Abstract
The recent coronavirus disease 2019 (COVID-19) pandemic outbreak has caused a serious global health emergency. Supporting evidence shows that COVID-19 shares a genomic similarity with other coronaviruses, such as severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), and that the pathogenesis and treatment strategies that were applied 17 years ago in combating SARS-CoV and other viral infections could be taken as references in today’s antiviral battle. According to the clinical pathological features of COVID-19 patients, patients can suffer from five steps of progression, starting with severe viral infection and suppression of the immune system and eventually progressing to cytokine storm, multi-organ damage, and lung fibrosis, which is the cause of mortality. Therefore, early prevention of disease progression is important. However, no specific effective drugs and vaccination are currently available, and the World Health Organization is urging the development of novel prevention and treatment strategies. Traditional Chinese medicine could be used as an alternative treatment option or in combination with Western medicine to treat COVID-19, due to its basis on historical experience and holistic pharmacological action. Here, we summarize the potential uses and therapeutic mechanisms of Chinese herbal formulas (CHFs) from the reported literature, along with patent drugs that have been recommended by institutions at the national and provincial levels in China, in order to verify their scientific foundations for treating COVID-19. In perspective, more basic and clinical studies with multiple high-tech and translational technologies are suggested to further confirm the therapeutic efficacies of CHFs.
Keywords: Coronavirus disease 2019, Severe acute respiratory syndrome coronavirus, Chinese herbal medicine, Antiviral, Cytokine storm, Lung fibrosis
1. Introduction
Since December 2019, an epidemic of coronavirus disease 2019 (COVID-19) has spread rapidly around the world, alarming international health emergency experts [1], [2], [3], [4]. The number of infection cases continues to rise due to the high infectivity of the virus, resulting in ongoing mortality. At present, there is still a lack of approved effective drugs or vaccination for this disease. Under these urgent circumstances, a large number of COVID-19 patients in China have received traditional Chinese medicine (TCM) treatment during different stages of the disease, with observable positive treatment outcomes [5]. Although basic and clinical research evidence might not align with the factual outcomes at present, the value of TCM is worth further investigation and utilization.
Typical clinical features of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-infected patients are fever, fatigue, cough, and acute pneumonia, which are similar to the symptoms caused by human severe acute respiratory syndrome coronavirus (SARS-CoV) [4], [6], [7]. The current epidemic is due to SARS-CoV-2 infection, which spreads through the respiratory tract and transmit through contact. The virus is highly contagious and the global population is generally susceptible. According to TCM theory, the etiology of COVID-19 is the feel of the pattern expression of qi within the basic theory of TCM. COVID-19 is considered to be a kind of strong contagious external evil in TCM. Therefore, the New Coronavirus Pneumonia Diagnosis and Treatment Program [5] points out that this disease can be categorized as plague in TCM, with the etiology of epidemic factor exposure. The disease is located in the lungs, and the basic pathogenesis is characterized by dampness, heat, toxicity, and blood stasis. The clinical treatment period of TCM is divided into five stages: mild case (cold-damp constraint in the lung pattern, damp-heat accumulation in the lung pattern), moderate case (damp-toxin constraint in the lung pattern, cold-damp obstructing the lung pattern), severe case (epidemic toxin blocking the lung pattern, blazing of both qi and yin pattern), critical case (internal blockage and external desertion pattern), and convalescence (lung–spleen qi deficiency pattern, deficiency of both qi and yin pattern). From the Western point of view, by using genomic sequencing, it was found that COVID-19 has a high similarity to bat coronaviruses and shares a high sequence identity with SARS-CoV [8], [9]. The pathological features of COVID-19 are also similar to those of SARS-CoV infection, while the entry of SARS-CoV-2 into cells is mediated by the interaction between spike glycoprotein (S protein) and the cellular receptor angiotensin-converting enzyme 2 (ACE2) [10], [11], [12]. Several important drug targets for the treatment of coronaviruses have been identified in previous studies on SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), such as S protein, ACE2, transmembrane protease serine 2 (TMPRSS2), coronavirus main proteinase (3CLpro), ribonucleic acid (RNA)-dependent RNA polymerase (RdRp), and papain-like protease (PLpro) [13], [14], [15], [16].
The major challenges of this new infectious disease include a poor understanding of the pathogenesis and treatment methods; furthermore, some patients show mild syndromes or even no syndrome but are still highly infectious [17]. Other patients will quickly progress to a severe/critical condition; yet there are still no effective drugs to relieve the condition. Many COVID-19 patients suffer from five steps of progression: first, severe viral infection, followed by suppression of immune system; then, when transitioning from a mild to a severe condition, they suffer from cytokine storm, resulting in multi-organ damage and dysfunction. Under a strong host immune response, the disease also causes complex adverse effects, with overall damage of multiple organs and lung fibrosis [1], [3], [7], [18], [19], [20].
Due to the similarities between SARS-CoV infection and COVID-19 infection, the treatment strategy of SARS-CoV infection using Chinese herbal medicine (CHM) that was used 17 years ago might provide insights to overcome today’s crisis. In particular, in the absence of specific effective Western medicine, the use of CHM should be treated as an important alternative. In addition, CHM has been extensively applied in China to combat COVID-19, and its efficacy and value have been admitted and documented in the Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7). [5].
The therapeutic mechanisms of CHMs work both similarly to and differently from conventional Western medicine, by directly intervening against the virus, viral-host interactions of viral cells, molecular targets, host receptors, signaling pathways, and micro-ecologies to exert a direct antiviral function. At the same time, CHMs may act on the body’s immune system to generate a holistic effect, protecting human tissues and organs and thereby enhancing the body’s ability to resist viruses and immune damage [21], [22], as CHMs are often composed of a variety of chemical ingredients that can act on multiple pathological pathways. In fact, Western medicine is not solely dependent on a single drug for treatment. Sometimes, in order to achieve comprehensive efficacy, Western medicine will also rely on combinational treatments and methods. For example, in COVID-19 treatment, the recommended treatment includes the application of a combination of antiviral drugs, interleukin (IL)-6 inhibitors, interferon (IFN)-γ, immune enhancers, oxygen delivery, and supportive therapies. Thus, Western medicine and TCM may achieve therapeutic outcomes through multidimensional common pathways and targets in the different stages of COVID-19 patients (Fig. 1 ). The current article aims to analyze and summarize the potential uses and treatment mechanisms of CHM from the reported literature, along with patent drugs recommended by institutions at the national and provincial levels in China.
