxide.
Also, roflumilast inhibited emphysema in polymeric Ig receptor (pIgR −/−) deficient mice which exhibited age dependent emphysema, developed through an innate neutrophilic response to airway microbiota [55]. Roflumilast has shown affinity towards 3C-like proteinase of SARS-CoV-2 at the concentration of 248.89 nM. Although the concentration is high, affinity of roflumilast on 3C-like proteinase cannot be ignored [56]. Accumulation of cAMP due to inhibition of PDE-4 enzyme by roflumilast leads to increased levels of anti-inflammatory cytokine (IL-10) and reduced secretion of TNF- α and IL-12 by dendritic cells [57], which indicate that roflumilast is not only effective in upstream suppression of several cytokine signalling pathways, but also in balancing the pro-inflammatory/anti-inflammatory equilibrium. As cytokines like IL-6, IL-8, IL-1β, and TNF-α have been shown in the primary stage of COVID-19 infection [58], preventing the storm of cytokines in the primary stage of COVID-19 pneumonia may be an appropriate treatment option. Therefore, it has been speculated that roflumilast can be considered as a potential therapeutic option against COVID-19 infection. Studies with roflumilast evidenced that it inhibited early and late allergic response and airway hyper-responsiveness in Aspergillus-fumigatus sensitized mice [59]. Recent finding suggested that roflumilast inhibit leukocyte-platelet interactions and also the pro-thrombotic functions in leukocytes and monocytes [60]. Thrombotic complications are largely reported in the pathogenesis of COVID -19 patients [61], which spicules scope of PDE-4 inhibitors like roflumilast as a potent therapy option for COVID-19. The cytokine storms in severe COVID-19 cases were known to be inhibited by PDE-4 inhibitors such as roflumilast. Hence, it is likely that use of roflumilast would be favorable in augmenting myriad of complications like inflammation and apoptosis along with immunomodulatory effect and bronchial smooth muscle relaxation in patients with COVID- 19 (Fig. 2 ). Tabaa and Tabaa has hypothesized that neprilysin (NEP)-mediated therapeutic properties of roflumilast could help to fight against COVID-19 associated inflammatory, coagulopathy and fibrotic cascades, since activation of NEP was previously reported to mitigate several airway inflammatory diseases [62].
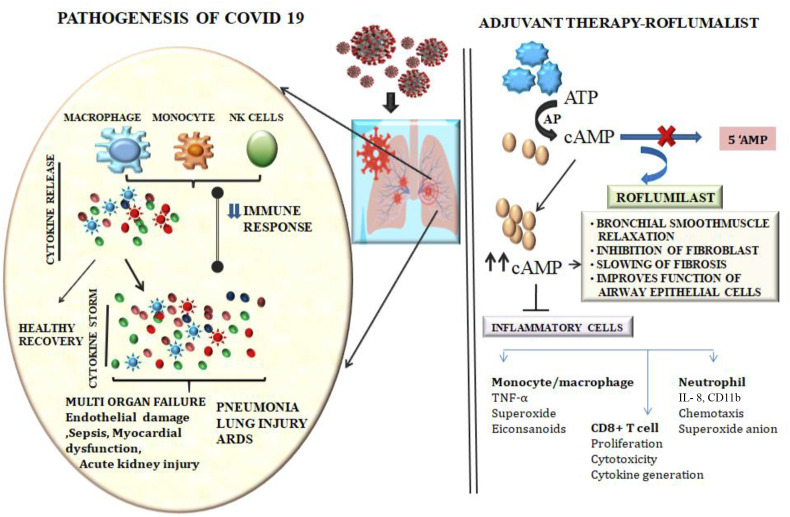
Fig. 2.
Pathogenesis of COVID-19 and potential adjuvant therapy of roflumilast. SARS-CoV-2 infections lead to elevation of inflammatory mediators and suppression of immune response which in turn triggers activation of cytokine storm. Overwhelming immune cell activation thus leads to ARDS, sepsis and multiorgan failure. In this context, Roflumilast might act as a potent adjuvant therapeutic agent by regulating the immune system, inflammation and bronchial smooth muscle relaxation through enhanced cAMP production and provide support for patients with ARDS and related complications. ARDS: Acute respiratory distress syndrome; PDE-4: Phosphodiestrase-4; AC: Adenylate Cyclase.
