Abstract
Background
Exposure, risks and immunity of healthcare workers (HCWs), a vital resource during the SARS-CoV-2 pandemic, warrant special attention.
Methods
HCWs at Helsinki University Hospital, Finland, filled in questionnaires and provided serum samples for SARS-CoV-2-specific antibody screening by Euroimmun IgG assay in March–April 2020. Positive/equivocal findings were confirmed by Abbott and microneutralization tests. Positivity by two of the three assays or RT-PCR indicated a Covid-19 case (CoV+).
Results
The rate of CoV(+) was 3.3% (36/1095) and seropositivity 3.0% (33/1095). CoV(+) was associated with contact with a known Covid-19 case, and working on a Covid-19-dedicated ward or one with cases among staff. The rate in the Covid-19-dedicated ICU was negligible. Smoking and age <55 years were associated with decreased risk. CoV(+) was strongly associated with ageusia, anosmia, myalgia, fatigue, fever, and chest pressure. Seropositivity was recorded for 89.3% of those with prior documented RT-PCR-positivity and 2.4% of those RT-PCR-negative. The rate of previously unidentified cases was 0.7% (8/1067) and asymptomatic ones 0% (0/36).
Conclusion
Undiagnosed and asymptomatic cases among HCWs proved rare. An increased risk was associated with Covid-19-dedicated wards. Particularly high rates were seen for wards with liberal HCW-HCW contacts, highlighting the importance of social distancing also among HCWs.
Keywords: Healthcare staff, HCW, SARS-CoV-2, Covid-19, Antibody response, Neutralizing antibodies
Abbreviations
- CoV(+)
a Covid-19 case
- CoV(−)
a non-Covid-19 case
- HCW
healthcare worker
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- Covid-19
Coronavirus disease 2019
1. Introduction
The upsurge of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1,2] poses a massive challenge to healthcare systems worldwide: by 21 November, 2020 the number of confirmed cases exceeded 57 million, with over 1 373 695 deaths reported [3]. Large-scale RT-PCR testing, preventive measures at hospitals and in society, mask wearing, isolation of positive cases, contact tracing, and quarantine for those exposed have been suggested as effective means of containing the epidemic [[4], [5], [6], [7], [8]]. As the virus is mainly transmitted from person to person, healthcare workers (HCWs) frequently exposed to Covid-19 patients constitute a vulnerable part of the workforce [9], and, if infected, may pose a risk to patients and other members of staff.
In Finland, the first Covid19-positive patient was a Chinese traveller from Wuhan diagnosed on 29 January 2020 [10]. As of 27 February, new cases occurred among travellers returning from Central and Southern Europe, most of them identified in the capital region, i.e. our hospital district. The epidemic peaked around the turn of March and April, after which a gradual decline was seen [11]. Investigations carried out by the Finnish Institute for Health and Welfare (THL), report that on weeks 17–19 the national incidence was 26.2 per 100 000 inhabitants [11], and according to a serological population study conducted since March, in the surrounding hospital district of Helsinki and Uusimaa the concomitant seroprevalence by MNT was 0.5% [12].
Several studies among HCWs have shown that while asymptomatic infections do occur [[13], [14], [15], [16], [17], [18], [19], [20], [21]], symptomatic cases have stronger potential to transmit the virus [22].
Most patients infected with SARS-CoV-2 develop antibodies against virus-specific proteins [23]. Antibodies are considered one of the key elements for protection against re-infections: some antibodies targeting the receptor-binding and N terminal domains of the spike protein can neutralize the virus [24]. Testing such antibodies may present a useful tool for identification of those recovered from Covid-19 and presumably at reduced risk of reinfection. Reports from various countries show differing seroprevalences among HCWs, with a rate of 9.3% recorded in Spain [13], 1.6% and 2.7% in Germany [20,25], 11.2% and 24.4% in the UK [22,26], 7.4% in Italy [27], 4.0% in Denmark [16], 7.6% and 13.7% in the USA [21,28], and 23.0% and 19.1% in Sweden [17,19]; The data are from studies mostly conducted in April–May 2020. Further understanding of factors associated with HCWs’ infection risk is needed. Here, we set out to study the SARS-CoV-2 serological response by a two-tiered testing protocol including a neutralization test, and to explore related exposure and clinical data among HCWs.
2. METHODS
2.1. Study design and data collection
To obtain data on prevalence of identified and unidentified SARS-CoV-2 infections, factors increasing/decreasing transmission risk, and antibody response among HCWs, we recruited HCWs on selected wards (part of them with known SARS-CoV-2 exposure, others with none identified) at Helsinki University Hospital (HUH), Finland. On 22 April 2020, a total of 1737 HCWs in selected working areas were invited to fill in a web-based questionnaire (accessible until 15 May) and provide one or more blood samples. The study protocol was approved by the Ethics Committee of HUH.
2.2. Covid-19 at HUH
HUH provides secondary and tertiary care for the 1.7 million population of Helsinki and Uusimaa region in Finland; there are 2805 beds, 559167 ED visits and 26536 members of staff (43.9% nurses/practical nurses and 12.9% physicians). In this article, ‘nurses' refer to registered nurses with a bachelor's degree and ‘practical nurses’ to professionals with vocational training in nursing.
The first Covid-19 patient was diagnosed 26 February, the number amounting to 4129 by 22 April, the date of invitation, with 527 laboratory-confirmed admitted patients, and 142 intensive care unit (ICU) periods. At the onset of the pandemic, limited laboratory capacity did not allow testing all members of staff with symptoms. Instead, they were advised to stay at home for 14 days from symptom onset. After 14 March, those with symptoms were all tested by PCR. On the ordinary wards, respirators were replaced by surgical masks in mid-March; both were worn together with face shield or safety eyewear and water-resistant gowns in all close contacts with risk patients. In ICUs or during aerosol-generating procedures, respirators were worn all the time. No shortage of protective equipment was reported over the study period, the brands varied, though.