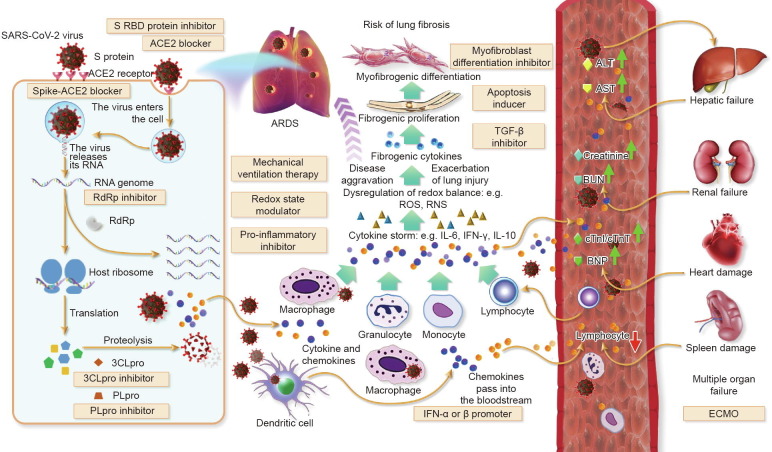
Fig. 1.
Therapeutic targets and potential treatment strategies for COVID-19. SARS-CoV-2 infects airway epithelial cells through interactions with the transmembrane enzyme ACE2. Blocking human ACE2 protein may be a promising therapeutic agent for patients with the virus. Once SARS-CoV-2 enters the cell, the infected cells undergo cell death and release virus particles together with intracellular components, which trigger the inflammatory response. Antiviral agents such as inhibitors of RNA polymerase and protease inhibitors are potential therapeutic strategies. Subsequently, the surge of pro-inflammatory cytokines and dysregulation of the redox balance causes edema and damages capillary and lung tissue, even leading to acute respiratory distress syndrome (ARDS); exacerbation of lung injury also increases the risk of lung fibrosis. Drugs inhibiting the pro-inflammatory cytokines, such as tocilizumab (an inhibitor of IL-6) are recommended in the cytokine storm phase of the disease, and modulators of the redox state may be used to restore the redox balance. When other organs, such as liver, kidney, heart, and spleen, are flooded with inflammatory cytokines and chemokine, organ failure ensues, with fatal consequences. Extracorporeal membrane oxygenation (ECMO) has a role in the treatment of severe COVID-19 at this stage. RBD: receptor-binding domain; TGF: transforming growth factor; ROS: reactive oxygen species; RNS: reactive nitrogen species; ALT: alanine aminotransferase; AST: aspartate transaminase; BUN: blood urea nitrogen; BNP: B-type natriuretic peptide; cTnI: cardiac troponin I; cTnT: cardiac troponin T.
2. Pathogenesis of COVID-19 and the treatment mechanism of CHM
A summary of the pathogenesis and therapeutic intervention mechanisms of CHM against COVID-19 is provided in Table 1 [21], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53]. According to the current network of pharmacology research and a few in vitro experiments, the mechanism of CHM on COVID-19 is multi-component, multi-target, and multi-pathway. The main mechanisms are direct anti-virus action, anti-inflammation, immune regulation, and the protection of target organs [54], [55]. A TCM is a complex compound that contains polysaccharides, flavonoids, saponins, alkaloids, and other ingredients with pharmacological activity.
Table 1.
Summary of the pathogenesis of COVID-19 and therapeutic mechanisms of CHM.
| Disease | Pathogenesis | Chinese herbal formulas and active components | Targets and signaling pathways | References |
|---|---|---|---|---|
| Severe viral infection | Virus replication | Lianhua Qingwen formula; Wogonin (Scutellariae Radix); Baicalin (Scutellariae Radix); L-methylephedrin, L-ephedrine, and D-pseudo-ephedrine (Ephedrae Herba); and patchouli alcohol (Pogostemonis Herba) | Inhibiting SARS-CoV-2, SARS-CoV, influenza A and B virus replication, inducing IFN-γ, modulating Toll-like receptors (TLRs), retinoic acid inducible gene-1 (RIG-1), adenosine monophosphate (AMP)-activated protein kinase (AMPK), phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt), and extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) pathways | [23], [24], [25], [26], [27] |
| Viral RNA synthesis | HouttuyniaeHerba | Inhibiting SRAS-CoV RdRp | [28] | |
| Virus invasion | Qingfei Paidu decoction and Huoxiang Zhengqi oral liquid, patchouli alcohol, tussilagone (Farfarae Flos), ergosterol, asarinin (Asari Radix et Rhizoma), ephedrine hydrochloride (Ephedrae Herba), shionone (Asteris Radix et Rhizoma), quercetin, isorhamnetin (Glycyrrhizae Radix et Rhizoma), and irisolidone (PogostemonisHerba) | Binding to ACE2 receptor | [29], [30] | |
| Viral protein proteins and particle assembly | Houttuyniae Herba, Qingfei Paidu decoction and Huoxiang Zhengqi oral liquid, patchouli alcohol (Pogostemonis Herba), saikosaponin B (Bupleuri Radix), ergosterol (Polyporus), shionone (Asteris Radix et Rhizoma), 23-acetate alisol B (Alismatis Rhizoma), licorice glycoside E, kaempferol, (2R)-7-hydroxy-2-(4-hydroxyphenyl)chroman-4-ketone, quercetin, isorhamnetin (Glycyrrhizae Radix et Rhizoma), naringenin (Citri Reticulatae Pericarpium), robinin (Platycodi Radix) and irisolidone (PogostemonisHerba), herbacetin (Rhodiolae Crenulatae Radix et Rhizoma), rhoifolin, apigenin, luteolin (Citri Reticulatae Pericarpium), quercetin, daidzein, and puerarin (Puerariae Radix) | Binding to 3CLpro and inhibiting the proteolytic activity of SARS-CoV 3CLpro | [28], [29], [30], [31] | |
| Inflammation and cytokine storm | Virus-infected alveolar cells release signals to recruit and activate immune cells, which secrete a variety of cytokines and chemokines and destroy the virus by releasing inflammatory mediators or phagocytosis. The excessive immune response initiates a “cytokine storm” that causes damage of lung tissue and exacerbation of pneumonia | Lianhua Qingwen capsule, Lonicerae Japonicae Flos, platycodin D (Platycodi Radix), and Moutan Cortex Radicis | Suppressing pro-inflammatory cytokines production | [32], [33], [34], [35] |
| Lonicerae Japonicae Flos | Enhancing anti-inflammatory cytokines production | [33] | ||
| Platycodin D (Platycodi Radix) | Suppressing apoptosis | [34] | ||
| Platycodin D (Platycodi Radix) and Moutan Cortex Radicis | Strengthening antioxidant | [34], [35] | ||
| Platycodin D (Platycodi Radix) and Moutan Cortex Radicis | Protecting host against acute lung injury | [34], [35] | ||
| Polysaccharides of Pinelliae Rhizoma | Regulating IL-4 and IFN-γ | [36] | ||
| Prevention of pulmonary fluids and obstruction | Acute lung inflammation increases the permeability of lung endothelial and epithelial barriers, impairs alveolar fluids clearance mechanisms, causes edema, blocks airways, and leads to hypoxia | Asteris Radix, Fritillaria cirrhosae Bulbus, Trichosanthis Fructus, Eriobotryae Japonicae Folium, polysaccharides of Pinelliae Rhizoma, and verticine | Dispelling phlegm and relieving cough, inhibiting mucus secretion in human airway epithelial cells | [36], [37], [38], [39] |
| Multi-organ dysfunction | ACE2 receptor attack, immune destruction | Astragalus and Angelica, Rheum and its components, and triptolide | Boosting the immune system, relieving diuresis, anti-oxidation, and inflammation | [40] |
| Qi deficiency | CodonopsisRadix and Panax ginseng | Replenishing qi–yin deficiency, promoting organ and tissue regeneration and recovery | [21], [40], [41] | |
| Activation of the airway inflammatory pathway | Xiyanping injection (andrographolide sulfonate) | Ameliorating airway inflammatory cell recruitment and inhibiting nuclear factor (NF)-κB and MAPK-mediated inflammatory responses | [42] | |