5. Roflumilast in humans
Several randomized, double-blind, placebo-controlled trials have demonstrated the safety and efficacy of roflumilast (500 μg orally once daily) in Chronic obstructive pulmonary disease (COPD) patients with the improvement in the lung function and reduced the rate of exacerbations [[63], [64], [65]] and decreased sputum neutrophils and eosinophils by 31% and 42%, respectively over one-month treatment [66]. Roflumilast also reduces allergen-induced inflammation in mild asthmatic subjects [67]. Several Phase III studies, single dose of roflumilast was associated with improvements from baseline in lung function, including increases in mean pre- and post- FEV1 and vital capacity [64,65,[68], [69], [70]]. The main clinical benefit of roflumilast in COPD is the reduction of exacerbations and a modest increase in FEV1 with improvement of symptoms [71]. This reduced exacerbation risk of roflumilast is directly correlated to its potential anti-inflammatory properties [72]. Further, Savelikhina et al. reported that roflumilast reduces IL-6 levels in bronchoalveolar lavage fluid (BALF) in severe COPD patients [73]. During July 2010, European Commission granted marketing authorisation for roflumilast (Daxas; Nycomed) in the European Union, for the maintenance treatment of severe COPD, due to its anti-inflammatory and bronchodilator properties [68,74]. Later, in March 2011, USFDA approved roflumilast (Daliresp; Forest) only for patients with COPD [75,76]. After nearly 20 years of efforts, roflumilast is the first class PDE-4 inhibitor to be marketed for treating COPD with severe airflow limitation, chronic bronchitis and history of exacerbations [74] and this could be attributed to its good efficacy/tolerability ratio. Further studies found that memory performance was significantly improved after acute administration of 0.1 mg roflumilast as a memory enhancer in humans without side effect. The plasma concentration of roflumilast and its active metabolite is 2.09 ng/ml (5.18 nM) and 1.91 ng/ml (4.73 nM) respectively [[77], [78], [79]]. Further, Phase I study have shown cognitive impairment associated with schizophrenia with roflumilast administration for 8 days at a dose of 100 or 250 μg, once daily in addition to second generation antipsychotics in participants with stable schizophrenia [80].
6. Pharmacokinetics of roflumilast
Roflumilast is completely absorbed after the oral administration and the bioavailability of roflumilast is approximately 80% with the Cmax of ~1 h after a single dose of 500 μg tablet in healthy volunteers. Plasma concentration (Cmax) of roflumilast was found to be 3.8 ng/ml (9.5 nM) when treated at 500 μg once daily in asthmatic and COPD patients [81]. Roflumilast is eliminated through hepatic metabolism by CYP1A2 and CYP3A4, primarily to the active metabolite roflumilast N-oxide (Fig. 1B) [82,83], which accounts for > 90% of roflumilast total PDE-4 inhibitory activity [42]. Parent and active metabolite of roflumilast are highly plasma protein bound (≥97%) and has a high volume of distribution, suggesting significant tissue penetration. Roflumilast N-oxide has approximately ten times more exposure than the parent drug. The plasma half-life (t½) of roflumilast and its active metabolite are ~17 h–30 h respectively [41]. Pharmacokinetics of roflumilast or roflumilast N-oxide was not affected by food in healthy volunteers [84]. This favorable pharmacokinetic profile of roflumilast could be anticipated to produce prolonged PDE-4 enzyme inhibition thereby contributing to its anti-inflammatory and immunomodulatory properties in immune cells. The suppression of inflammatory mediators and cytokines usually translates into benefits for patients infected with corona virus who often have elevated markers of inflammation compared to patients with baseline disease.
7. Roflumilast safety
Roflumilast is usually well tolerated with adverse effects (AEs) constant with those expected for PDE-4 inhibitors [85]. However, few studies have reported increased frequency of roflumilast linked adverse events and further discontinuation of the drug [[86], [87], [88]]. Clinical trials of roflumilast revealed that the most common AEs were diarrhoea and nausea and these symptoms were related with an increased risk of decreased appetite, weight loss, insomnia, abdominal pain and significant increase of the risk of headache [89] at COPD dose of 500 μg. The typical side effects of PDE-4 inhibitors were not observed at the 100 μg dose of roflumilast (five times less than COPD dose) in the recent studies on cognition enhancement in young adults. However, at the 300 μg dosage, nausea was reported by some participants [77], indicating that roflumilast might have a favorable safety margin. Corona virus infection may directly affect heart disease, those with Cardiac diseases who are infected by the coronavirus have an elevated risk of adverse outcomes, and infection itself is associated with Cardio vascular complications [90]. Notably, roflumilast was associated with a significantly lower risk of major adverse cardiovascular events in patients with COPD versus placebo-treated patients [91]. Although, the safety of roflumilast has been demonstrated in many of the clinical studies, its beneficial effect when given to patients with COVID-19 should be carefully observed even though the roflumilast is highly tolerable.
