Editor—The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 continues to spread, infecting millions worldwide. Critically ill COVID-19 patients with profound respiratory failure often require tracheal intubation. To minimise peri-intubation healthcare worker infection risk from COVID-19, an additional protection barrier known as an aerosol box or intubation box was introduced. Although hospitals worldwide have used various prototypes of aerosol boxes, their effect on intubation remains unclear, with studies suggesting these barriers may hinder and potentially delay airway management.1 Initial reports raised concerns, such as restricted range of motion and increased intubation difficulty.2 The aim of this systematic review and meta-analysis was to evaluate the impact of an aerosol box on time to tracheal intubation (TTI). Other factors influencing TTI, such as skill level (experienced proceduralists [consultants] vs less experienced proceduralists [residents]) and type of laryngoscope used, were also analysed.
This review was reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses framework and was registered on the International Prospective Register of Systematic Reviews (CRD42020220378). Studies evaluating the impact of aerosol box use on TTI were included. Two authors independently searched the COVID-19 living systematic review from January 1, 2020 to November 10, 2020, using the search terms ‘barrier’, ‘box’, ‘intubate’, or ‘intubation’. Statistical analyses were performed using Review Manager 5.4 (Cochrane Collaboration). Comparisons between aerosol box and no aerosol box were analysed using mean difference (MD). An estimation formula was used to convert median values to mean values with standard deviation to facilitate statistical analyses.3 The Cochrane risk-of-bias tool for randomised trials was used.
A total of 54 studies were identified, with 40 studies selected for full-text review (Supplementary Fig 1). Twelve studies reporting on 351 proceduralists were included (Supplementary Table 1). Supplementary table 2 outlines the risk of bias assessment for the selected studies. Supplementary Table 3 summarises the characteristics of selected studies.
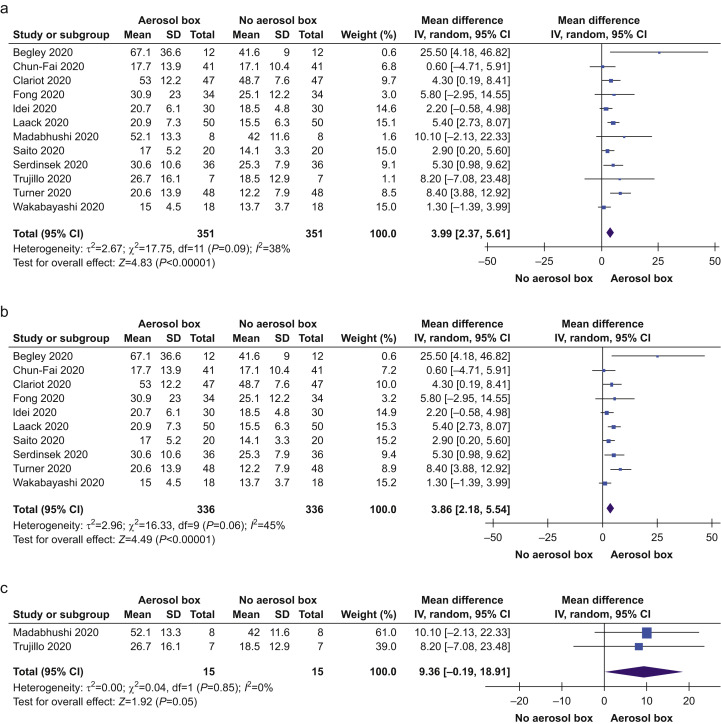
All studies provided data on TTI with and without an aerosol box (Supplementary Table 3). Eight studies reported statistically significant (P<0.05) increases in TTI with an aerosol box. Fig. 1 demonstrates the impact of aerosol boxes during intubation. TTI was longer when an aerosol box was used (MD=4.0 s; 95% confidence interval [CI]: 2.4–5.6; P<0.001). Heterogeneity was moderate (I 2=38%). Amongst 10 studies, where TTI was measured using manikins, TTI was longer with an aerosol box (MD=3.9 s; 95% CI: 2.2–5.5; P<0.001; I 2=45%). In two studies, where TTI was measured using patients, the observed TTI was more than double that of when manikins were used, however, not statistically significant (MD=9.4 s; 95% CI: –0.2 to 18.9; P=0.05; I 2=0%).
Fig 1.
Primary outcome: time to intubation: (a) all selected studies, (b) studies using simulations and manikins, and (c) amongst patients. CI, confidence interval; sd, standard deviation; IV, Inverse Variance.
Amongst 136 residents in six studies, the MD in TTI reported remained similar to the overall TTI reported in the primary outcome (Supplementary Fig 2a; MD=4.0 s; 95% CI: 2.1–5.9; P<0.001; I 2=21%). However, amongst 159 consultants, TTI with the aerosol box was comparatively lower (Supplementary Fig 2b; MD=2.6 s; 95% CI: 0.8–4.5; P=0.005; I 2=20%) than that of residents.