| Over-secretion of inflammatory cytokines | Xuebijing injection (Carthamus tinctorius, Ligusticum wallichii, and Salvia miltiorrhiza) | Suppressing inflammatory cytokine secretion | [43] | |
| Lung fibrosis | Induction of lipogenesis | Naringenin | Inhibiting autophagy and suppressing lung inflammation and fibrosis | [44] |
| Wnt signaling activation | Morusin | Alleviating mycoplasma pneumonia via the inhibition of Wnt/β-catenin and NF-κB signaling | [45] | |
| TGF-β and integrin activation | Yupingfeng formula (Astragalus and Atractylodes macrocephala) | Blocking fibroblast activation, collagen production, and extracellular matrix (ECM) degradation signaling pathway | [46] | |
| Tissue damage due to viral binding to ACE2 | Tanshinone IIA | Attenuating bleomycin-induced pulmonary fibrosis via modulating ACE2 | [47] | |
| p38 MAPK activation | Oxymatrine | Inhibiting phosphorylated p38 MAPK and blocking fibroblast activation and collagen production | [48] | |
| Activation of ECM | Honokiol | Inhibiting ECM and pro-inflammatory factors | [49], [50] | |
| Induction of ROS and protein oxidation | Resveratrol and berberine | Acting as ROS scavenger, maintaining redox balance, and preventing of protein oxidation | [51], [52], [53] | |
2.1. Antiviral activity targeting SARS-CoV-2 and its host receptor ACE2
SARS-CoV-2 is an enveloped, single-stranded, positive-sense RNA beta coronavirus. The S protein on the surface of SARS-CoV-2 induces the attachment and invasion of SARS-CoV-2 to the host cells by recognizing the ACE2 receptor [15]. The invaded virus then takes control of the host cell’s genetic reproduction tools to create new virus RNA with RdRp; synthesizes glycoproteins by the host ribosome, which are cleaved to non-structural proteins and structural proteins (S proteins) by virus proteinases (3CLpro and PLpro); and assembles new viral particles to be released to infect other host cells [56], [57]. Therefore, the ACE2 receptor, RdRp, spike protein, 3CLpro, and PLpro are essential in the invasion and replication of SARS-CoV-2, and could be potential targets for the treatment of COVID-19 by CHM [31], [58]. The etiology of COVID-19 is that SARS-CoV-2 spreads through the respiratory tract, infects the lungs, causes pneumonia, and produces inflammatory factors; the virus replicates and releases in host cells, circulates in the blood, binds to ACE2 on the surface of multiple organs in the body, disrupts the balance of the RAS signal pathway, and causes damage to multiple organs throughout the body. The virus can also cause an excessive immune response in the body, resulting in an inflammatory storm, which further aggravates the disease. Inflammation in the lungs causes a large number of secretions that block the airway and exacerbate the body’s hypoxia.
The potential therapeutic effect of TCM is to directly inhibit the adsorption of the virus to host cells and its replication by binding to the ACE2 receptor and 3CLpro. In a review by Hao et al. [23], the researchers noted that CHM might be used as a complementary and alternative approach to the primary and secondary prevention of cardiovascular disease. The cardiovascular protective actions of CHM have been mainly ascribed to their antioxidant, anti-inflammatory, and anticytotoxic effects [23], [59]. The heart is an ACE2-rich organ, so we speculate that the protective mechanism of CHM on the heart is also related to blocking the contact of virus with ACE2 target. For example, reduning injection is used to treat severe cases of COVID-19. This injection contains Salvia miltiorrhiza. Tanshinone from Salvia miltiorrhiza is a representative example of terpenoids with cardiovascular protective effects in CHM. Therefore, we hypothesize that the tanshinone in reduning injection is an active ingredient in the treatment of COVID-19 to protect target organs.
In addition, Lianhua Qingwen capsule has been shown to significantly and dose-dependently inhibit the replication of SARS-CoV-2 (half maximal inhibitory concentration (IC50): 411.2 μg·mL−1) in Vero E6 cells infected by SARS-CoV-2 [32]. The water extract of Houttuyniae Herba exhibited a significant inhibitory effect on SARS-CoV 3CLpro and RdRp [28]. Network pharmacology analysis also showed that the major active compounds contained in Qingfei Paidu decoction and Huoxiang Zhengqi oral liquid, such as patchouli alcohol (Pogostemonis Herba), saikosaponin B (Bupleuri Radix), ergosterol (Polyporus), shionone (Asteris Radix et Rhizoma), 23-acetate alisol B (Alismatis Rhizoma), licorice glycoside E, kaempferol, (2R)-7-hydroxy-2-(4-hydroxyphenyl)chroman-4-ketone, quercetin, isorhamnetin (Glycyrrhizae Radix et Rhizoma), naringenin (Citri Reticulatae Pericarpium), robinin (Platycodi Radix), and irisolidone (Pogostemonis Herba), could bind to 3CLpro, while patchouli alcohol, tussilagone (Farfarae Flos), ergosterol, asarinin (Asari Radix et Rhizoma), ephedrine hydrochloride (Ephedrae Herba), shionone (Asteris Radix et Rhizoma), quercetin, isorhamnetin, and irisolidone could bind to ACE2 receptor, thus blocking SARS-CoV-2 virus invasion and replication [29], [30]. In addition, herbacetin (Rhodiolae Crenulatae Radix et Rhizoma), rhoifolin, apigenin, luteolin (Citri Reticulatae Pericarpium), quercetin, daidzein, puerarin (Puerariae Radix), and kaempferol were reported to inhibit the proteolytic activity of SARS-CoV 3CLpro [31]. Moreover, many active components of CHMs used to treat COVID-19 showed significant antiviral effect against influenza virus, which could contribute to their effect in controlling COVID-19. For example, wogonin (Scutellariae Radix) effectively suppressed both influenza A and B virus replication in Madin–Darby canine kidney (MDCK) cells and human lung epithelial (A549) cells via modulation of adenosine monophosphate (AMP)-activated protein kinase (AMPK) pathways [24]. Baicalin (Scutellariae Radix) exhibited anti-influenza virus A (H1N1) activity in vitro and in vivo as a potent inducer of IFN-γ in major IFN-γ-producing cells [25]. L-methylephedrin, L-ephedrine, and D-pseudo-ephedrine (Ephedrae Herba) inhibited the replication of influenza A virus in vitro and protected virus-infected mice by adjusting the host’s Toll-like receptors (TLRs) and retinoic acid inducible gene-1 (RIG-1) pathways [26]. Patchouli alcohol significantly inhibited the in vitro multiplication of different influenza virus A strains and may block influenza virus A infection by inactivating virus particles directly and interfering with the early stages after virus adsorption through cellular phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt), and extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) signaling pathways [27].