2.3. Volunteers, sampling and questionnaires
We invited all HCWs (symptomatic and asymptomatic) from selected working areas at HUH. These comprised two emergency departments; Covid-19-dedicated units (two ICUs and four infectious diseases/pulmonary/new cohort wards); units with no such patients (one ICU and two oncological wards); units with no such patients but with known HCW-HCW exposure among staff (later wards A with 25 and B with 32 exposed HCWs; the two index cases on these two wards were not included in the study). For inclusion, a serum sample was required and an online questionnaire was to be filled in. Those with equivocal antibody result by the first serological assay (Euroimmun) were asked to provide a new sample two weeks later. The results of previous SARS-CoV-2 RT-PCR tests, if taken, were retrieved from the laboratory database.
The questionnaires covered background data on demographics, working area, profession, history of Covid-19 and exposures to SARS-CoV-2, symptoms, etc. (Supplementary Table 1, Supplementary Table 2).
2.4. Serological methods
We used three serological assays. All samples were screened by SARS-CoV-2 IgG ELISA (Euroimmun, Lübeck, Germany) with EUROLabworkstation (Euroimmun), the assay we previously reported to have a specificity of 87% and sensitivity of 71% [29]. For positive and equivocal samples, a further analysis was conducted by both an automated chemiluminescent microparticle immunoassay (CMIA) for SARS-CoV-2 IgG (Abbott, Illinois, USA) with Architect Plus i2000sr Analyzer (Abbott) and a microneutralization (MNT) test with SARS-COV Fin-1 strain on Vero E6 cells essentially as described previously [10]. The Abbot assay proved to present 95% specificity and 80% sensitivity in our use [29]. Both the Euroimmun and Abbott assays were conducted according to manufacturers’ instructions (Euroimmun; Abbott). In the MNT, the sera were titrated to endpoint starting from the dilution of 1:20 in duplicates. Titres 1:20 and above were considered positive.
As an internal control to examine the success of the Euroimmun test in screening, we tested 216 first samples, regardless of result, also by MNT (data not shown).
2.5. Definitions and categorizations
Serology was considered as positive if the results were positive by two of the three assays: Euroimmun (positive/equivocal in primary screening), Abbott and MNT (positive in confirming secondary tests).
A confirmed Covid-19 case, CoV(+), was defined as one with positive serology or a recorded positive SARS-CoV-2 RT-PCR result. An asymptomatic case was defined as one recorded as CoV(+) but with no symptoms.
The working areas were categorized by potential exposure to SARS-CoV-2 as follows: 1) Covid-19-dedicated ICU; 2) regular ICU (no patients with suspected/confirmed Covid-19); 3) Covid-19-dedicated ward; 4) non-Covid-19 ward; 5) ward A: non-Covid-19-dedicated ward but case(s) identified among members of staff not having socialized outside working hours; 6) ward B: non-Covid-19 ward but case(s) identified among staff and members known to have attended a common get-together; 7) emergency department. For analyses of the impact of working area, only nurses, practical nurses, and physicians were selected.
2.6. Statistical analyses
SPSS v. 22.0 (IBM Corp., Armonk, NY) was used for all statistical analyses, for categorical variables the χ2-test, Fisher's exact test or binary logistic regression, as appropriate, and for continuous variables, Mann-Whitney U test.
3. RESULTS
3.1. Study population and background data
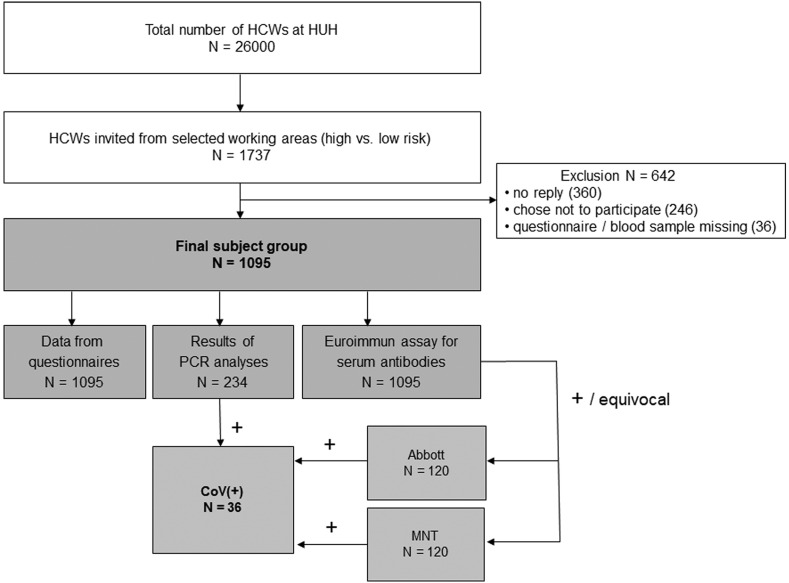
Of the 1737 HCWs initially invited by the HUH occupational healthcare, 1131 (65.1%) gave an informed consent. Blood samples were missing for 34 and two did not fill in the questionnaire (Fig. 1 ). The final study population comprised 1095 HCWs, 895 (82.7%) females and 187 (17.3%) males, and the median age was 38 years (IQR 31–48). Of all, 23.0% were physicians, 58.5% nurses, 5.1% practical nurses, 4.2% clerks, and 4.5% ward domestics (Supplementary Table 1). Of the respondents, 28 (2.8%) had tested positive for SARS-CoV-2 by RT-PCR before recruitment, 206 (18.8%) had tested negative, and 861 (78.6%) had not taken the test. In total, we identified 63 HCW-HCW exposures and 12 Covid-19 patient-HCW exposures (data not shown).
