Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic has seen transplant volume decrease nationwide, resulting in a 2.2-fold increase in waitlist mortality. In particular, solid organ transplant patients are subjected to increased morbidity and mortality from infection. In the face of these challenges, transplant centers need to develop innovative protocols to ensure high-quality care.
Methods
A multidisciplinary protocol was developed that included the following: virtual selection meetings, coronavirus disease 2019 negative donors, pretransplant symptom screening, rapid testing on presentation, telehealth follow-up, and weekly community outreach town halls. All orthotopic liver transplants completed between January 2018 and August 2020 were included in the study (n = 344). The cohort was stratified from January 2018 to February 2020 as “pre-COVID-19,” and from March 2020 to August 2020 as “COVID-19.” Patient demographics and postoperative outcomes were compared.
Results
From March 2020 to August 2020, there was a significant decrease in average monthly referrals for orthotopic liver transplantation (29.8 vs 37.1, P = .01). However, listings (11.0 vs 14.3, P = .09) and transplant volume remained unchanged (12.2 vs 10.6, P = .26). Rapid testing was utilized on arrival for transplant, zero patients tested positively preoperatively, and median time from test result until abdominal incision was 4.5 h [interquartile range, 1.2, 9.2]. Simultaneously, telehealth visits increased rapidly, peaking at 85% of all visits. It is important to note that there was no difference in outcomes between cohorts.
Conclusion
Orthotopic liver transplant can be accomplished safely and effectively in the COVID-19 era without compromising outcomes through increasing utilization of telehealth, rapid COVID-19 testing, and multidisciplinary protocols for managing immunosuppressed patients.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has had dramatic effects across our health care system, altering the way we practice medicine in every field. Transplant patients have been significantly affected, with increased morbidity and mortality from infection attributable to immunosuppression and comorbidities, making this patient population high risk.1, 2, 3, 4, 5 Given the inherent risks of COVID-19 to both patients and providers alike, our health care system has struggled to maintain pre-COVID-19 surgical volumes during this difficult time.6, 7, 8, 9
Solid organ transplant volume is down nationwide—across heart, kidney, and orthotopic liver transplants (OLTs)—leaving potential recipients exposed to potentially increased rates of waitlist mortality.8 , 10, 11, 12 Furthermore, patients in the 5 states hardest hit by the COVID-19 pandemic experienced a 2.2-fold increase in waitlist mortality compared with expected.8 Not only have transplant volumes been affected by regional infection rates, but center variability has also been a driver for differences in transplant volume when compared with neighboring transplant programs.13 One hypothesized cause for this was the lack of federal regulations, leading to center-level variability within the same region.13 Moreover, ethical concerns regarding continuing our mission to care for these patients during the COVID-19 pandemic, while also being good stewards of available resources, have been raised in the transplant community.14 , 15 Further complicating the landscape, many experts have predicted a new surge in COVID-19 cases in the coming months.5 Despite the expedient vaccine development efforts of Operation Warp Speed, currently finishing accrual with multiple candidates in phase III trials, mass vaccination in the United States is predicted to take until at least mid- to late 2021. Therefore, we will need to define a new normal for continuing to care for our patients in the years ahead.
Ohio Governor Mike DeWine declared a state of emergency attributable to the coronavirus on March 9, 2020, cancelled all elective medical care procedures on March 15, 2020, and declared a stay-at-home order on March 22, 2020. This unprecedented shutdown of standard surgical procedures left the OLT community with new challenges in the decision-making and management of patients with end stage liver disease (ESLD). At the end of the study period, Hamilton County, OH, had experienced 47,940 cases per 1 million people, which is above the US average of 49,110 cases per 1 million.16 The aim of this study is to report our center’s (University of Cincinnati Medical Cente, Cincinnati, OH) protocolized response to the COVID-19 pandemic. Our mission was and remains to continue to offer safe OLT for patients with ESLD throughout the pandemic. Through leveraging multidisciplinary expertise, we report our COVID-19 testing protocols and results, telehealth utilization, and perioperative outcomes.