2.2. Suppressing pro-inflammatory cytokines to block cytokine storm
Accumulating evidence reveals that a cytokine storm occurs in severe COVID-19 patients, in which the cytokine levels of IL-1B, IL-1RA, IL-6, IL-7, IL-8, IL-9, IL-10, fibroblast growth factor (FGF), granulocyte macrophage colony stimulating factor (GM-CSF), IFN-γ, granulocyte colony stimulating factor (G-CSF), IFN-γ-inducible protein (IP)-10, monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein-1α (MIP1A), platelet-derived growth factor (PDGF), tumor necrosis factor (TNF)-α, and vascular endothelial growth factor (VEGF) significantly increased [60], [61]. IL-6 elevation was significant in critically ill or non-survival patients [2], [62], [63], [64]. Anti-inflammatory treatment—including glucocorticoids, Tocilizumab (recombinant human IL-6 monoclonal antibody), Baricitinib (Janus kinase (JAK) inhibitor), chloroquine, and hydroxychloroquine—has been employed in clinics and was shown to be effective in reducing fever and the exacerbation of pneumonia, promoting the absorption of pneumonia, obtaining better oxygenation, improving lung imaging findings, and increasing the negative rate of virus nucleic acid testing [65]. Compelling evidence has revealed that, when used to treat COVID-19, CHMs and their active ingredients can suppress pro-inflammatory cytokines and thus relieve cytokine storm in COVID-19 patients. Li et al. [32] showed that Lianhua Qingwen capsule markedly reduced the production of TNF-α, IL-6, C–C motif chemokine ligand (CCL)-2/MCP-1, and chemokine (C–X–C motif) ligand (CXCL)-10/IP-10 in a concentration-dependent manner in SARS-CoV-2-infected Huh-7 cells at the messenger RNA (mRNA) level. Ethanol extract of Lonicerae Japonicae Flos increased nuclear specificity protein 1 (Sp1) binding activity and thereby enhanced the expression of IL-10, while decreasing nuclear factor (NF)-κB binding activity and thereby inhibiting the expression of TNF-α, IL-1β, and IL-6 in acute lung inflammation induced by lipopolysaccharide (LPS) in mice [33]. Platycodin D (Platycodi Radix) improved the acute lung injury induced by LPS or bleomycin by suppressing apoptosis (caspase (CASP)-3 and Bax down-regulated) and inflammation (decreased TNF-α, IL-6, and NF-κB) and strengthening anti-oxidation (decreased myeloperoxidase (MPO) activity and improved superoxide dismutase activity) [34]. Extract of Moutan Cortex Radicis also improved LPS-induced acute lung injury in rats by reducing IL-1β, MIP-2, IL-6, and MOP activity [35]. Polysaccharides of Pinelliae Rhizoma exhibited significant suppression of LPS-induced airway inflammation by regulating levels of IL-4 and IFN-γ and inhibiting mucus secretion in human airway epithelial human lung carcinoma cell 292 (NCI-H292) cells [36]. It has been reported that quercetin, kaempferol, luteolin, isorhamnetin, baicalein, naringenin, and wogonin could be the main active ingredients of CHM for the management of COVID-19 by inhibiting inflammatory mediators, regulating immunity, and eliminating free radicals. These ingredients target IL-17, arachidonic acid, hypoxia inducible factor (HIF)-1, NF-κB, RAS, and TNF through cyclooxygenase (COX)-2, CASP-3, IL-6, MAPK1, MAPK14, MAPK8, and RelA in the signaling pathways [54].
2.3. Suppressing lung inflammation to reduce lung epithelial secretion and prevent pulmonary obstruction
Although most COVID-19 patients primarily have fever, fatigue, and a dry cough with a favorable prognosis [61], some patients with severe illness may develop dyspnea and hypoxemia, and quickly progress to acute respiratory distress syndrome (ARDS) [63], requiring mechanical ventilation. The pathophysiology of ARDS is complex and involves acute lung inflammation that increases the permeability of lung endothelial and epithelial barriers, impairs the alveolar fluid clearance mechanism, causes edema, and blocks airways, resulting in hypoxia [66]. The recommended CHMs and their active components not only suppress lung inflammation and slow down cytokine storm, but also reduce lung epithelial secretion and thereby prevent pulmonary obstruction. For example, extracts of Asteris Radix, Fritillaria cirrhosae Bulbus, Trichosanthis Fructus, or Eriobotryae Japonicae Folium, which have been used in CHMs for more than 1000 years to dispel phlegm and relieve cough, and their active component verticine demonstrated significant expectorant, antitussive, and anti-inflammatory effects [37], [38], [39]. CHM has been reported to act on the lungs to reduce the exudation of alveolar cells and vascular epithelial cells, thereby alleviating airway obstruction by secretions and fibrosis. This is also a possible reason why CHM can improve the hypoxic state of patients with new coronary pneumonia. These factors may also contribute to the low rate of mild COVID-19 patients becoming severe and critically ill cases within the treatment group of patients in China who received integrative therapy of Chinese and Western medicine.