Ten studies reported on TTI using videolaryngoscopy. In these studies, TTI with an aerosol box was significantly longer compared with intubation without an aerosol box (Supplementary Fig 2c; MD=3.7 s; 95% CI: 1.7–5.7; P=0.0004; I 2=54%). Intubation with direct laryngoscopy yielded longer TTI compared with videolaryngoscopy (Supplementary Fig 2d; MD=4.5 s; 95% CI: 2.4–6.6; P<0.001; I 2=45%). Where consultants performed the intubation with a videolaryngoscope, an increase in TTI was not statistically significant (Supplementary Fig 2e; MD=1.9 s; 95% CI: –0.04 to 3.8; P=0.06; I 2=26%). In a post hoc analysis, first-pass success was significantly lower amongst intubations with an aerosol box (482/533; 90.4%; 95% CI: 87.6–92.8%) than without an aerosol box (499/521; 95.8%; 95% CI: 93.7–97.3%). Personal protective equipment (PPE) breaches were reported in three studies, where breaches were significantly more common when an aerosol box was used (19/58; 32.8%; 95% CI: 21.0–46.3%) than when no aerosol box was used (0/46; 0.0%; 95% CI: 0.0–7.7%).
We observed a significant increase in TTI when an aerosol box was used. Multiple factors, such as increased procedural difficulty, lack of experience, and cognitive overload for the proceduralist, may prolong TTI.4 , 5 Although a mean delay of 4 s may seem negligible in the overall intubation time sequence, it is important to consider that simulated studies do not fully capture the influence human factors can play in real-life situations. This prolonged TTI should be considered in the context of critically ill patients with COVID-19, where risk of hypoxaemia can be higher, underpinning the importance of minimising apnoea time amongst these patients.6 , 7 The finding of relatively shorter TTI by consultants supports the recommendation that the most experienced physician should be involved with intubating suspected or confirmed COVID-19 patients.8
A meaningful interpretation of these findings necessitates a careful risk–benefit analysis from both patient and proceduralist perspectives. On the one hand, the use of an aerosol box may prolong TTI to a variable degree based on proceduralist experience, exposing patients to a risk of hypoxaemia.4 On the other hand, damage to conventional PPE when using the box potentially increases aerosol exposure, placing the proceduralist and others assisting in airway management at risk of infection.9 Hence, with the current available evidence, it is important that proceduralists need to consider with caution the ongoing use of aerosol boxes when its use is delaying TTI without improving safety for healthcare professionals.
The limitations of this systematic review include the use of manikins to simulate intubation in most studies, variability in the definition for TTI between studies, and lack of evaluation of clinical or patient-centred outcomes.
In conclusion, TTI when an aerosol box was used was significantly longer compared with intubation without an aerosol box. TTI was relatively shorter when intubation was performed by more experienced proceduralists using videolaryngoscopy. These findings should be interpreted in the context of increased infection risks to the proceduralist and other healthcare workers assisting with airway management.
Declarations of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
The authors thank Nejc Umek, Praneeth Madabhushi, and Alexander Trujillo for providing additional information that made this systematic review and meta-analysis possible.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.11.036.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sorbello M., Rosenblatt W., Hofmeyr R., Greif R., Urdaneta F. Aerosol boxes and barrier enclosures for airway management in COVID-19 patients: a scoping review and narrative synthesis. Br J Anaesth. 2020;125:880–894. doi: 10.1016/j.bja.2020.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gould C.L., Alexander P.D.G., Allen C.N., McGrath B.A., Shelton C.L. Protecting staff and patients during airway management in the COVID-19 pandemic: are intubation boxes safe? Br J Anaesth. 2020;125:e292–e293. doi: 10.1016/j.bja.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong Kong Baptist University Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range 2020. http://www.math.hkbu.edu.hk/∼tongt/papers/median2mean.html Available from: [DOI] [PMC free article] [PubMed]
- 4.Begley J.L., Lavery K.E., Nickson C.P., Brewster D.J. The aerosol box for intubation in coronavirus disease 2019 patients: an in-situ simulation crossover study. Anaesthesia. 2020;75:1014–1021. doi: 10.1111/anae.15115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fong S., Li E., Violato E., Reid A., Gu Y. Impact of aerosol box on intubation during COVID-19: a simulation study of normal and difficult airways. Can J Anaesth. 2020:1–9. doi: 10.1007/s12630-020-01825-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao W., Wang T., Jiang B. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: lessons learnt and international expert recommendations. Br J Anaesth. 2020;125:e28–37. doi: 10.1016/j.bja.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kearsley R. Intubation boxes for managing the airway in patients with COVID-19. Anaesthesia. 2020;75:969. doi: 10.1111/anae.15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson J.P., Wong D.N., Verco L., Carter R., Dzidowski M., Chan P.Y. Measurement of airborne particle exposure during simulated tracheal intubation using various proposed aerosol containment devices during the COVID-19 pandemic. Anaesthesia. 2020;75:1587–1595. doi: 10.1111/anae.15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.