Methods
Protocolized response to COVID-19
A uniform, multidisciplinary protocol was efficiently established at the onset of the global pandemic to safely serve patients from waitlist through their transplant and into their postoperative follow-up. Paramount to this effort was the principle of safely maintaining a high volume of life-saving transplants during this trying time, putting no providers or patients at undue risk. With this in mind, we adopted a series of additional methods to care for liver transplant patients, in addition to appropriate standard precautions. All multidisciplinary meetings were held virtually. This included selection meetings, quality meetings, education conferences, and multi-team rounds. Because of the unknown effects of COVID-19 infection on potential organs, all organ procurements were only conducted on COVID-19 negative donors. In addition, transplant coordinators conducted weekly pretransplant symptom screening on all listed patients. Any patient who complained of fevers, chills, cough, or shortness of breath, was asked to take a COVID-19 test. Similarly, testing of the transplant team was conducted for symptomatic individuals or after experiencing a COVID-19 positive exposure.
Rapid COVID-19 testing played a significant role in minimizing organ cold ischemia time during OLTs. The rapid test utilized was the Xpert Xpress severe acute respiratory syndrome coronavirus 2 test (Cepheid Innovation, Sunnyvale, CA, USA), which is an amplified nucleic acid assay authorized by the US Food and Drug Administration (FDA) under an Emergency Use Authorization. The limit of detection of this test was set at 95% accuracy, and the test was validated by the US FDA with a 97.8% positive percent agreement and a 95.6% negative percent agreement.17 All potential transplant recipients were required to take a rapid COVID-19 test on presentation for transplant. They were then placed in contact, droplet, and airborne precautions, and had a non-contrast chest computed-tomography scan to rule out infection (Supplemental Fig 1). If the rapid COVID-19 test had not resulted before incision, N95 masks were worn throughout the operation. Moreover, repeat COVID-19 testing was done before any additional invasive procedures.
To minimize infection risk to patients and providers, all preoperative transplant clinic visits were conducted on a virtual telehealth platform. In addition, postoperative liver transplant patients had a telehealth visit on postoperative day 14 and were not seen in clinic physically until postoperative day 21 for staple removal. The telehealth platform was expanded to include transplant coordinators and pharmacists who met with patients virtually for patient education visits. Weekly virtual town halls were held with the entire pretransplant and post-transplant population to inform patients on best practices to minimize infection risk and whom to contact if they became symptomatic. If patients became infected with COVID-19, an antiviral algorithm was followed. Transplant immunology developed this algorithm that detailed workup, diagnosis, and management of COVID-19 infection.
Given the unprecedented demand for intensive care unit (ICU) beds and ventilators, patient selection for transplantation included consideration for patients who were likely to have a rapid recovery. Patient selection was not focused on selecting the most ill patients, but also patients who were anticipated to have shorter ICU stays and who avoid tying up resources during the pandemic. Specifically, patients without significant cardiac and respiratory comorbidities were preferentially selected. The need to ensure continued care for patients with ESLD and organ utilization, was coupled with the need to minimize hospital resource utilization.
Data collection and statistical analysis
All OLTs completed between January 1, 2018, and August 30, 2020, were included in the study. Baseline demographic information and patient characteristics were collected. The following data were also collected: COVID-19 screening test results; referrals for transplant; transplant listings; transplants completed; and short-term outcomes including length of stay, early allograft dysfunction, primary non-function, reoperations, bile leak, hepatic artery thrombosis, and readmission rates. Outpatient preoperative and postoperative clinic visits were collected and stratified by type of visit—in-person, telephone, or video. Subsequently, we divided our patient population into two cohorts: pre-COVID-19 and COVID-19. The pre-COVID-19 cohort was as all patients who underwent OLT from January 1, 2018, to February 28, 2020. The COVID-19 cohort was defined as all patients who underwent OLT from March 1, 2020, to August 30, 2020.
Statistical analysis was performed utilizing JMP, v Pro 15 (SAS Institute Inc, Cary, NC, USA). Patient baseline demographics, clinical characteristics, and postoperative outcomes were compared between those in the pre-COVID-19 and COVID-19 cohorts, using the Pearson χ2 for categorical variables and the Student t test for continuous variables. This project was approved by the University of Cincinnati Institutional Review Board.
Results
Patient population
From January 2018 to August 2020, our center performed 344 OLTs. In the pre-COVID-19 cohort 274 OLTs were completed, and—between March 1, 2020, and August 30, 2020—a total of 70 OLTs (COVID-19 cohort) were completed. Patient demographics and baseline clinical characteristics are presented in Table I . The data indicated no difference in age, sex, or race between the pre-COVID-19 and COVID-19 cohorts. However, the average match Model for End-Stage Liver Disease (MELD) score of transplanted patients during COVID-19 was significantly decreased from the average match MELD score pre-COVID-19 (18.9 ± 7.5 vs 22.6 ± 7.6, P < .01). The data indicated no difference in the percentage of patients called in from home versus those hospitalized at the time of transplant or difference in patients admitted to the ICU at time of transplant (Table I). Of note, there was a decrease in patients with malignancy undergoing transplant during COVID-19 (1.1% vs 9.9%, P < .01).