2.4. Protecting lungs and multiple organs from damage
It was reported that COVID-19 patients with comorbidities and the elderly have a higher death rate, as these patients have a greater possibility of developing multi-organ failure and then progressing into critical condition. One of the major reasons causing SARS-CoV-2 infection to be lethal is multi-organ dysfunction, since SARS-CoV-2 attacks not only the lung tissues, but also many key organs of the body, such as the heart, kidneys, and testes, and is broadly distributed in the lung, liver, colon, and brain as well [1], [13], [16], [17], [19], [67]. These key organs express a high level of ACE2, which becomes a target of SARS-CoV-2 attack [14]. Most hospitalized COVID-19 patients show the typical characteristics of ground-glass opacity and bilateral patchy shadowing in chest computed tomography [40], [68]. However, when the virus infects the heart and kidneys, it binds to ACE2 and causes sudden cardiac arrest and renal failure, which are the cause of death in most patients—even severely ill patients that are treated in the intensive care unit (ICU). Therefore, it is essential to reduce the viral load in the early stage and relieve the syndromes to prevent disease progression. When CHMs were applied early, they demonstrated effectiveness in protecting key organs and then preventing disease progression from mild to severe/critical condition. For example, Astragalus, Angelica, Rheum officinale, and their components (emodin, rhein, and triptolide) have been reported to treat chronic kidney disease by boosting the immune system and relieving diuresis, and through anti-oxidation and anti-inflammation by the alteration of T helper cell 17 (Th17) and Th17/regulatory T-cell ratios [44]. Codonopsis Radix and Panax ginseng can be applied to patients with qi deficiency in critical condition, and promote cellular autophagy and glucose metabolic pathways [21], [22], [69]. Xiyanping injection, with its active component of andrographolide sulfonate, ameliorates airway inflammatory cell recruitment and then inhibits NF-κB and MAPK-mediated inflammatory responses, where NF-κB is a major transcriptional factor that can stimulate inflammation [42]. Furthermore, Xuebijing injection (Carthamus tinctorius, Ligusticum wallichii, and Salvia miltiorrhiza) suppressed inflammatory cytokine secretion and then replenished qi [43]. Although these CHMs do not directly target ACE2, they can holistically relieve the syndromes of patients, increase their comfort level, and prevent further disease progression [70].
2.5. Preventing lung fibrosis
Based on past experience with SARS-CoV infection and recent reports on COVID-19, although the death rate of COVID-19 is not high, many patients suffer from long-term lung function damage. Severe patients usually develop acute viral infection, severe inflammation, alveolar epithelial cell (AEC) injury, fibroblast activation, collagen production, extracellular matrix (ECM) degradation inhibition, and eventual lung fibrosis, resulting in pulmonary obstruction and scarring on the lung tissues [71], [72]. Long-term pulmonary obstruction and the reduction of pulmonary capacity will affect the daily activities and quality of life of COVID-19 patients after discharge. Therefore, effective prevention of lung fibrosis and rehabilitation care are also critical challenges, while CHM may contribute to relieve or even reverse this condition [44], [45], [73]. CHMs are thus indispensable and should be promoted for use during the rehabilitation stage. Moreover, although some patients reach a discharge standard, such as a negative viral load and relief of respiratory syndromes, they are usually still suffering from qi and yin deficiency, according to TCM diagnosis.
When SARS-CoV-2 invades the respiratory tract and the lung, the cell proliferative pathways of the lung epithelial cells are often activated due to the demand for tissue regeneration during the host immune defense response and inflammation. Under the infected and inflamed conditions, the mucin (MUC)-5B, transforming growth factor (TGF)-β, p38 MAPK, integrin signaling, and Wnt signaling pathways are activated [46], [47], [74], [75], [76], [77], [78], [79]. SARS-CoV-2 also causes induction of reactive oxygen species (ROS), which turns on cell cycle regulatory genes, leading to lung fibroblast proliferation. ROS also induce protein oxidation, which is associated with cellular damage-related inflammatory response, resulting in pulmonary fibrosis and obstruction [48], [71], [72], [74]. In addition, cell senescence, mitochondrial dysfunction, and dysregulated proteostasis are among the etiologies of pulmonary fibrosis [71], [72]. Medications that can suppress these factors may be able to prevent lung fibrosis, especially in the early stage of the disease.
Previous studies have shown that a number of Chinese medicinal herbs, CHMs, and their active components relieve lung fibrosis under various conditions of pneumonia including SARS-CoV infection. Hence, we suggest the use of CHM in the early stage of COVID-19 to block these pathways and lung fibrosis. Yupingfeng, an herbal formula composed of Astragalus and Atractylodes macrocephala, was reported to have inhibitory potency on TGF-β cell proliferation signaling, leading to blockage of fibroblast activation and collagen production [49]. Furthermore, naringenin was reported to inhibit autophagy to suppress lung inflammation and fibrosis by attenuating ROS [74], while morusin alleviates mycoplasma pneumonia via the inhibition of Wnt/ β-catenin and NF-κB signaling [75]. Tanshinone IIA attenuates bleomycin-induced pulmonary fibrosis via modulation of ACE2, by preventing viral binding [50], and oxymatrine inhibits phosphorylated p38 MAPK cell proliferation signaling [51].
Even after discharge from hospital, patients may have qi–yin deficiency syndrome according to TCM, along with depression or other disorders that require further treatment. Panax ginseng and Astragalus, two Chinese medicinal herbs that are commonly used in clinics, have been shown to be effective in preventing experimental pulmonary fibrosis, and so forth [21], [22], [69], [70]. Honokiol was reported to inhibit the ECM and pro-inflammatory factors, while also relieving lung fibrosis [52], [53]. Moreover, some chemical compounds derived from CHM may act as ROS scavengers and as drugs targeting redox imbalance in order to relieve lung inflammation and fibrosis, such as resveratrol and berberine [80], [81], [82].
In sum, by focusing on the pathogenesis of COVID-19, therapeutic targets and potential treatment strategies have been identified. It is recommended to apply a combination of antiviral drugs, IL-6 inhibitors, IFN-γ, immune enhancers, oxygen delivery, and supportive therapies for the treatment of COVID-19 patients (Fig. 1). TCM can achieve therapeutic outcomes through multidimensional common pathways and targets in COVID-19 patients at different stages.
3. Opinions and perspectives
Under these emergent circumstances and with the current lack of effective medication against COVID-19, integrated therapy of Chinese and Western medicine should be adopted to treat COVID-19 patients, with appropriate safety guidance and measures for TCM. In TCM development, we should take our past experiences into account and combine them with today’s innovative findings and knowledge to fight this global antiviral battle. To make an appropriate formulation and appropriately use CHMs, TCM doctors should conduct a comprehensive analysis of the clinical manifestations, pattern differentiation, and individual conditions of individual patients. This will enable them to make a well-defined prescription, in which the safe use of herbs should be given great attention and priority, based on both literature records and modern experimental data.
According to the experiences of clinical experts such as Professor Boli Zhang and Professor Xiaolin Tong in treating COVID-19 patients in Wuhan, CHMs should be applied in the earliest possible stage in order to achieve multidimensional therapeutic outcomes, including slowing down or preventing the progression of the disease. TCM doctors can also prescribe different CHMs to treat patients in different stages of the disease based on practical needs, resulting in optimal therapeutic efficacy of the CHMs. Thus, TCM treatment should be used throughout all the entire treatment processes to address different stages of the disease.