Fig. 1.
Flow chart of study conduct. Healthcare workers (HCWs) were invited to participate in the study by an email sent by the occupational healthcare of Helsinki University Hospital (HUH).
3.2. Serology
A positive SARS-CoV-2 serology (positive result by two of the three assays, Euroimmun, Abbott and MNT) was recorded for 33/1095 (3.0%) HCWs. An initial positive result was obtained for 73/1095 (6.7%) by Euroimmun; of these, 32 (43.8%) were also positive by the Abbott test, and 29 (39.7%) had neutralizing antibodies. In addition, one sample equivocal with Euroimmun yet positive by Abbott and MNT was considered seropositive. Three previously RT-PCR-positive cases proved seronegative: two had negative results and one positive using the Euroimmun assay, the latter tested negative by Abbott and MNT.
When scrutinizing the three RT-PCR-positive/seronegative cases, the following was observed: In the case negative by Euroimmun and MNT but positive by Abbott, the serum sample was taken 12 days after RT-PCR positivity. However, follow-up serum samples taken five weeks after the PCR-positive result tested positive by both Euroimmun and Abbott. In addition, one patient with positive PCR 11 days earlier was negative by both Euroimmun and Abbott but a follow-up sample taken 79 days later tested positive by both assays. Moreover, one case found RT-PCR-positive 46 days earlier proved positive only by Euroimmun but not by Abbott and MNT and was thus judged as seronegative; no follow-up sample was available.
Of CoV(+) cases, 30/36 (83.3%) proved positive by MNT. Of the 33 seropositives, 25 (75.8%) had previously been tested positive and five (15.2%) negative by RT-PCR; three (9.1%) had not been tested. Of the 28 RT-PCR-positive HCWs, 25 (89.3%) had positive serology. In total, 8/1067 (0.7%) can be considered new diagnoses: Among the 206 RT-PCR-negatives, five had positive serology (2.4%) despite being tested negative at the time of symptoms. Among the 861 with no record of RT-PCR testing, three (0.3%) were seropositive. Of these three, one had merely reported headache and myalgia, one was febrile with rhinorrhoea, and the third had a sore throat, rhinorrhoea, fatigue, and breathlessness.
Analysis of the 216 first consecutive samples showed for Euroimmun 92.9% sensitivity and 76.1% specificity in comparison with the MNT assay (data not shown).
3.3. Characteristics of CoV(+) cases
A total of 3.3% (36/1095) were considered to have had a Covid-19 infection (at least two of the three antibody assays positive or recorded positive RT-PCR), with no gender differences. CoV(+) status was more common among those aged 55 years or older (6.8%) than the younger (2.9%; p = 0.022; OR 2.4, 95% CI 1.1–5.2). None of the underlying illnesses reported by 34.0% of the participants were associated with CoV(+).
3.4. Potential risk factors
3.4.1. Professional group and working areas, occupational/other exposure
Nearly all CoV(+) participants reported a known contact with another laboratory-confirmed case. CoV(+) rates were not found to differ significantly between the various professional groups (Supplementary Table 1). Among physicians, nurses, and practical nurses differences were associated with working area: the CoV(+) rates among those working on Covid-19-dedicated wards were higher in non-ICUs than ICUs (9.1% vs. 0.9%; p = 0.002, OR 11.0, 95% CI 1.3–89.7).
In addition to patient-HCW transmission, an increased risk was also associated with HCW-HCW contacts outside working hours: higher rates were recorded for ward B (Covid-19 cases among staff plus get-together) but not for ward A (cases among staff but no gatherings) (22.6% vs. 0%). Ward B showed the highest rates of all in the study (Table 1 ). HCW-HCW contacts accounted for most transmissions: HCWs on Ward B constituted 55.9% of all cases.
Table 1.
Factors associated with CoV(+) (SARS-CoV-2 infection) among 1095 HCWs.