Table I.
Recipient demographics and clinical characteristics stratified by cohort
| Category | Pre-COVID-19 (n = 274) | COVID-19 (n = 70) | P value |
|---|---|---|---|
| Age, y, n (SD) | 53.2 (17.1) | 56.2 (10.9) | .17 |
| Sex, female, n (%) | 105 (38.3%) | 20 (29.0%) | .14 |
| Race, n (%) | .25 | ||
| Non-Hispanic white | 251 (91.6%) | 63 (91.3%) | |
| African American | 13 (4.7%) | 6 (8.7%) | |
| MELD score—match, mean (SD) | 22.6 (7.6) | 18.9 (7.51) | <.01 |
| Meld score—lab, mean (SD) | 19.8 (8.7) | 17.4 (7.8) | .04 |
| Etiology of liver disease, n (%) | <.01 | ||
| NASH | 98 (35.7%) | 27 (38.0%) | |
| Alcoholic liver disease | 85 (30.9%) | 22 (31.0%) | |
| Viral liver disease | 38 (13.8%) | 3 (4.23%) | |
| Biliary disease | 21 (7.6%) | 1 (1.4%) | |
| Malignancy | 3 (1.1%) | 7 (9.9%) | |
| Fulminant | 3 (1.1%) | 2 (2.8%) | |
| Other | 27 (9.8%) | 9 (12.7%) | |
| Admitted at time of OLT, yes, n (%) | 54 (19.6%) | 11 (15.5%) | .43 |
| ICU at time of OLT, yes, n (%) | 20 (17.4%) | 6 (8.5%) | .09 |
| Hospitalized in previous 90 days, yes, n (%) | 92 (33.5%) | 17 (23.9%) | .12 |
| Intubated at time of OLT, yes, n (%) | 6 (2.2%) | 4 (5.6%) | .12 |
| Current smoker, yes, n (%) | 3 (3.1%) | 1 (1.5%) | .49 |
COVID-19, coronavirus disease 2019; MELD, model of end-stage liver disease; NASH, non-alcoholic steatohepatitis; ICU, intensive care unit; OLT, orthotopic liver transplantation; SD, standard deviation.
The COVID-19 pandemic, coupled with the recent change in organ allocation policy, were correlated with a shift in donor organ procurement location from predominantly local-regional to national (Table II ). In addition, the percent of organ donation offers that were accepted under expedited placement was increased during COVID-19 (29.6% vs 16.8%, < 0.05). Expedited placement organ offers are organs that are offered while procurement is ongoing, and the primary center has declined the offer. This increase may illustrate the strain on transplant centers during these times and on the uncertainty regarding outcomes of higher risk donor organs and recipients. Even with a higher percentage of organs being accepted through the expedited process, we observed no significant difference in cold ischemia time between cohorts (5.0 ± 2.8 h vs 5.1 ± 3.5 h P = .87).
Table II.
Organ donor characteristics
| Category | Pre-COVID-19 (n = 274) | COVID-19 (n = 70) | P value |
|---|---|---|---|
| Organ location, n (%) | <.01 | ||
| Local | 107 (38.9%) | 12 (16.9%) | |
| Regional | 105 (38.2%) | 22 (31.0%) | |
| National | 63 (22.9%) | 37 (52.1%) | |
| Donor age, y, mean (SD) | 43.1 (15.0) | 40.5 (15.0) | .20 |
| Donor BMI, mean (SD) | 29.4 (8.5) | 30.6 (9.5) | .29 |
| Donation after circulatory death, yes, n (%) | 23 (8.4%) | 8 (11.3%) | .45 |
| PHS increased risk, yes, n (%) | 116 (42.2%) | 32 (45.1%) | .66 |
| Expedited placement, yes, n (%) | 17 (16.8%) | 21 (29.6%) | .05 |
| Cold ischemia time, h, mean (SD) | 5.0 (2.8) | 5.1 (3.5) | .87 |
BMI, body mass index; COVID-19, coronavirus disease 2019; PHSs, public health services; SD, standard deviation.