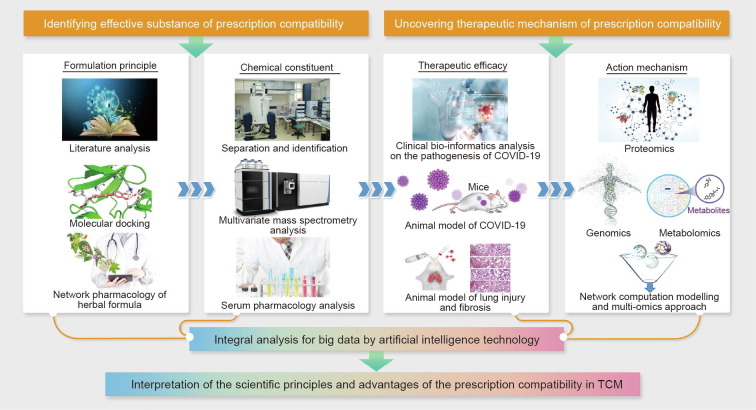
Key emphasis should also be placed on comparative research of severe acute respiratory syndrome (SARS) and COVID-19; in particular, the similarities and differences in terms of drug prescriptions should be clarified. Based on the current regulations of the national drug agency, although SARS and COVID-19 share similar genomic sequences, the approved prescribed drugs are still very different. Therefore, global agreement on the specific drugs approved for treating COVID-19 has not been reached. At present, randomized clinical trials (RCTs) of CHMs for COVID-19 treatment are still insufficient; therefore, a claim regarding their “obvious curative effectiveness” based on clinical observations would raise academic and public concern and limit global acceptance of TCM for treating COVID-19 patients. As such, it is necessary to carry out additional in-depth and well-designed clinical trials in order to further prove the efficacy of specific prescriptions or chemicals with in-depth solid scientific evidence. It is also essential to use multidisciplinary advanced technologies from conventional medicine and modern science to conduct multidimensional in-depth studies in order to identify effective substances for prescription compatibility. Research could be conducted based on traditional formulation principles, and then proceed to an investigation of the chemical constituents, therapeutic efficacy, and action mechanism in order to firmly convince the public of prescription efficacy, based on objective clinical evidence and detailed scientific data (Fig. 2 ).
Fig. 2.
Elucidating the scientific foundations of Chinese herbal formulas by multiple high-tech technologies. Multiple high-tech technologies, such as network pharmacology and multivariate mass spectrometry analysis, could be applied to identify the formulation principles and chemical constituents of Chinese herbal formulas, so as to recognize effective substances and prescription compatibility. Also, to uncover the therapeutic mechanisms of prescription compatibility, a multi-omics approach is recommended to identify the multiple targets of Chinese herbal formulas in treating complex diseases. With integral analysis of big data by artificial intelligence technology, the scientific principles and advantages of the prescription compatibility in TCM will be better elucidated.
4. Concluding remarks
In conclusion, the treatment value of TCM for COVID-19 is fundamentally supported by the literature and by clinical experience. CHMs have been prescribed alone or in combination with Western medicine for COVID-19 patients in China at different stages of the disease, and have shown positive efficacy. However, since COVID-19 is a new infectious disease, its pathogenesis and treatment could be similar to or different from other pandemic diseases that occurred in the past, and specific effective drugs and treatments remain a major challenge. We believe that TCM is a treasure of humanity against disease, including the current pandemic, and that its multidisciplinary cutting-edge technologies are powerful. Further uncovering the scientific foundations of TCM treatments would help us to open this treasure box. It is our sincere hope to combat the COVID-19 pandemic by joining hands with clinicians and scientists around the world.
Acknowledgments
Acknowledgements
This work was supported by the Macao Science and Technology Development Fund (0053/2020/A, 0057/2020/A, 0059/2020/A, and 0003/2019/AKP).
Compliance with ethics guidelines
Elaine Lai-Han Leung, Hu-Dan Pan, Yu-Feng Huang, Xing-Xing Fan, Wan-Ying Wang, Fang He, Jun Cai, Hua Zhou, and Liang Liu declare that they have no conflict of interest or financial conflicts to disclose.
References
- 1.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . World Health Organization; Geneva: 2020. Coronavirus disease 2019 (COVID-19) situation report—33. [Google Scholar]
- 4.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Health Commission of the People’s Republic of China, State Administration of Traditional Chinese Medicine. Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7) [Internet]. Beijing: National Health Commission of the People’s Republic of China; 2020 Mar 3 [cited 2020 Mar 3]. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf. Chinese.
- 6.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang S., Xia S., Ying T., Lu L. A novel coronavirus (2019-nCoV) causing pneumonia-associated respiratory syndrome. Cell Mol Immunol. 2020;17(5):554. doi: 10.1038/s41423-020-0372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 12.Hamming I., Cooper M.E., Haagmans B.L., Hooper N.M., Korstanje R., Osterhaus A.D. The emerging role of ACE2 in physiology and disease. J Pathol. 2007;212(1):1–11. doi: 10.1002/path.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016–1035. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gembardt F., Sterner-Kock A., Imboden H., Spalteholz M., Reibitz F., Schultheiss H.P. Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides. 2005;26(7):1270–1277. doi: 10.1016/j.peptides.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuba K., Imai Y., Ohto-Nakanishi T., Penninger J.M. Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 2010;128(1):119–128. doi: 10.1016/j.pharmthera.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rolling updates on coronavirus disease (COVID-19) [Internet]. Geneva: World Health Organization; c2020 [updated 2020 Jul 31; cited 2020 Jul 3]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen.