8. Conclusion
At present, there are no specific treatments available for COVID-19. Considering the consistent effects of roflumilast on respiratory diseases from numerous preclinical and clinical studies, roflumilast holds great intrinsic value for future clinical applications. Although, there is no preclinical evidence supporting efficacy of roflumilast against COVID- 19 infection, there are ample data on the efficacy and safety of roflumilast in human respiratory diseases. We speculate the scope of roflumilast as a potent adjuvant therapy to manage the severe clinical manifestations in severely infected COVID- 19 patients.
Funding
No funding sources.
Ethical approval
Not required.
Declaration of competing interest
The author declares no conflict of interest.
References
- 1.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . 2020. WHO Director-General's Opening Remarks at the Media Briefing on COVID-19 - 27 July 2020.https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-27-july-2020 [Google Scholar]
- 3.UNCATD, Coronavirus update COVID-19 likely to cost economy $1 trillion during 2020, says UN trade agency. https://news.un.org/en/story/2020/03/1059011 March 9 (2020) Available at:
- 4.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J. Pediatr. 2020;87(4):281–286. doi: 10.1007/s12098-020-03263-6. https://doi/10.1007/s12098-020-03263-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.Q., Wang Q., Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. doi: 10.1038/s41392-020-0148-4. https://doi/10.1038/s41392-020-0148-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;1395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. https://doi/10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. J. Am. Med. Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. https://doi/10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harapan H., Itoh N., Yufika A., Winardi W., Keam S., Te H., Megawati D. Coronavirus disease 2019 (COVID-19): a literature review. J. Infect. Public Health. 2020;(5):667–673. doi: 10.1016/j.jiph.2020.03.019. https://doi/10.1016/j.jiph.2020.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colson P., Rolain J.-M., Raoult D. Chloroquine for the 2019 novel coronavirus SARSCoV- 2. Int. J. Antimicrob. Agents. 2020:105923. doi: 10.1016/j.ijantimicag.2020.105923. https://doi/10.1016/j.ijantimicag.2020.105923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. J. Am. Med. Assoc. 2020 Apr 13 doi: 10.1001/jama.2020.6019. https://doi/10.1001/jama.2020.6019 [Published online ahead of print, 2020 Apr 13] [DOI] [PubMed] [Google Scholar]
- 11.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;(3):269–271. doi: 10.1038/s41422-020-0282-0. https://doi/10.1038/s41422-020-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Wit E., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T., Scott D., Cihlar T., Feldmann H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. U.S.A. 2020;117(12):6771–6776. doi: 10.1073/pnas.1922083117. https://doi/10.1073/pnas.1922083117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S., Kao R.Y., Poon L.L. HKU/UCH SARS Study Group. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. https://doi/10.1136/thorax.2003.012658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. https://doi:10/1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020;12 doi: 10.1093/cid/ciaa248. https://doi/10.1093/cid/ciaa248 ciaa 248. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. https://doi/10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao W., Li T. COVID-19: towards understanding of pathogenesis. Cell Res. 2020;30(5):367–369. doi: 10.1038/s41422-020-0327-4. https://doi/10.1038/s41422-020-0327-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao X. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. https://doi/10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary pathology of Early-phase 2019 Novel Coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 2020;15(5):700–704. doi: 10.1016/j.jtho.2020.02.010. https://doi/10.1016/j.jtho.2020.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin. Immunol. 2020;215:108427. doi: 10.1016/j.clim.2020.108427. https://doi/10.1016/j.clim.2020.108427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schudt C., Tenor H., Hatzelmann A. PDE isoenzymes as targets for anti-asthma drugs. Eur. Respir. J. 1995;(8):1179–1183. doi: 10.1183/09031936.95.08071179. https://doi/10.1183/09031936.95.08071179 [DOI] [PubMed] [Google Scholar]