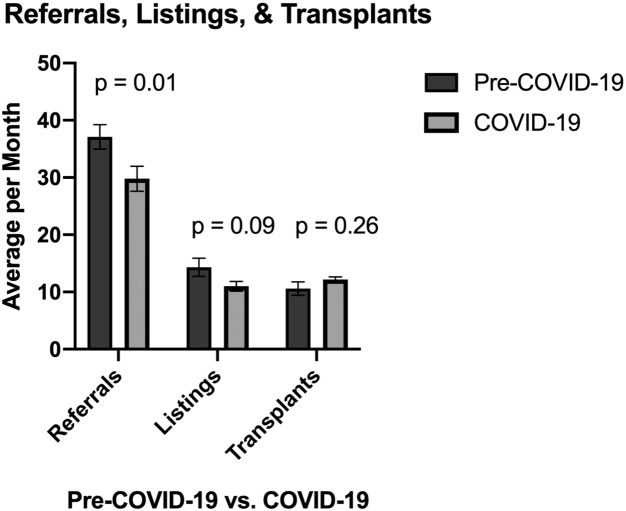
Referrals, listings, transplants
The COVID-19 pandemic resulted in a significant decrease in patients referred for OLT. The average referral rate per month was 37.1 in the pre-COVID-19 era and decreased to 29.8 during the COVID-19 pandemic (P = .01[Fig 1 ]). The average number of listings for OLT per month in the pre-COVID-19 era was 14.3 and this decreased to 11.0 per month (P = .09) during the COVID-19 pandemic. However, even though there was a decrease in OLT referrals, the number of listings and OLTs performed did not change. The average number of OLTs performed per month was 10.6 in the pre-COVID-19 era and was 12.2 in the COVID-19 era (P = .26).
Fig 1.
Liver transplant referrals, listings, and transplants performed per month stratified by cohort. Referrals were significantly decreased during COVID-19 (P = .01), and listings (P = .09) and transplants (P = .26) were unchanged. COVID-19, coronavirus disease 2019.
COVID-19 rapid testing
All OLT candidates underwent rapid testing on presentation for transplant beginning in mid-March 2020 (n = 62). Of 62 tested patients, 0 tested positive preoperatively. The median time from COVID-19 test result until abdominal incision was 4.5 h [interquartile range, 1.2, 9.2]. Of all the patients, 6 had their OLTs initiated before the result of their COVID-19 tests were known.. All 6 donor organs were non-local, and their cases commenced to minimize cold ischemia time because the expedited offer process. The average number of preoperative COVID-19 tests per patient was 1.96 ± 1.6, and the average number of postoperative COVID-19 tests per patient was 2.3 ± 2.8. We observed that 2 patients tested positive in the postoperative setting as outpatients. The first patient was asymptomatic, and the second had mild respiratory symptoms. Both patients were managed as outpatients, had their immunosuppression (mycophenolate mofetil) dosage decreased, and recovered without complications.
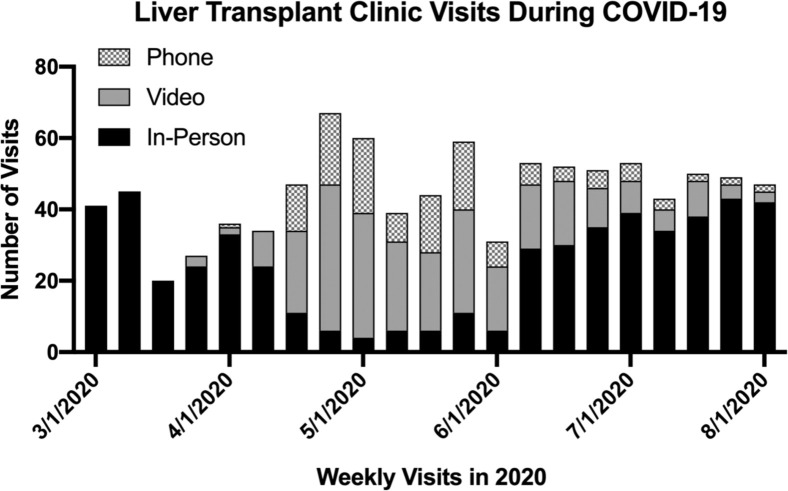
Telehealth
On April 1, 2020, the solid organ transplant division instituted a number of changes to combat the COVID-19 pandemic. Among them, all in-person patient selection meetings, education conferences, and clinics were moved to a virtual telehealth platform (Fig 2 ). Of the previously scheduled visits, the 100% in-person clinic pre-COVID-19 was converted to an average of 45% virtual visits per week in the COVID-19 era. During the worst weeks of the pandemic, up to 85% of clinic visits were converted from in-person to virtual. Pretransplant liver evaluation clinic visits were conducted virtually with both the transplant hepatologist and the transplant surgeon. In addition, pre-COVID-19 postoperative clinic visits were typically scheduled in-person at 7- and 14-days postdischarge, and the COVID-19 cohort was not seen in person until postoperative day 21. These visits accounted for the majority of in-person clinic visits during the pandemic through the end of August 2020. The flexibility of the telehealth platform allowed us to continue to serve the vulnerable pretransplant and post-transplant population through the COVID-19 pandemic, minimizing their risk of exposure in the clinic.