| Total |
CoV(+) (%) |
CoV(−) (%) |
p-value |
OR (95% CI) |
|
|---|---|---|---|---|---|
| Total | 1095 | 36 (100) | 1059 (100) | ||
| Working area (only registered nurses, practical nurses, physiciansa) | |||||
| Covid-19 ICU | 111 (11.7) | 1 (2.9) | 110 (12.0) | 1.0 | |
| Other ICU | 245 (25.8) | 0 (0) | 245 (26.8) | 0.995 | N/A |
| Covid-19 ward | 88 (9.3) | 8 (23.5) | 80 (8.7) | 0.025 | 11.0 (1.3–89.7) |
| Non-Covid-19 ward Ab with case(s) among staff | 23 (2.4) | 0 (0) | 23 (2.5) | 0.998 | N/A |
| Non-Covid-19 ward Bc with case(s) among staff | 84 (8.9) | 19 (55.9) | 65 (7.1) | 0.001 | 32.2 (4.2–245.8) |
| Other non-Covid-19 ward | 178 (18.8) | 1 (2.9) | 177 (19.3) | 0.738 | 0.6 (0.04–10.0) |
| Emergency department | 206 (21.7) | 5 (14.7) | 201 (22.0) | 0.351 | 2.7 (0.3–23.7) |
| Other working area | 14 (1.5) | 0 (0) | 14 (1.5) | 0.998 | N/A |
| Known contacts with Covid-19 patientsd | |||||
| Treated Covid-19 patients | 653 (60.0) | 12 (35.3) | 641 (60.8) | 0.003 | 0.4 (0.2–0.7) |
| Treated Covid-19 patient(s) without adequate protection | 52 (4.8) | 5 (14.7) | 30 (2.9) | 0.020 | 3.7 (1.4–10.0) |
| Treated patients without known Covid-19 | 497 (45.7) | 18 (52.9) | 479 (45.4) | 0.388 | 1.4 (0.7–2.7) |
| Covid-19 ward, no patient contact | 182 (16.7) | 1 (2.9) | 282 (17.2) | 0.029 | 0.1 (0.02–1.1) |
| Other working areas, no patient contact | 122 (11.2) | 2 (5.9) | 120 (11.4) | 0.317 | 0.5 (0.1–2.1) |
| Contact with persons with Covid-19/suspicion of Covid-19/travel abroad | |||||
| -No known contact | 573 (52.3) | 7 (19.4) | 566 (53.4) | 1.0 | |
| -Contact with a person with Covid-19 suspicion or travel abroad | 195 (17.8) | 2 (5.6) | 193 (18.2) | 0.826 | 0.8 (0.2–4.1) |
| -Contact with a confirmed Covid-19 case | 327 (29.8) | 27 (75.0) | 300 (28.3) | <0.001 | 7.3 (3.1–16.9) |
| Isolation | |||||
| -None | 850 (79.2) | 13 (3.1) | 837 (80.7) | 1.0 | |
| -Respondent in quarantine | 167 (15.5) | 12 (33.3) | 155 (14.9) | <0.001 | 5.0 (2.2–11.1) |
| -Household contact in quarantine | 35 (3.3) | 1 (2.8) | 34 (3.3) | 0.544 | 1.9 (0.2–14.9) |
| -Respondent plus family member in quarantine | 12 (1.1) | 1 (2.8) | 11 (1.1) | 0.102 | 5.9 (0.7–48.7) |
| - Covid-19-positive household member | 9 (0.8) | 9 (25.0) | 0 (0) | 0.998 | N/A |
| Used public transportation | 532 (49.5) | 18 (50.0) | 514 (49.6) | 0.959 | 1.0 (0.5–2.0) |
| Travel abroade | |||||
| -No | 728 (66.5) | 24 (66.7) | 704 (66.5) | 1.0 | |
| -Yes | 367 (33.5) | 12 (33.3) | 355 (33.5) | 0.981 | 1.0 (0.5–2.0) |
| Household members | |||||
| No others | 270 (24.7) | 10 (27.8) | 260 (24.6) | 0.661 | 1.0/0.8 (0.4–1.8) |
| One other adult | 658 (60.1) | 21 (58.3) | 637 (60.2) | 0.782 | 0.9 (0.4–1.9) |
| Two or more other adults | 113 (10.3) | 5 (13.9) | 108 (10.2) | 0.740 | 1.2 (0.4–3.6) |
| Children 10–18 years | 251 (22.9) | 15 (41.7) | 236 (22.3) | 0.230 | 1.7 (0.7–3.8) |
| Children 6–9 years | 168 (15.3) | 7 (19.4) | 161 (15.2) | 0.807 | 1.1 (0.4–3.0) |
| Children 0–5 years | 178 (16.2) | 0 (0) | 178 (16.8) | 0.987 | N/A |
Total number of CoV (+) = 34 and CoV(−) = 915.
No socializing outside working hours.
Socializing outside working hours.
Missing data 17.
No significant differences were seen between the various regions as destinations.
We found no association between number of daily contacts outside workplace and risk of CoV(+) (data not shown).
3.4.2. History of respiratory tract infections and upper respiratory tract surgical interventions
CoV(+) cases were not found more common among those with a history of frequent upper respiratory tract infections, pneumonia in lifetime or upper respiratory tract surgical interventions (such as tympanostomy or adenoidectomy in childhood).
3.4.3. Smoking status and vitamin D supplementation
None of the 36 who tested CoV(+) were current smokers (smoking at least once a week); 31.4% were ex-smokers, and 60.0% had never smoked. Current smoking was associated with lower rates of CoV(+) (p = 0.027). Likewise, smokeless tobacco products were used by 3.2%, none of these CoV(+) (Supplementary Table 1).
In our data, supplementation with vitamin D, regardless of dose, did not provide protection against Covid-19 (Supplementary Table 1).
3.5. Symptoms
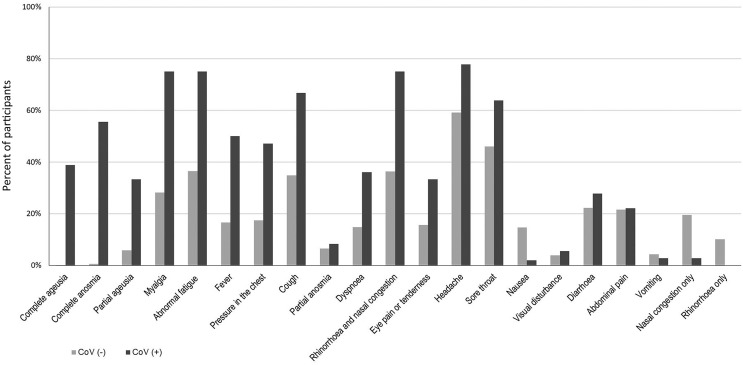
The great majority of our Covid-19 cases (35/36; 97.2%) were mild; only one (2.8%) was hospitalized and treated in ICU. Some of the symptoms proved significantly more common in the CoV(+) (n = 36) than the CoV(−) (n = 1059) group (Supplementary Table 2, Fig. 2 ). These included complete anosmia and ageusia (55.6% versus 0.6% and 38.9% versus 0.2%); myalgia (75.0% versus 28.2%); fatigue (75.0% versus 36.5%); fever (50.0% versus 16.7%); pressure in the chest (47.2% versus 17.4%); cough (66.7% versus 34.8%); and dyspnoea (36.1% versus 14.9%). No association was found between CoV(+) and gastrointestinal symptoms.