- 23.Tenor H., Hatzelmann A., Church M.K., Schudt C., Shute J.K. Effects of theophylline and rolipram on leukotriene C4 (LTC4) synthesis andchemotaxis of human eosinophils from normal and atopic subjects. Br. J. Pharmacol. 1996;118(7):1727–1735. doi: 10.1111/j.1476-5381.1996.tb15598.x. https://doi/10.1111/j1476-5381.1996.tb15598.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torphy T.J. Phosphodiesterase isozymes: molecular targets for novel antiasthma agents. Am. J. Respir. Crit. Care Med. 1998;157(2):351–370. doi: 10.1164/ajrccm.157.2.9708012. https://doi/10.1164/ajrccm.157.2.9708012 [DOI] [PubMed] [Google Scholar]
- 25.Cortijo J., Villagrasa V., Navarrete C., Sanz C., Berto L., Michel A., Bonnet P.A., Morcillo E.J. Effects of SCA40 on human isolated bronchus and human polymorphonuclear leukocytes: comparison with rolipram, SKF94120 and levcromakalim. Br. J. Pharmacol. 1996;11:99–106. doi: 10.1111/j.1476-5381.1996.tb15682.x. https://doi/10.1111/j.1476-5381.1996.tb15682.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siegmund B., Eigler A., Moeller J., Greten T.F., Hartmann G., Endres S. Suppression of tumour necrosis factor-alpha production by interleukin- 10 is enhanced by cAMP-elevating agents. Eur. J. Pharmacol. 1997;321:231–239. doi: 10.1016/s0014-2999(96)00947-8. https://doi/10.1016/s0014-2999(96)00947-8 [DOI] [PubMed] [Google Scholar]
- 27.Au B.T., Teixeira M.M., Collins P.D., Williams T.J. Effect of PDE-4 inhibitors on zymosan-induced IL-8 release from human neutrophils: synergism with prostanoids and salbutamol. Br. J. Pharmacol. 1998;123:1260–1266. doi: 10.1038/sj.bjp.0701723. https://doi/10.1038/sj.bjp.0701723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brideau C., Van Staden C., Styhler A., Rodger I.W., Chan C.C. The effects of phosphodiesterase type 4 inhibitors on tumour necrosis factor-alpha and leukotriene B4 in a novel human whole blood assay. Br. J. Pharmacol. 1999;126(4):979–988. doi: 10.1038/sj.bjp.0702387. https://doi/10.1038/sj.bjp.0702387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatzelmann A., Morcillo E.J., Lungralla G., Adnot S., Sanjar S., Beume R., Schudt C., Tenor H. The pre-clinical pharmacology of roflumilast, a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm. Pharmcol. Ther. 2010;23(4):235–256. doi: 10.1016/j.pupt.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Wittmann M., Helliwell P.S. Phosphodiesterase 4 inhibition in the treatment of psoriasis, psoriatic arthritis and other chronic inflammatory diseases. Dermatol. Ther. 2013;3(1):1–15. doi: 10.1007/s13555-013-0023-0. https://doi/10.1007/s13555-013-0023-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner C.R., Esser K.M., Wheeldon E.B. Therapeutic intervention in a rat model of ARDS: IV. Phosphodiesterase IV inhibition. Circ. Shock. 1993;39(3):237–245. [PubMed] [Google Scholar]
- 32.Miotla J.M., Teixeira M.M., Hellewell P.G. Suppression of acute lung injury in mice by an inhibitor of phosphodiesterase type 4. Am. J. Respir. Cell Mol. Biol. 1998;18(3):411–420. doi: 10.1165/ajrcmb.18.3.2913. https://doi/10.1165/ajrcmb.18.3.2913 [DOI] [PubMed] [Google Scholar]
- 33.Toward T.J., Broadley K.J. Chronic lipopolysaccharide exposure on airway function, cell infiltration, and nitric oxide generation in conscious Guinea pigs: effect of rolipram and dexamethasone. J. Pharmacol. Exp.Ther. 2001;298(1):298–306. [PubMed] [Google Scholar]
- 34.Toward T.J., Johnson F.J., Boult J.E., Maillard J.Y. Airway function and reactivity, leukocyte influx and nitric oxide after inoculation with parainfluenza-3 virus: effects of dexamethasone or rolipram. Int. Immunopharm. 2005;5(4):771–782. doi: 10.1016/j.intimp.2004.12.006. https://doi/10.1016/j.intimp.2004.12.006 [DOI] [PubMed] [Google Scholar]