Fig 2.
Weekly liver transplant clinic visits by type. The number of telehealth visits increased during April 1, 2020, through July 1, 2020. After this period, clinic visits began a return to primarily in-person visits. COVID-19, coronavirus disease 2019.
Clinical outcomes
Patients who underwent OLT during the COVID-19 pandemic (n = 70) did not experience a significant change in short-term outcomes compared with the pre-COVID-19 era (Table III ). No difference was observed in 30-day or 90-day patient survival. Similarly, no significant difference was observed in 30-day or 90-day graft survival. As Table III shows, the rates of primary nonfunction, relisting, unplanned reoperations, hepatic artery thrombosis, early allograft dysfunction, and bile leak were unchanged between cohorts. In addition, data indicated a significant decrease in the number of patients who remained ventilated at 24 h postoperatively from OLT during COVID-19 (8.6% vs 18.6%, P = .04) as well as a decrease in the hospital length of stay for patients undergoing OLT (8.5 days ± 5.1 vs 13.1 days ± 15.2, P = .02). Although COVID-19 posed unprecedented challenges to OLT, with multidisciplinary protocols and added safety measures, we did not observe a change in patient outcomes.
Table III.
Short-term outcomes of patients after orthotopic liver transplantation
| Category | Pre-COVID-19 (n = 274) | COVID-19 (n = 70) | P value |
|---|---|---|---|
| 30-day Patient survival, n (%) | 97.1% | 98.6% | .48 |
| 90-day Patient survival, n (%) | 96.0% | 93.6% | .46 |
| 30-day Graft survival, n (%) | 97.1% | 97.2% | .97 |
| 90-day Graft survival, n (%) | 96.0% | 91.5% | .21 |
| Primary nonfunction, n (%) | 2 (0.7%) | 1 (1.4%) | .57 |
| Relisted, n (%) | 7 (2.6%) | 0 (0.0%) | .17 |
| Reoperation, yes, n (%) | 75 (27.4%) | 20 (28.6%) | .84 |
| Hepatic artery thrombosis, yes, n (%) | 3 (1.1%) | 1 (1.4%) | .81 |
| Early allograft dysfunction, yes, n (%) | 18 (6.6%) | 2 (2.9%) | .23 |
| Bile leak within 90 days, yes, n (%) | 22 (8.0%) | 8 (11.4%) | .36 |
| Length of stay, days, mean (SD) | 13.1 (15.2) | 8.5 (5.1) | .02 |
| Readmissions within 30 days, yes, n (%) | 26 (41.9%) | 20 (61.5%) | .09 |
| Indication for readmission, n (%) | .42 | ||
| Acute kidney injury | 1 (3.8%) | 1 (5.0%) | |
| Abdominal pain | 3 (11.5%) | 3 (15.0%) | |
| Wound infection | 2 (7.7%) | 2 (10.0%) | |
| Ascites | 1 (3.8%) | 1 (5.0%) | |
| Altered mental status | 2 (7.7%) | 3 (15.0%) | |
| Bile leak or stricture | 2 (7.7%) | 5 (25.0%) | |
| Metabolic abnormalities | 3 (11.5%) | 1 (5.0%) | |
| Urinary tract infections | 1 (3.8%) | 1 (5.0%) | |
| Fever | 5 (19.2%) | 1 (5.0%) | |
| Acute rejection episode | 1 (3.8%) | 0 (0.0%) | |
| Other | 5 (19.2%) | 2 (10.0%) | |
| Ventilated > 24 h, yes, n (%) | 51 (18.6%) | 6 (8.6%) | .04 |
COVID-19, coronavirus disease 2019; SD, standard deviation.