Fig. 2.
Proportion of healthcare workers with given symptoms in groups considered as Covid-19 positive (CoV+; black) and Covid-19 negative cases (CoV-; gray). Symptoms are listed by the order of decreasing OR (for more detailed information, see Supplementary Table 2.
4. Discussion
Combining positive serology (3.0%) and recorded positive RT-PCR (2.8%) for our HCWs, the proportion of Covid-19 cases added up to 3.3%. This percentage appears small compared to HCW studies reporting rates between 1.6% and 44% [13,14,16,17,19,20,22,[25], [26], [27], [28]], presumably reflecting epidemiological situation, availability of personal protective equipment, and differences in diagnostic methods used. According with increased risk reported for HCWs [16,[18], [19], [20],22,30], our rate exceeded sixfold the seroprevalence of 0.5% as confirmed by MNT among the general population in our hospital district [12].
4.1. Newly diagnosed cases
Interestingly, eight (22.9%) of those found CoV(+) had not been diagnosed earlier; five (14.3%) had tested negative and three (8.6%) had not taken any tests (each reported only minor symptoms). Increasing evidence suggests limited clinical sensitivity of RT-PCR for SARS-CoV-2 testing [31]. Possible false negative PCR results are, of course, a great concern. Our five PCR-negative CoV(+) cases may not all have been true false negatives, as the patients may have contracted the disease on another occasion, or the negative result could be attributed to late sampling [32]. However, if serology was taken as a reference and all these were interpreted as false negatives, it is evident that the rate remained low: at the maximum only 2.4% (5/206) of all PCR tests.
4.2. Asymptomatic cases
While some studies report asymptomatic/presymptomatic transmission to account for half of all Covid-19 infections [33], we found no truly asymptomatic cases: all our participants had Covid-19 symptoms; three cases were so mild that the HCW saw no need to take a test. Indeed, in investigations looking at seropositive HCWs the proportion of those showing no symptoms varies considerably, from 9.0% up to 64% [13,14,16,17,[19], [20], [21]]. On the other hand, studies carried out in Spain, Italy, and the UK among fully asymptomatic HCWs report PCR-positive rates not higher than 0.2–2.4% [18,22,26,[34], [35], [36]]. In our data, only the symptomatic HCWs were tested by RT-PCR, whereas our rates of those asymptomatic were based on seropositivity. The lack of asymptomatic infections may partly be attributed to the longish period (up to two months) covered in the questionnaires: the CoV(+) individuals may have experienced, besides an asymptomatic Covid-19 disease, some respiratory tract infection and solely report symptoms related to that. Our strict definition of asymptomatic disease – not even allowing atypical symptoms – may to some extent also explain the low rates. Indeed, in numerous studies as many as half of the “asymptomatic” report respiratory tract symptoms in questionnaires or in detailed interviews [18,22]. Furthermore, the rates reported may depend on the methods used: had we solely relied on the Euroimmun assay yielding the highest rates of seropositivity, our asymptomatic infection rate would have amounted to 17.8%. Moreover, as recent literature suggests that >90% of those infected develop antibody responses that persist for months [37], it is unlikely that we could have missed any significant number of CoV(+) cases since the blood samples were drawn within a few weeks after symptom onset.
Our negligible proportion of asymptomatic cases supports the policy of only testing HCWs having symptoms, not overlooking even mild ones, though. Indeed, in a study by Eyre et al. PCR-positive asymptomatic HCWs did not transmit the infection to their co-workers [22].
4.3. Symptoms of Covid-19
Overall, Covid-19 among our HCWs was in most cases not found severe: one (1/36; 2.8%) was hospitalized (ICU). According with many other studies [16,17,19,35,[38], [39], [40]], in our data anosmia (complete among CoV(+) versus CoV(−) 55.6% versus 0.6%; partial 8.3% versus 6.5%) and ageusia (complete 38.9% versus 0.2%; partial 33.3% versus 6.0%) were strongly associated with CoV(+). The proportion of CoV(+) HCWs with olfactory and/or taste disorders varies greatly between studies: in an investigation by Lan et al. 15.7% of the PCR-positive [41] and Lindahl et al. 12.0% of the seropositive [17] show olfactory/taste disorders, whereas Villareal et al. and Lombardi et al. present considerably higher rates ranging from 70.0 to 76.9% [35,40] that accord with our data, where the respective figure is 77.1% for olfactory or taste disorders. Increasing awareness of these symptoms being related to Covid-19 and their suggested association with milder disease may account for the rates differing [[42], [43], [44]]. Younger age may also explain some of the variation [44]. Unlike many previous studies [35,45,46] our data with mostly mild cases did not show significant differences between CoV(+) and CoV(−) HCWs in the prevalence of gastrointestinal symptoms. Indeed, gastrointestinal symptoms have been associated with severe clinical picture [46], while in non-severe cases the data are inconsistent [35,41].