- 35.Pan J.B., Hou Y.H., Zhang G.J. Rolipram attenuates bleomycin A5-induced pulmonary fibrosis in rats. Respirology. 2009;14(7):975–982. doi: 10.1111/j.1440-1843.2009.01606.x. https://doi/10.1111/j.1440-1843.2009.01606.x [DOI] [PubMed] [Google Scholar]
- 36.Chang W., Chen J., Schlueter C.F., Rando R.J., Pathak Y.V., Hoyle G.W. Inhibition of chlorine- induced lung injury by the type 4 phosphodiesterase inhibitor rolipram. Toxicol. Appl. Pharmacol. 2012;263(2):251–258. doi: 10.1016/j.taap.2012.06.017. https://doi/10.1016/j.taap.2012.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma G., Champalal Sharma D., Hwei Fen L., Pathak M., Bethur N., Pendharkar V., Peiris M., Altmeyer R. Reduction of influenza virus-induced lung inflammation and mortality in animals treated with a phosophodisestrase-4 inhibitor and a selective serotonin reuptake inhibitor. Emerg. Microb. Infect. 2013;2(8):e54. doi: 10.1038/emi.2013.52. https://doi/10.1038/emi.2013.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalamaga M., Karampela I., Mantzoros C.S. Commentary: phosphodiesterase 4 inhibitors as potential adjunct treatment targeting the cytokine storm in COVID-19. Metabolism. 2020;109:S0026–S0495. doi: 10.1016/j.metabol.2020.154282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woo T., Kuzel P. Crisaborole 2% ointment (Eucrisa) for atopic dermatitis. Skin Ther. Lett. 2019;24(2):4–6. [PubMed] [Google Scholar]
- 40.Mugheddu C., Pizzatti L., Sanna S., Atzori L., Rongioletti F. COVID-19 pulmonary infection in erythrodermic psoriatic patient with oligodendroglioma: safety and compatibility of apremilast with critical intensive care management. J. Eur. Acad. Dermatol. Venereol. 2020;34(8):e376–e378. doi: 10.1111/jdv.16625. https://doi/10.1111/jdv.16625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rabe K.F. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br. J. Pharmacol. 2011;163(1):53–67. doi: 10.1111/j.1476-5381.2011.01218.x. https://doi/10.1111/j.1476-5381.2011.01218.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hatzelmann A., Schudt C. Anti-inflammatory and immunomodulatory potential of the novel PDE4 inhibitor roflumilast in vitro. J. Pharmacol. Exp.Ther. 2001;297:267–279. [PubMed] [Google Scholar]
- 43.Togo S., Liu X., Wang X., Sugiura H., Kamio K., Kawasaki S., Kobayashi T. PDE-4 inhibitors roflumilast and rolipram augment PGE2 inhibition of TGF-β1-stimulated fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;296:959–969. doi: 10.1152/ajplung.00508.2007. https://doi/10.1152/ajplung.00508.2007 [DOI] [PubMed] [Google Scholar]
- 44.Sanz M.J., Cortijo J., Morcillo E.J. PDE-4 inhibitors as new anti-inflammatory drugs: effects on cell trafficking and cell adhesion molecules expression. Pharmacol. Ther. 2005;106(3):269–297. doi: 10.1016/j.pharmthera.2004.12.001. https://doi/10.1016/j.pharmthera.2004.12.001 [DOI] [PubMed] [Google Scholar]
- 45.Buenestado A., Chaumais M.C., Grassin-Delyle S., A Risse P., Naline E., Longchampt E., Tenor H., Devillier P. Roflumilast inhibits lipopolysaccharide-induced tumor necrosis factor-α and chemokine production by human lung parenchyma. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0074640. https://doi/10.1371/journal.pone.0074640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cortijo J., Iranzo A., Milara X., Mata M., Cerdá-Nicolás M., Ruiz-Saurí A., Tenor H., Hatzelmann A., Morcillo E.J. Roflumilast, a phosphodiesterase 4 inhibitor, alleviates bleomycin-induced lung injury. Br. J. Pharmacol. 2009;156:534–544. doi: 10.1111/j.1476-5381.2008.00041.x. https://doi/10.1111/j.1476-5381.2008.00041.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeo C.D., Kim Y.A., Lee H.Y., Kim J.W., Kim S.J., Lee S.H., Kim Y.K. Roflumilast treatment inhibits lung carcinogenesis in benzo(a)pyrene-induced murine lung cancer model. Eur. J. Pharmacol. 2017;812:189–195. doi: 10.1016/j.ejphar.