Discussion
This analysis of the policies, procedures, and short-term outcomes of a busy OLT center provides preliminary evidence that it is safe to continue to care for these high-risk patients in the COVID-19 era. This was accomplished by an extensive reliance on telehealth for patient visits, a rapid testing policy for all new transplant patients on arrival, and weekly community outreach education sessions for keeping our patient population informed during the pandemic. High-volume centers have previously demonstrated the safety of performing OLT during the pandemic, using different strategies to maintain patient safety.1 , 18, 19, 20, 21 However, this review is the largest series of consecutive liver transplants in the COVID-19 era, highlighting the significant role that telehealth and rapid testing can play to maintain high-quality care for solid organ transplant patients.
One of the most concerning findings from the analysis was the significantly decreased rate of referral for patients with ESLD. Similar to recent publications describing decreased cancer diagnosis,22 , 23 decreased acute myocardial infarction hospital admissions,24 and decreased incidence of appendicitis during COVID-19,25 our patient population with ESLD is not being referred for evaluation at the same rate as they were before COVID-19. This cohort may be a delayed victim of the COVID-19 pandemic, and they may not survive to transplant referral and subsequent listing. Moreover, once listed, they are subjected to increased waitlist mortality.11 Referring physicians have suggested this delay in care is likely multifactorial for the following reasons: increased patient anxiety regarding visiting a hospital, decreasing physician and interventionalist availability for workup, and decreased physician referrals. Not only are patients with cirrhosis at risk for complications from their underlying disease, but they also are subject to high rates of 30-day mortality when co-infected with COVID-19.26 , 27 The implications of this finding could lead to significantly increased morbidity and mortality for patients with ESLD who do not make it to transplantation. In our single-center experience, we were underpowered to detect a difference in waitlist mortality between cohorts (5.4% vs 1.5%, P = .17).
As Agopian et al13 point out in their review of liver transplant practices during COVID-19, there is significant variation between centers and regions regarding OLTs. Our center, in Region 10, had lower rates of COVID-19 as compared with other centers in our region.28 This created decreased competition for donor organs, and may in part explain the rise in OLTs performed despite a decrease in referrals. In addition, the decrease in local-regional donor procurements, and subsequent increase in national donor procurements, highlights the new organ allocation policy.29 Furthermore, the rise in expedited placement liver organs—from longer distances away—might be attributable to the significant stresses COVID-19 placed on transplant centers, leaving many to turn down otherwise healthy organs. The protocolized use of the rapid COVID-19 tests immediately on patient arrival helped minimize organ exposure to cold ischemia time. Furthermore, asymptomatic testing of waitlisted patients and recent transplant recipients may be beneficial to avoid missing asymptomatic carriers of COVID-19.
The ability to safely perform OLTs requires in-hospital multidisciplinary teams, surgical ICU beds, mechanical ventilators, and blood products that were accessible throughout the pandemic. However, because of a scarcity of designated COVID-19 positive ICU beds in the medical ICU and cardiovascular ICU, the surgical ICU was forced to accept COVID-19 positive and COVID-19 negative patients in the same unit. Fortunately, this did not increase COVID-19 infections in our cohort. Cross-infection was best prevented with personal protective equipment, standard infection control guidelines, and minimizing the number of providers physically seeing patients. It is important to note that patients who were more likely to have a rapid recovery and decreased ICU length of stay were preferentially selected for transplant, and this is reflected in the decreased number of patients ventilated > 24 h (8.6% vs 18.6%, P = .04) and decreased hospital length of stay (8.5 days vs 13.1 days, P = .02 [Table II]). Furthermore, the decrease in average MELD score of patients transplanted during COVID-19 may reflect a trend toward organ allocation to lower MELD patients during the pandemic, leaving patients with higher MELD scores at increased waitlist morbidity and mortality.