4.4. Does smoking decrease risk of contracting SARS-CoV-2?
Remarkably, none of the participants in our CoV(+) group were current smokers. In the literature, the impact of smoking appears controversial [45,[47], [48], [49], [50], [51], [52]]. Despite several studies presenting lower rates of COVID-19 among smokers than non-smokers [49,52], the habit should by no means be advocated, as this potential benefit is definitely outweighed by its harmfulness [52].
4.5. Does treating Covid-19 patients involve increased risk for HCWs?
Higher risk of contracting SARS-CoV-2 is reported in several investigations both for HCWs treating [16,[18], [19], [20],22] and not treating Covid-19 patients [19,22], while many other researchers have found no such association [25,27,53]. In our data, of those working in ICUs caring for known/suspected COVID-19 patients, only one (0.9%) of the 111 HCWs was CoV(+), consistent with recent research among HCWs in ICUs [22,26,54]. Of our participants on non-ICU wards caring for known/suspected COVID-19 patients and wearing surgical masks 8 (9.1%) were positive. While the difference may simply be explained by varying quality of personal protection (respirators in ICU, surgical mask on ward), it may also be associated with degree of social distancing, since unlike HCWs on wards, those in ICUs mostly stay at their patients’ bedside, not in nursing areas together with coworkers. Since there was no shortage in supply of personal protective devices like respirators, our data serve particularly well to unravel the true occupational risks. What our results show is that in ICU settings protection and practices were adequate, whereas on Covid-19-dedicated non-ICU wards more cases were recorded.
Our highest CoV(+) rates were seen for those who worked in a unit without known COVID-19 patients but cases among staff who had a gathering after work. No such increase was seen on a ward with cases among staff but no socializing after work day. This result brings HCW-HCW transmission to the fore, demonstrating that attention should also be paid to social distancing outside working hours [54].
Indeed, contact tracing on our Covid-19 wards (data not shown) also suggest that although a HCW initially contracted the virus from a brief unprotected exposure when caring for a patient, the subsequent HCW-HCW transmissions demonstrate that socializing at work and during spare time may pose greater risk than interaction with patients.
Although our results do not associate number of household contacts (children or other adults) and risk of Cov(+), the data accord with previous studies [22,54] in showing significant association with CoV(+) cases at home, an obvious source of transmission.
4.6. Limitations and strengths
Our low Covid-19 rate – presumably ascribable to vigorous domestic lockdown measures – may have impacted the assessment of many of the risk factors. In fact, our rate should not be considered as an estimate of all employees, either since the working areas were not picked randomly but had been selected so as to cover ward, emergency departments, and ICU, either with or without Covid-19 patients and wards with known Covid-19 cases among the staff (the two index cases not included). However, this selection was designed to comprise wards of all kinds and thus allow rough comparisons between the wards.
One limitation concerns the analyses of symptoms. The questionnaire covered a period with high rates of respiratory tract infections of any kind. While this very time spans enables comparisons between symptoms of Covid-19 and other respiratory tract infections, some volunteers with CoV(+) may have had both and thus list symptoms of both. As this study was cross-sectional, a recall bias should be considered. However, since the first patient case at HUH was not recorded until late February, the numbers peaking in March–April, for most participants the recall period remained rather short (average 31 days).
One of our strengths was that the volunteers responded prudently to practically all questions; this may partly explain the low proportion of asymptomatic cases. It should be noted that our study probably differs from many others, since no shortage of personal protective devices was reported.
5. Conclusions
The CoV(+) rates among our HCWs exceeded the background level. A positive serology was seen for the majority of those with confirmed Covid-19, most of whom also had neutralizing antibodies. The low rates of possible false negative PCR results and lack of asymptomatic infections support active testing of HCWs with symptoms as the principal approach for identifying Covid-19 cases among HCWs. In our HCW data, a particularly high risk of contracting SARS-CoV-2 pertains to socializing with co-workers. In addition to demonstrating the importance of protective precautions when treating patients, our results highlight the necessity for social distancing between co-workers.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tmaid.2020.101949.
Funding
This work was supported by the Academy of Finland (grants 1336490, 336439 and 335527), the Juho Vainio Foundation, the Finnish Medical Foundation, private donors through UH, the Jane and Aatos Erkko Foundation, the EU Horizon 2020 programme VEO (grant 874735) and Helsinki University Hospital Funds (TYH 2018322). The funding sources were not involved in study design, data collection, data interpretation, writing of the manuscript, or the decision to submit the article for publication. The corresponding author had full access to all the study data and ultimate responsibility for the decision to submit for publication.