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Schick M.A., Wunder C., Wollborn J., Roewer N., Waschke J., Germer C.T., Schlegel N. Phosphodiesterase-4 inhibition as a therapeutic approach to treat capillary leakage in systemic inflammation. J. Physiol. 2012;590(11):2693–2708. doi: 10.1113/jphysiol.2012.232116. https://doi/10.1113/jphysiol.2012.232116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Izikki M., Raffestin B., Klar J., Hatzelmann A., Marx D., Tenor H., Zadigue P., Adnot S., Eddahibi S. Effects of roflumilast, a phosphodiesterase-4 inhibitor, on hypoxia- and monocrotaline-induced pulmonary hypertension in rats. J. Pharmacol. Exp. Ther. 2009;330:54–62. doi: 10.1124/jpet.108.148742. https://doi/10.1124/jpet.108.148742 [DOI] [PubMed] [Google Scholar]
- 50.Jabaris S.G., Sumathy H., Kumar R.S., Narayanan S., Thanikachalam S., Babu C.S. Effects of rolipram and roflumilast, phosphodiesterase-4 inhibitors, on hypertension-induced defects in memory function in rats. Eur. J. Pharmacol. 2015;746:138–147. doi: 10.1016/j.ejphar.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 51.Martorana P.A., Beume R., Lucattelli M., Wollin L., Lungarella G. Roflumilast fully prevents emphysema in mice chronically exposed to cigarette smoke. Am. J. Respir. Crit. Care Med. 2005;172(7):848–853. doi: 10.1164/rccm.200411-1549OC. https://doi/10.1164/rccm.200411-1549OC [DOI] [PubMed] [Google Scholar]
- 52.Martorana P.A., Lunghi B., Lucattelli M., De Cunto G., Beume R., Lungarella G. Effect of roflumilast on inflammatory cells in the lungs of cigarette smoke-exposed mice. BMC Pulm. Med. 2008;28:8–17. doi: 10.1186/1471-2466-8-17. https://doi/10.1186/1471-2466-8-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herbert C., Hettiaratchi A., Webb D.C., Thomas P.S., Foster P.S., Kumar R.K. Suppression of cytokine expression by roflumilast and dexamethasone in a model of chronic asthma. Clin. Exp. Allergy. 2008;(5):847–856. doi: 10.1111/j.1365-2222.2008.02950.x. https://doi/10.1111/j.1365-2222.2008.02950.x [DOI] [PubMed] [Google Scholar]
- 54.Feng H., Chen J., Wang H., Cheng Y., Zou Z., Zhong Q., Xu J. Roflumilast reverses polymicrobial sepsis-induced liver damage by inhibiting inflammation in mice Lab. Invest. 2017;97(9):1008–1019. doi: 10.1038/labinvest.2017.59. https://doi/10.1038/labinvest.2017.59 [DOI] [PubMed] [Google Scholar]
- 55.Richmond B.W., Du R.H., Han W., Benjamin J.T., van der Meer R., Gleaves L., Guo M., McKissack A. Bacterial-derived neutrophilic inflammation drives lung remodeling in a mouse model of chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2018;58(6):736–744. doi: 10.1165/rcmb.2017-0329OC. https://doi/10.1165/rcmb.2017-0329OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu F., Jiang J., Yin P. 2020. Prediction of Potential Commercially Inhibitors against SARSCoV-2 by Multi-Task Deep Model. arXiv preprint arXiv:2003.00728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X., Chen Y., Fan L., Ye J., Fan J., Xu X., You D., Liu S., Chen X., Luo P. Pharmacological mechanism of roflumilast in the treatment of asthma-COPD overlap. Drug Des. Dev. Ther. 2018;1(12):2371–2379. doi: 10.2147/DDDT.S165161. https://doi/10.2147/DDDT.S165161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moghadam S.O. A review on currently available potential therapeutic options for COVID-19. Int. J. Gen. Med. 2020;24(13):443–467. doi: 10.2147/IJGM.S263666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoymann H.G., Wollin L., Müller M., Korolewitz R., Krug N., Braun A., Beume R. Effects of the phosphodiesterase type 4 inhibitor roflumilast on early and late allergic response and airway hyperresponsiveness in aspergillus-fumigatus sensitized mice. Pharmacology. 2009;83:188–195. doi: 10.1159/000196814. https://doi/10.1159/000196814 [DOI] [PubMed] [Google Scholar]
- 60.Totani L., Amore C., Di Santo A., Dell'Elba G., Piccoli A., Martelli N., Tenor H., Beume R., Evangelista V. Roflumilast inhibits leukocyte-platelet interactions and prevents the prothrombotic functions of polymorphonuclear leukocytes and monocytes. J. Thromb. Haemost. 2016;14(1):191–204. doi: 10.1111/jth.13173. https://doi/10.1111/jth.13173 [DOI] [PubMed] [Google Scholar]