- 18.Liang W., Guan W., Chen R., Wang W., Li J., Xu K. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rismanbaf A., Zarei S. Liver and kidney injuries in COVID-19 and their effects on drug therapy; a letter to editor. Arch Acad Emerg Med. 2020;8(1) [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang W.Y. Therapeutic wisdom in traditional Chinese medicine: a perspective from modern science. Trends Pharmacol Sci. 2005;26(11):558–563. doi: 10.1016/j.tips.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Yuan R., Lin Y. Traditional Chinese medicine: an approach to scientific proof and clinical validation. Pharmacol Ther. 2000;86(2):191–198. doi: 10.1016/s0163-7258(00)00039-5. [DOI] [PubMed] [Google Scholar]
- 23.Hao P.P., Jiang F., Chen Y.G., Yang J., Zhang K., Zhang M.X. Traditional Chinese medication for cardiovascular disease. Nat Rev Cardiol. 2015;12(2):115–122. doi: 10.1038/nrcardio.2014.177. [DOI] [PubMed] [Google Scholar]
- 24.Seong R.K., Kim J.A., Shin O.S. Wogonin, a flavonoid isolated from Scutellaria baicalensis, has anti-viral activities against influenza infection via modulation of AMPK pathways. Acta Virol. 2018;62(1):78–85. doi: 10.4149/av_2018_109. [DOI] [PubMed] [Google Scholar]
- 25.Chu M., Xu L., Zhang M., Chu Z., Wang Y. Role of Baicalin in anti-influenza virus A as a potent inducer of IFN-γ. BioMed Res Int. 2015;2015 doi: 10.1155/2015/263630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei W., Du H., Shao C., Zhou H., Lu Y., Yu L. Screening of antiviral components of Ma Huang Tang and investigation on the ephedra alkaloids efficacy on influenza virus type A. Front Pharmacol. 2019;10:961. doi: 10.3389/fphar.2019.00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu Y., Zhang Y., Wang S., Liu W., Hao C., Wang W. Inhibition effects of patchouli alcohol against influenza a virus through targeting cellular PI3K/Akt and ERK/MAPK signaling pathways. Virol J. 2019;16(1):163. doi: 10.1186/s12985-019-1266-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau K.M., Lee K.M., Koon C.M., Cheung C.S.F., Lau C.P., Ho H.M. Immunomodulatory and anti-SARS activities of Houttuynia cordata. J Ethnopharmacol. 2008;118(1):79–85. doi: 10.1016/j.jep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang D., Zhang B., Lv J.T., Sa R.N., Zhang X.M., Lin Z.J. The clinical benefits of Chinese patent medicines against COVID-19 based on current evidence. Pharmacoll Res. 2020;157 doi: 10.1016/j.phrs.2020.104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng Y., Liu B., He Z., Liu T., Zheng R., Yang A. Study on active compounds from Huoxiang Zhengqi Oral Liquid for prevention of coronavirus disease 2019 (COVID-19) based on network pharmacology and molecular docking. Chin Tradit Herbal Drugs. 2020;51(5):1113–1122. [Google Scholar]
- 31.Jo S., Kim S., Shin D.H., Kim M.S. Inhibition of SARS-CoV 3CL protease by flavonoids. J Enzyme Inhib Med Chem. 2020;35(1):145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li R., Hou Y., Huang J., Pan W., Ma Q., Shi Y. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol Res. 2020;156 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kao S.T., Liu C.J., Yeh C.C. Protective and immunomodulatory effect of flos Lonicerae japonicae by augmenting IL-10 expression in a murine model of acute lung inflammation. J Ethnopharmacol. 2015;168:108–115. doi: 10.1016/j.jep.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Tao W., Su Q., Wang H., Guo S., Chen Y., Duan J. Platycodin D attenuates acute lung injury by suppressing apoptosis and inflammation in vivo and in vitro. Int Immunopharmacol. 2015;27(1):138–147. doi: 10.1016/j.intimp.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Fu P.K., Yang C.Y., Tsai T.H., Hsieh C.L. Moutan cortex radicis improves lipopolysaccharide-induced acute lung injury in rats through anti-inflammation. Phytomedicine. 2012;19(13):1206–1215. doi: 10.1016/j.phymed.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Hu M., Liu Y., Wang L., Wang J., Li L., Wu C. Purification, characterization of two polysaccharides from Pinelliae Rhizoma Praeparatum cum Alumine and their anti-inflammatory effects on mucus secretion of airway epithelium. Int J Mol Sci. 2019;20(14):3553. doi: 10.3390/ijms20143553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Y., Ming T.W., Gaun T.K.W., Wang S., Ye B. A comparative assessment of acute oral toxicity and traditional pharmacological activities between extracts of Fritillaria cirrhosae Bulbus and Fritillaria pallidiflora Bulbus. J Ethnopharmacol. 2019;238 doi: 10.1016/j.jep.2019.111853. [DOI] [PubMed] [Google Scholar]
- 38.Yu P., Cheng S., Xiang J., Yu B., Zhang M., Zhang C. Expectorant, antitussive, anti-inflammatory activities and compositional analysis of Aster tataricus. J Ethnopharmacol. 2015;164:328–333. doi: 10.1016/j.jep.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 39.Yu X., Tang L., Wu H., Zhang X., Luo H., Guo R. Trichosanthis Fructus: botany, traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 2018;224:177–194. doi: 10.1016/j.jep.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 40.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szodoray P., Nakken B., Barath S., Csipo I., Nagy G., El-Hage F. Altered Th17 cells and Th17/regulatory T-cell ratios indicate the subsequent conversion from undifferentiated connective tissue disease to definitive systemic autoimmune disorders. Hum Immunol. 2013;74(12):1510–1518. doi: 10.1016/j.humimm.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Peng S., Hang N., Liu W., Guo W., Jiang C., Yang X. Andrographolide sulfonate ameliorates lipopolysaccharide-induced acute lung injury in mice by down-regulating MAPK and NF-κB pathways. Acta Pharm Sin B. 2016;6(3):205–211. doi: 10.1016/j.apsb.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song Y., Yao C., Yao Y., Han H., Zhao X., Yu K. XueBiJing injection versus placebo for critically III patients with severe community-acquired pneumonia: a randomized controlled trial. Crit Care Med. 2019;47(9):e735–e743. doi: 10.1097/CCM.0000000000003842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y., Nie Y., Luo Y., Lin F., Zheng Y., Cheng G. Protective effects of naringin against paraquat-induced acute lung injury and pulmonary fibrosis in mice. Food Chem Toxicol. 2013;58:133–140. doi: 10.1016/j.fct.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 45.Cui W., Li L., Li D., Mo X., Zhou W., Zhang Z. Total glycosides of Yupingfeng protects against bleomycin-induced pulmonary fibrosis in rats associated with reduced high mobility group box 1 activation and epithelial-mesenchymal transition. Inflamm Res. 2015;64(12):953–961. doi: 10.1007/s00011-015-0878-x. [DOI] [PubMed] [Google Scholar]
- 46.Xia H., Diebold D., Nho R., Perlman D., Kleidon J., Kahm J. Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Exp Med. 2008;205(7):1659–1672. doi: 10.1084/jem.20080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stewart A.G., Thomas B., Koff J. TGF-β: master regulator of inflammation and fibrosis. Respirology. 2018;23(12):1096–1097. doi: 10.1111/resp.13415. [DOI] [PubMed] [Google Scholar]