The ability to continue care and minimize infection risk to patients and providers was made possible by increasing use of a previously validated and implemented telehealth platform.30 , 31 Telehealth has been demonstrated to increase patient satisfaction, increase immunosuppressive medication adherence, and increase quality of life scores.30 , 32 , 33 At the beginning of the COVID-19 pandemic, the accessibility of the telehealth platform allowed patients recently discharged from the hospital to avoid routine clinic visits, offering providers familiar interfaces for monitoring these complex patients. Furthermore, telehealth was utilized in the following new arenas: patient education with pharmacy and weekly community outreach sessions to stay in close contact with the entire post-transplant population and relay safe guidelines during the pandemic. These novel approaches were associated with a decreased hospital length of stay but may come at the cost of increased 30-day readmissions. Providers exhibited increased caution in this immunosuppressed population, and the inability to physically evaluate a patient may contribute to increased readmissions. Although no statistically significant difference was demonstrated, there was an increase from 41.9% to 61.5% in 30-day readmissions, that may be explained by this approach.
This single-center review of the policies, procedures, and outcomes of liver transplantation during COVID-19 has limitations. First and foremost, COVID-19 has affected different geographic regions and centers with varying severity, and our center has maintained ICU, ventilator, and operating room availability throughout the pandemic. Transplant centers without these resources may have to adopt different policies and be less aggressive in offering OLT. Furthermore, this protocolized response may need to adapt in settings without access to rapid testing and telehealth. Although our study was conducted at a single center with an established telehealth program, it may be more challenging for other centers to build a sustainable platform in a short amount of time. In addition, this early retrospective analysis does not assess 1-y patient and allograft survival. The long-term risks and benefits of solid organ transplantation in the era of COVID-19 are still unknown and will require additional monitoring and close analysis moving forward.
In conclusion, COVID-19 poses a unique set of challenges for the liver transplant community, and this analysis is the largest single-center experience in the literature documenting no change in short-term outcomes for patients undergoing liver transplant during the pandemic. The ability of liver transplant centers to continue to provide care for patients with ESLD will increasingly become reliant on center flexibility, rapid testing, telehealth, and virtual platforms for the delivery of high-quality care to this high-risk population. As the expected winter surge of COVID-19 cases occurs,34 it becomes even more necessary to share multidisciplinary protocols and experiences between liver transplant centers to ensure optimal preparedness and the best possible outcomes for patients with ESLD.
Funding/Support
None of the authors have any funding or support to disclose.
Conflict of interest/Disclosure
None of the authors have any conflicts of interest to report.
Footnotes
Supplementary material associated with this article can be found, in the online version, at [https://doi.org/10.1016/j.surg.2020.12.044].
Supplementary Materials
References
- 1.Yi S.G., Rogers A.W., Saharia A., et al. Early experience with COVID-19 and solid organ transplantation at a US high-volume transplant center. Transplantation. 2020;104:2208–2214. doi: 10.1097/TP.0000000000003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu L., Xu X., Ma K., et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant. 2020;20:1859–1863. doi: 10.1111/ajt.15869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang J.F., Zheng K.I., George J., et al. Fatal outcome in a liver transplant recipient with COVID-19. Am J Transplant. 2020;20:1907–1910. doi: 10.1111/ajt.15909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pereira M.R., Mohan S., Cohen D.J., et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20:1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVID 19 Forecasts CDC.gov. Available from https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days. Accessed October 15, 2020.
- 6.Cholankeril G., Podboy A., Alshuwaykh O.S., et al. Early impact of COVID-19 on solid organ transplantation in the United States. Transplantation. 2020;104:2221–2224. doi: 10.1097/TP.0000000000003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angelico R., Trapani S., Manzia T.M., Lombardini L., Tisone G., Cardillo M. The COVID-19 outbreak in Italy: initial implications for organ transplantation programs. Am J Transplant. 2020;20:1780–1784. doi: 10.1111/ajt.15904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyarsky B.J., Werbel W.A., Durand C.M., et al. Early national and center-level changes to kidney transplantation in the United States during the COVID-19 epidemic. Am J Transplant. 2020;20:3131–3139. doi: 10.1111/ajt.16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen L.H., Drew D.A., Graham M.S., et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5:e475–e483. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeFilippis E.M., Sinnenberg L., Reza N., et al. Trends in US heart transplant waitlist activity and volume during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Cardiol. 2020;5:1048–1052. doi: 10.1001/jamacardio.2020.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyarsky B.J., Po-Yu Chiang T., Werbel W.A., et al. Early impact of COVID-19 on transplant center practices and policies in the United States. Am J Transplant. 2020;20:1809–1818. doi: 10.1111/ajt.15915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halazun K.J., Rosenblatt R. Lest we forget. Am J Transplant. 2020;20:1785–1786. doi: 10.1111/ajt.15888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agopian V., Verna E., Goldberg D. Changes in liver transplant center practice in response to coronavirus disease 2019: unmasking dramatic center-level variability. Liver Transpl. 2020;26:1052–1055. doi: 10.1002/lt.25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaffe A., Schilsky M.L., Deshpande R., Batra R. Liver transplantation in the time of COVID19: barriers and ethical considerations for management and next steps. Hepatol Commun. 2020;4:1242–1256. doi: 10.1002/hep4.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chew C.A., Iyer S.G., Kow A.W.C., et al. An international multicenter study of protocols for liver transplantation during a pandemic: a case for quadripartite equipoise. J Hepatol. 2020;73:873–881. doi: 10.1016/j.jhep.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohio coronavirus map and casecount. The New York Times Web site. https://www.nytimes.com/interactive/2020/us/ohio-coronavirus-cases.html
- 17.Cepheid Xpert Xpress SARS-CoV-2: Cepheid.US Food and Drug Administration Web site. https://www.fda.gov/media/136314/download
- 18.Muller X., Tilmans G., Chenevas-Paule Q., et al. Strategies for liver transplantation during the SARS-CoV-2 outbreak: preliminary experience from a single center in France. Am J Transplant. 2020;20:2989–2996. doi: 10.1111/ajt.16082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galvan N.T.N., Moreno N.F., Garza J.E., et al. Donor and transplant candidate selection for solid organ transplantation during the COVID-19 pandemic. Am J Transplant. 2020;20:3113–3122. doi: 10.1111/ajt.16138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lembach H., Hann A., McKay S.C., et al. Resuming liver transplantation amid the COVID-19 pandemic. Lancet Gastroenterol Hepatol. 2020;5:725–726. doi: 10.1016/S2468-1253(20)30187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fix O.K., Hameed B., Fontana R.J., et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD Expert Panel consensus statement. Hepatology. 2020;72:287–304. doi: 10.1002/hep.31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufman H.W., Chen Z., Niles J., Fesko Y. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maringe C., Spicer J., Morris M., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia S., Albaghdadi M.S., Meraj P.M., et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tankel J., Keinan A., Blich O., et al. The decreasing incidence of acute appendicitis during COVID-19: a retrospective multi-centre study. World J Surg. 2020;44:2458–2463. doi: 10.1007/s00268-020-05599-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iavarone M., D’Ambrosio R., Soria A., et al. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063–1071. doi: 10.1016/j.jhep.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webb G.J., Marjot T., Cook J.A., et al. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5:1008–1016. doi: 10.1016/S2468-1253(20)30271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johns Hopkins University of Medicine Coronavirus Resource Center Web site. https://coronavirus.jhu.edu/us-map
- 29.Organ Procurement and Transplantation Network. US Department of Health and Human Services Web site. https://optn.transplant.hrsa.gov/
- 30.Lee T.C., Kaiser T.E., Alloway R., Woodle E.S., Edwards M.J., Shah S.A. Telemedicine based remote home monitoring after liver transplantation: results of a randomized prospective trial. Ann Surg. 2019;270:564–572. doi: 10.1097/SLA.0000000000003425. [DOI] [PubMed] [Google Scholar]
- 31.Ertel A.E., Kaiser T.E., Abbott D.E., Shah S.A. Use of video-based education and tele-health home monitoring after liver transplantation: results of a novel pilot study. Surgery. 2016;160:869–876. doi: 10.1016/j.surg.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Le L.B., Rahal H.K., Viramontes M.R., Meneses K.G., Dong T.S., Saab S. Patient satisfaction and healthcare utilization using telemedicine in liver transplant recipients. Dig Dis Sci. 2019;64:1150–1157. doi: 10.1007/s10620-018-5397-5. [DOI] [PubMed] [Google Scholar]
- 33.Levine D., Torabi J., Choinski K., Rocca J.P., Graham J.A. Transplant surgery enters a new era: increasing immunosuppressive medication adherence through mobile apps and smart watches. Am J Surg. 2019;218:18–20. doi: 10.1016/j.amjsurg.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 34.Moghnieh R., Abdallah D., Bizri A.R. COVID-19: Second wave or multiple peaks, natural herd immunity or vaccine-we should be prepared. Disaster Med Public Health Prep. 2020:1–18. doi: 10.1017/dmp.2020.349. [e-pub ahead of print] Accessed October 15, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.