Authors’ contributions
Study concept and design AK, OV; acquisition of data AK, LK, SHP, KB, SM, AP, JV, RU, ML, AJ, SK, AJ, OV, TS; analysis and interpretation of results AK, TL, AJ, OV, TS; statistical analysis TL; literature search AK, TL, OV; drafting of manuscript AK, TL; critical comments: OV, TS; final approval of version published: all.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeng H., Deng S., Zhou Z., Qiu X., Jia X., Li Z. Diagnostic value of combined nucleic acid and antibody detection in suspected COVID-19 cases. Publ Health. 2020;186:1–5. doi: 10.1016/j.puhe.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ECDC. ECDC . 2020. COVID-19 situation update worldwide.https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases as of 21 November 2020. Accessed. Accessed 22 Nov 2020. [Google Scholar]
- 4.Ferretti L., Wymant C., Kendall M., Zhao L., Nurtay A., Abeler-Dörner L. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science. 2020;368 doi: 10.1126/science.abb6936. Epub 2020 Mar 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong L.X., Lin A., He Z.B., Zhao H.H., Zhang J.G., Zhang C. Mask wearing in pre-symptomatic patients prevents SARS-CoV-2 transmission: an epidemiological analysis. Trav Med Infect Dis. 2020;36:101803. doi: 10.1016/j.tmaid.2020.101803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang M., Gao L., Cheng C., Zhou Q., Uy J.P., Heiner K. Efficacy of face mask in preventing respiratory virus transmission: a systematic review and meta-analysis. Trav Med Infect Dis. 2020;36:101751. doi: 10.1016/j.tmaid.2020.101751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sousa-Pinto B., Fonseca J.A., Oliveira B., Cruz-Correia R., Rodrigues P.P., Costa-Pereira A. Simulation of the effects of COVID-19 testing rates on hospitalizations. Bull World Health Organ. 2020;98:299. doi: 10.2471/BLT.20.258186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. J Am Med Assoc. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 9.Kambhampati A.K., O'Halloran A.C., Whitaker M., Magill S.S., Chea N., Chai S.J. COVID-19-associated hospitalizations among health care personnel - COVID-NET, 13 states, March 1-May 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1576–1583. doi: 10.15585/mmwr.mm6943e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haveri A., Smura T., Kuivanen S., Österlund P., Hepojoki J., Ikonen N. Serological and molecular findings during SARS-CoV-2 infection: the first case study in Finland, January to February 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.11.2000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finnish Institute for Health and Welfare. Hybridistrategian seuranta – tilannearvioraportti, Accessed 15 October 2020.
- 12.Finnish Institute for Health and Welfare Koronaepidemian väestöserologiatutkimuksen viikkoraportti (in Finnish) https://www.thl.fi/roko/cov-vaestoserologia/sero_report_weekly.html Accessed.
- 13.Garcia-Basteiro A.L., Moncunill G., Tortajada M., Vidal M., Guinovart C., Jimenez A. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun. 2020;11:3500. doi: 10.1038/s41467-020-17318-x. 4020-17318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houlihan C.F., Vora N., Byrne T., Lewer D., Kelly G., Heaney J. Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health-care workers. Lancet. 2020;396:e6–e7. doi: 10.1016/S0140-6736(20)31484-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunter E., Price D.A., Murphy E., van der Loeff I.S., Baker K.F., Lendrem D. First experience of COVID-19 screening of health-care workers in England. Lancet. 2020;395:e77–e78. doi: 10.1016/S0140-6736(20)30970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iversen K., Bundgaard H., Hasselbalch R.B., Kristensen J.H., Nielsen P.B., Pries-Heje M. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindahl J.F., Hoffman T., Esmaeilzadeh M., Olsen B., Winter R., Amer S. High seroprevalence of SARS-CoV-2 in elderly care employees in Sweden. Infect Ecol Epidemiol. 2020;10:1789036. doi: 10.1080/20008686.2020.1789036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivett L., Sridhar S., Sparkes D., Routledge M., Jones N.K., Forrest S. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife. 2020;9 doi: 10.7554/eLife.58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudberg A.S., Havervall S., Manberg A., Jernbom Falk A., Aguilera K., Ng H. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020;11:5064. doi: 10.1038/s41467-020-18848-0. 020-18848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt S.B., Gruter L., Boltzmann M., Rollnik J.D. Prevalence of serum IgG antibodies against SARS-CoV-2 among clinic staff. PloS One. 2020;15 doi: 10.1371/journal.pone.0235417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stubblefield W.B., Talbot H.K., Feldstein L., Tenforde M.W., Rasheed M.A.U., Mills L. Seroprevalence of SARS-CoV-2 among frontline healthcare personnel during the first month of caring for COVID-19 patients - nashville, Tennessee. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eyre D.W., Lumley S.F., O'Donnell D., Campbell M., Sims E., Lawson E. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. Elife. 2020;9 doi: 10.7554/eLife.60675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel R., Theel E.S., Storch G.A., Pinsky B.A., St George K., Smith T.C. Report from the American society for microbiology COVID-19 international summit, 23 March 2020: value of diagnostic testing for SARS-CoV-2/COVID-19. mBio. 2020;11 doi: 10.1128/mBio.00722-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L., Wang P., Nair M.S., Yu J., Rapp M., Wang Q. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584:450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- 25.Korth J., Wilde B., Dolff S., Anastasiou O.E., Krawczyk A., Jahn M. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128:104437. doi: 10.1016/j.jcv.2020.104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shields A., Faustini S.E., Perez-Toledo M., Jossi S., Aldera E., Allen J.D. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020 doi: 10.1136/thoraxjnl-2020-215414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sotgiu G., Barassi A., Miozzo M., Saderi L., Piana A., Orfeo N. SARS-CoV-2 specific serological pattern in healthcare workers of an Italian COVID-19 forefront hospital. BMC Pulm Med. 2020;20:203. doi: 10.1186/s12890-020-01237-0. 020-01237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moscola J., Sembajwe G., Jarrett M., Farber B., Chang T., McGinn T. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York city area. J Am Med Assoc. 2020;324:893–895. doi: 10.1001/jama.2020.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jääskelainen A.J., Kuivanen S., Kekäläinen E., Ahava M.J., Loginov R., Kallio-Kokko H. Performance of six SARS-CoV-2 immunoassays in comparison with microneutralisation. J Clin Virol. 2020;129:104512. doi: 10.1016/j.jcv.2020.104512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen L.H., Drew D.A., Graham M.S., Joshi A.D., Guo C.G., Ma W. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020 doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woloshin S., Patel N., Kesselheim A.S. False negative tests for SARS-CoV-2 infection - challenges and implications. N Engl J Med. 2020;383:e38. doi: 10.1056/NEJMp2015897. [DOI] [PubMed] [Google Scholar]