- 48.Somogyi V., Chaudhuri N., Torrisi S.E., Kahn N., Müller V., Kreuter M. The therapy of idiopathic pulmonary fibrosis: what is next? Eur Respir Rev. 2019;28(153) doi: 10.1183/16000617.0021-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L., Li D., Xu L., Zhao P., Deng Z., Mo X. Total extract of Yupingfeng attenuates bleomycin-induced pulmonary fibrosis in rats. Phytomedicine. 2015;22(1):111–119. doi: 10.1016/j.phymed.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 50.Wu H., Li Y., Wang Y., Xu D., Li C., Liu M. Tanshinone IIA attenuates bleomycin-induced pulmonary fibrosis via modulating angiotensin-converting enzyme 2/angiotensin-(1–7) axis in rats. Int J Med Sci. 2014;11(6):578–586. doi: 10.7150/ijms.8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong J., Ma Q. Osteopontin enhances multi-walled carbon nanotube-triggered lung fibrosis by promoting TGF-β1 activation and myofibroblast differentiation. Part Fibre Toxicol. 2017;14(1):18. doi: 10.1186/s12989-017-0198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiang C.K., Sheu M.L., Lin Y.W., Wu C.T., Yang C.C., Chen M.W. Honokiol ameliorates renal fibrosis by inhibiting extracellular matrix and pro-inflammatory factors in vivo and in vitro. Br J Pharmacol. 2011;163(3):586–597. doi: 10.1111/j.1476-5381.2011.01242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weng T.I., Wu H.Y., Kuo C.W., Liu S.H. Honokiol rescues sepsis-associated acute lung injury and lethality via the inhibition of oxidative stress and inflammation. Intensive Care Med. 2011;37(3):533–541. doi: 10.1007/s00134-010-2104-1. [DOI] [PubMed] [Google Scholar]
- 54.Huang Y.F., Bai C., He F., Xie Y., Zhou H. Review on the potential action mechanisms of Chinese medicines in treating coronavirus disease 2019 (COVID-19) Pharmacol Res. 2020;158 doi: 10.1016/j.phrs.2020.104939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang WY, Xie Y, Zhou H, Liu L. Contribution of traditional Chinese medicine to the treatment of COVID-19. Phytomedicine 2020. In press. [DOI] [PMC free article] [PubMed]
- 56.Kiemer L., Lund O., Brunak S., Blom N. Coronavirus 3CLpro proteinase cleavage sites: possible relevance to SARS virus pathology. BMC Bioinf. 2004;5(1):72. doi: 10.1186/1471-2105-5-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thiel V., Ivanov K.A., Putics Á., Hertzig T., Schelle B., Bayer S. Mechanisms and enzymes involved in SARS coronavirus genome expression. J Gen Virol. 2003;84(9):2305–2315. doi: 10.1099/vir.0.19424-0. [DOI] [PubMed] [Google Scholar]
- 58.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Discov. 2020;19(3):149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 59.Hao P., Jiang F., Cheng J., Ma L., Zhang Y., Zhao Y. Traditional Chinese medicine for cardiovascular disease: evidence and potential mechanisms. J Am Coll Cardiol. 2017;69(24):2952–2966. doi: 10.1016/j.jacc.2017.04.041. [DOI] [PubMed] [Google Scholar]
- 60.Wan S, Yi Q, Fan S, Lv J, Zhang X, Guo L, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). 2020. medRxiv:2020.02.10.20021832.
- 61.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ware L.B. Pathophysiology of acute respiratory distress syndrome. In: Webb A., Angus D., Finfer S., Gattinoni L., Singer M., editors. Oxford textbook of critical care. Oxford University Press; Oxford: 2016. pp. 497–500. [Google Scholar]
- 67.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. 2020. bioRxiv:2020.02.03.931766.
- 68.Xu Y.H., Dong J.H., An W.M., Lv X.Y., Yin X.P., Zhang J.Z. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect. 2020;80(4):394–400. doi: 10.1016/j.jinf.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuen M.F., Tam S., Fung J., Wong D.K.H., Wong B.C.Y., Lai C.L. Traditional Chinese medicine causing hepatotoxicity in patients with chronic hepatitis B infection: a 1-year prospective study. Aliment Pharmacol Ther. 2006;24(8):1179–1186. doi: 10.1111/j.1365-2036.2006.03111.x. [DOI] [PubMed] [Google Scholar]
- 70.Kam P.C., Liew S. Traditional Chinese herbal medicine and anaesthesia. Anaesthesia. 2002;57(11):1083–1089. doi: 10.1046/j.1365-2044.2002.02823.x. [DOI] [PubMed] [Google Scholar]
- 71.McLean-Tooke A., Moore I., Lake F. Idiopathic and immune-related pulmonary fibrosis: diagnostic and therapeutic challenges. Clin Transl Immunol. 2019;8(11) doi: 10.1002/cti2.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Otoupalova E., Smith S., Cheng G., Thannickal V.J. Oxidative stress in pulmonary fibrosis. Compr Physiol. 2020;10(2):509–547. doi: 10.1002/cphy.c190017. [DOI] [PubMed] [Google Scholar]
- 73.Divya T., Dineshbabu V., Soumyakrishnan S., Sureshkumar A., Sudhandiran G. Celastrol enhances Nrf2 mediated antioxidant enzymes and exhibits anti-fibrotic effect through regulation of collagen production against bleomycin-induced pulmonary fibrosis. Chem Biol Interact. 2016;246:52–62. doi: 10.1016/j.cbi.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 74.Nakahira K., Pabon Porras M.A., Choi A.M. Autophagy in pulmonary diseases. Am J Respir Crit Care Med. 2016;194(10):1196–1207. doi: 10.1164/rccm.201512-2468SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen C., Wang J., Chen J., Zhou L., Wang H., Chen J. Morusin alleviates mycoplasma pneumonia via the inhibition of Wnt/β-catenin and NF-κB signaling. Biosci Rep. 2019;39(6) doi: 10.1042/BSR20190190. BSR20190190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Williamson J.D., Sadofsky L.R., Hart S.P. The pathogenesis of bleomycin-induced lung injury in animals and its applicability to human idiopathic pulmonary fibrosis. Exp Lung Res. 2015;41(2):57–73. doi: 10.3109/01902148.2014.979516. [DOI] [PubMed] [Google Scholar]
- 77.Kitamura H., Cambier S., Somanath S., Barker T., Minagawa S., Markovics J. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin αvβ8-mediated activation of TGF-β. J Clin Invest. 2011;121(7):2863–2875. doi: 10.1172/JCI45589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weng T., Ko J., Masamha C.P., Xia Z., Xiang Y., Chen N.Y. Cleavage factor 25 deregulation contributes to pulmonary fibrosis through alternative polyadenylation. J Clin Invest. 2019;129(5):1984–1999. doi: 10.1172/JCI122106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Borok Z. Role for α3 integrin in EMT and pulmonary fibrosis. J Clin Invest. 2009;119(1):7–10. doi: 10.1172/JCI38084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sato N., Takasaka N., Yoshida M., Tsubouchi K., Minagawa S., Araya J. Metformin attenuates lung fibrosis development via NOX4 suppression. Respir Res. 2016;17(1):107. doi: 10.1186/s12931-016-0420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kheirollahi V., Wasnick R.M., Biasin V., Vazquez-Armendariz A.I., Chu X., Moiseenko A. Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nat Commun. 2019;10(1):2987. doi: 10.1038/s41467-019-10839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rangarajan S., Bone N.B., Zmijewska A.A., Jiang S., Park D.W., Bernard K. Metformin reverses established lung fibrosis in a bleomycin model. Nat Med. 2018;24(8):1121–1127. doi: 10.1038/s41591-018-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]