- 61.Dolhnikoff M., Duarte-Neto A.N., de Almeida Monteiro R.A., da Silva L.F.F., de Oliveira E.P., Saldiva P.H.N., Mauad T., Negri E.M. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J. Thromb. Haemost. 2020;18(6):1517–1519. doi: 10.1111/jth.14844. https://doi/10.1111/jth.14844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El Tabaa M.M., El Tabaa M.M. New putative insights into neprilysin (NEP)-dependent pharmacotherapeutic role of roflumilast in treating COVID-19. Eur. J. Pharmacol. 2020;15(889):173615. doi: 10.1016/j.ejphar.2020.173615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calverley P.M., Sanchez-Toril F., McIvor A., Teichmann P., Bredenbroeker D., Fabbri L.M. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007;176:154–161. doi: 10.1164/rccm.200610-1563OC. [DOI] [PubMed] [Google Scholar]
- 64.Calverley P.M., Rabe K.F., Goehring U.M., Kristiansen S., Fabbri L.M., Martinez F.J. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685–694. doi: 10.1016/S0140-6736(09)61255-1. https://doi/10.1016/S0140-6736(09)61255-1 [DOI] [PubMed] [Google Scholar]
- 65.Martinez F.J., Calverley P.M., Goehring U.M., Brose M., Fabbri L.M., Rabe K.F. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet. 2015;385:857–866. doi: 10.1016/S0140-6736(14)62410-7. https://doi/10.1016/S0140-6736(14)62410-7 [DOI] [PubMed] [Google Scholar]
- 66.Grootendorst D.C., Gauw S.A., Verhoosel R.M., Sterk P.J., Hospers J.J., Bredenbröker D., Bethke T.D., Hiemstra P.S., Rabe K.F. Reduction in sputum neutrophil and eosinophil numbers by the PDE-4 inhibitor roflumilast in patients with COPD. Thorax. 2007;62(12):1081–1087. doi: 10.1136/thx.2006.075937. https://doi/10.1136/thx.2006.075937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gauvreau G.M., Boulet L.P., Schmid-Wirlitsch C., Côté J., Duong M., Killian K.J., Milot J. Roflumilast attenuates allergen-induced inflammation in mild asthmatic subjects. Respir. Res. 2011;12(1):140. doi: 10.1186/1465-9921-12-140. https://doi/10.1186/1465-9921-12-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fabbri L.M., Beghé B., Yasothan U., Kirkpatrick P. Roflumilast. Nat. Rev. Drug Discov. 2010;9:761–762. doi: 10.1038/nrd3276. https://doi/10.1038/nrd3276 [DOI] [PubMed] [Google Scholar]
- 69.Rennard S.I., Calverley P.M., Goehring U.M., Bredenbröker D., Martinez F.J. Reduction of exacerbations by the PDE4 inhibitor roflumilast – the importance of defining different subsets of patients with COPD. Respir. Res. 2011;12:18. doi: 10.1186/1465-9921-12-18. https://doi/10.1186/1465-9921-12-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng J., Yang J., Zhou X., Zhao L., Hui F., Bai H. Wang C. Roflumilast for the treatment of COPD in an Asian population: a randomized, double-blind, parallel-group study. Chest. 2014;145(1):44–52. doi: 10.1378/chest.13-1252. https://doi/10.1378/chest.13-1252 [DOI] [PubMed] [Google Scholar]
- 71.Wedzicha J.A., Calverley P.M.A., Rabe K.F. Roflumilast: a review of its use in the treatment of COPD. Int. J. COPD. 2016;11:81–90. doi: 10.2147/COPD.S89849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rabe K.F., Calverley P.M.A., Martinez F.J., Fabbri L.M. Effect of roflumilast in patients with severe COPD and a history of hospitalisation. Eur. Respir. J. 2017;50(1):1700158. doi: 10.1183/13993003.00158-2017. https://doi/10.1183/13993003.00158-2017 [DOI] [PubMed] [Google Scholar]
- 73.Savelikhina I., Ostrovskyy M., Ostrovska K., Kulynych-Miskiv M., Varunkiv O. Proinflammatory cytokine IL-6 detetion in severe COPD patients: focus on roflumilast. Eur. Respir. J. 2018;52:OA3267. https://doi/10.1183/13993003.congress-2018.OA3267 [Google Scholar]
- 74.Baye J. Roflumilast (daliresp) A novel phosphodiesterase-4 inhibitor for the treatment of severe chronic obstructive pulmonary disease. P T. 2012;37(3):149–161. [PMC free article] [PubMed] [Google Scholar]
- 75.FDA Approves new drug to treat chronic obstructive pulmonary disease, March 1. 2011. www.fda.gov Available at: November 21.