- 32.Xiao A.T., Tong Y.X., Zhang S. Profile of RT-PCR for SARS-CoV-2: a preliminary study from 56 COVID-19 patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 34.Arriola-Villalobos P., Fernandez-Perez C., Arino-Gutierrez M., Fernandez-Vigo J.I., Benito-Pascual B., Cabello-Clotet N., Letter J. Response to article in journal of infection: "High SARS-CoV-2 antibody prevalence among healthcare workers exposed to COVID-19 patients. J Infect. 2020 doi: 10.1016/j.jinf.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lombardi A., Consonni D., Carugno M., Bozzi G., Mangioni D., Muscatello A. Characteristics of 1573 healthcare workers who underwent nasopharyngeal swab testing for SARS-CoV-2 in Milan, Lombardy, Italy. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olalla J., Correa A.M., Martin-Escalante M.D., Hortas M.L., Martin-Sendarrubias M.J., Fuentes V. Search for asymptomatic carriers of SARS-CoV-2 in healthcare workers during the pandemic: a Spanish experience. QJM. 2020 doi: 10.1093/qjmed/hcaa238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wajnberg A., Amanat F., Firpo A., Altman D.R., Bailey M.J., Mansour M. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020 doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makaronidis J., Mok J., Balogun N., Magee C.G., Omar R.Z., Carnemolla A. Seroprevalence of SARS-CoV-2 antibodies in people with an acute loss in their sense of smell and/or taste in a community-based population in London, UK: an observational cohort study. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tostmann A., Bradley J., Bousema T., Yiek W.K., Holwerda M., Bleeker-Rovers C. Strong associations and moderate predictive value of early symptoms for SARS-CoV-2 test positivity among healthcare workers, The Netherlands, March 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.16.2000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villarreal I.M., Morato M., Martinez-RuizCoello M., Navarro A., Garcia-Chilleron R., Ruiz A. Olfactory and taste disorders in healthcare workers with COVID-19 infection. Eur Arch Oto-Rhino-Laryngol. 2020 doi: 10.1007/s00405-020-06237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lan F.Y., Filler R., Mathew S., Buley J., Iliaki E., Bruno-Murtha L.A. COVID-19 symptoms predictive of healthcare workers' SARS-CoV-2 PCR results. PloS One. 2020;15 doi: 10.1371/journal.pone.0235460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Avci H., Karabulut B., Farasoglu A., Boldaz E., Evman M. Relationship between anosmia and hospitalisation in patients with coronavirus disease 2019: an otolaryngological perspective. J Laryngol Otol. 2020;134:710–716. doi: 10.1017/S0022215120001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siso-Almirall A., Kostov B., Mas-Heredia M., Vilanova-Rotllan S., Sequeira-Aymar E., Sans-Corrales M. Prognostic factors in Spanish COVID-19 patients: a case series from Barcelona. PloS One. 2020;15 doi: 10.1371/journal.pone.0237960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Bartheld C.S., Hagen M.M., Butowt R. Prevalence of chemosensory dysfunction in COVID-19 patients: a systematic review and meta-analysis reveals significant ethnic differences. ACS Chem Neurosci. 2020;11:2944–2961. doi: 10.1021/acschemneuro.0c00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brendish N.J., Poole S., Naidu V.V., Mansbridge C.T., Norton N., Borca F. Clinical characteristics, symptoms and outcomes of 1054 adults presenting to hospital with suspected COVID-19: a comparison of patients with and without SARS-CoV-2 infection. J Infect. 2020 doi: 10.1016/j.jinf.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mao R., Qiu Y., He J.S., Tan J.Y., Li X.H., Liang J. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gupta S., Hayek S.S., Wang W., Chan L., Mathews K.S., Melamed M.L. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ioannou G.N., Locke E., Green P., Berry K., O'Hare A.M., Shah J.A. Risk factors for hospitalization, mechanical ventilation, or death among 10131 US veterans with SARS-CoV-2 infection. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Lusignan S., Dorward J., Correa A., Jones N., Akinyemi O., Amirthalingam G. Risk factors for SARS-CoV-2 among patients in the oxford royal college of general practitioners research and surveillance centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meini S., Fortini A., Andreini R., Sechi L.A., Tascini C. Nicotine Tob Res; 2020. The paradox of the low prevalence of current smokers among covid-19 patients hospitalized in non-intensive care wards: results from an Italian multicenter case-control study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soares R.C.M., Mattos L.R., Raposo L.M. Risk factors for hospitalization and mortality due to COVID-19 in espirito santo state, Brazil. Am J Trop Med Hyg. 2020 doi: 10.4269/ajtmh.20-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simons D., Shahab L., Brown J., Perski O. The association of smoking status with SARS-CoV-2 infection, hospitalisation and mortality from COVID-19: a living rapid evidence review with Bayesian meta-analyses (version 7) Addiction. 2020 doi: 10.1111/add.15276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hunter B.R., Dbeibo L., Weaver C., Beeler C., Saysana M., Zimmerman M. Seroprevalence of SARS-CoV-2 antibodies among healthcare workers with differing levels of COVID-19 patient exposure. Infect Control Hosp Epidemiol. 2020:1–7. doi: 10.1017/ice.2020.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lentz R.J., Colt H., Chen H., Cordovilla R., Popevic S., Tahura S. Assessing coronavirus disease 2019 (COVID-19) transmission to healthcare personnel: the global ACT-HCP case-control study. Infect Control Hosp Epidemiol. 2020:1–7. doi: 10.1017/ice.2020.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.