- 76.Choi C.H., Schoenfeld B.P., Bell A.J., Hinchey J., Rosenfelt C., Gertner M.J., Campbell S.R. Multiple drug treatments that increase cAMP signaling restore long-term memory and aberrant signaling in fragile X syndrome models. Front. Behav. Neurosci. 2016;10:136. doi: 10.3389/fnbeh.2016.00136. https://doi/10.3389/fnbeh.2016.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Duinen M.A., Sambeth A., Heckman P.R.A., Smit S., Tsai M., Lahu G., Uz T., Blokland A., Prickaerts J. Acute administration of roflumilast enhances immediate recall of verbal word memory in healthy young adults. Neuropharmacology. 2018;15:31–38. doi: 10.1016/j.neuropharm.2017.12.019. https://doi/10.1016/j.neuropharm.2017.12.019 [DOI] [PubMed] [Google Scholar]
- 78.Sugin Lal Jabaris S., Murugesan A., Bindu M., Sunil K.N. Roflumilast: a potential drug for the treatment of cognitive impairment? Neurosci. Lett. 2020;736:135281. doi: 10.1016/j.neulet.2020.135281. [DOI] [PubMed] [Google Scholar]
- 79.Van Duinen M., Heckman P., Vanmierlo T., Sambeth A., Ogrinc F., Tsai M., Lahu G., Uz T., Blokland A., Prickaerts J. The PDE4-inhibitor roflumilast improves episodic memory: findings from a translational perspective. Eur. Neuropsychopharmacol. 2017;27:S1024–S1025. [Google Scholar]
- 80. NCT02079844 ClinicalTrialsgov. 2016 October 3. [Google Scholar]
- 81.Reid P. Roflumilast altana pharma. Curr. Opin. Invest. Drugs. 2002;3:1165–1170. [PubMed] [Google Scholar]
- 82.Bethke T.D., Böhmer G.M., Hermann R., Hauns B., Fux R., Morike K., David M. Dose-proportional intra individual single- and repeated-dose pharmacokinetics of roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor. J. Clin. Pharmacol. 2007;47:26–36. doi: 10.1177/0091270006294529. https://doi/10.1177/0091270006294529 [DOI] [PubMed] [Google Scholar]
- 83.Lahu G., Hünnemeyer A., Herzog R., McCracken N., Hermann R., Elmlinger M., Zech K. Effect of repeated dose of erythromycin on the pharmacokinetics of roflumilast and roflumilast N-oxide. Int. J. Clin. Pharm. Ther. 2009;47:236–245. doi: 10.5414/cpp47236. https://doi/10.5414/cpp47236 [DOI] [PubMed] [Google Scholar]
- 84.Hauns B., Hermann R., Hunnemeyer A., Herzog R., Hauschke D., Zech K., Bethke T.D. Investigation of a potential food effect on the pharmacokinetics of roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor, in healthy subjects. J. Clin. Pharmacol. 2006;46:1146–1153. doi: 10.1177/0091270006291621. https://doi/10.1177/0091270006291621 [DOI] [PubMed] [Google Scholar]
- 85.Wedzicha J.A., Calverley P.M., Rabe K.F. Roflumilast: a review of its use in the treatment of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2016;6(11):81–90. doi: 10.2147/COPD.S89849. https://doi/10.2147/COPD.S89849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mackay A.J., C Patel A.R., Singh R., Sapsford R.J., Donaldson G.C., Prasad N., Goehring U.M., Nip T.K., Wedzicha J.A. Randomized double-blind controlled trial of roflumilast at acute exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2017;196(5):656–659. doi: 10.1164/rccm.201612-2518LE. https://doi/10.1164/rccm.201612-2518LE [DOI] [PubMed] [Google Scholar]
- 87.Kim K.H., Kang H.S., Kim J.S., Yoon H.K., Kim S.K., Rhee C.K. Risk factors for the discontinuation of roflumilast in patients with chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2017;12:3449–3456. doi: 10.2147/COPD.S143967. https://doi/10.2147/COPD.S143967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martinez F.J., Calverley P.M., Goehring U.M., Brose M., Fabbri L.M., Rabe K.F. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet. 2015;385(9971):857–866. doi: 10.1016/S0140-6736(14)62410-7. https://doi/10.1016/S0140-6736(14)62410-7 [DOI] [PubMed] [Google Scholar]
- 89.Michalski J.M., Golden G., Ikari J., Rennard S.I. PDE-4: a novel target in the treatment of chronic obstructive pulmonary disease. Clin. Pharmacol. Ther. 2012;91:134–142. doi: 10.1038/clpt.2011.266. https://doi/10.1038/clpt.2011.266 [DOI] [PubMed] [Google Scholar]
- 90.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Biondi-Zoccai G., Brown T.S. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J. Am. Coll. Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. https://doi/10.1016/j.jacc.2020.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.White W.B., Cooke G.E., Kowey P.R., Calverley P.M.A., Bredenbröker D., Goehring U.M., Zhu H. Cardiovascular safety in patients receiving roflumilast for the treatment of COPD. Chest. 2013;144(3):758–765. doi: 10.1378/chest.12-2332. https://doi/10.1378/chest.12-2332 [DOI] [PubMed] [Google Scholar]