Abstract
Background
Coronavirus disease 2019 (COVID-19) global pandemic has strikingly high mortality rate with hypercoagulability state being part of the imputed mechanisms. We aimed to compare the rates of in hospital mortality in propensity score matched cohorts of COVID-19 patients in chronic anticoagulation versus those that were not.
Methods
In this population-based study in the Veneto Region, we retrospectively reviewed all patients aged 65 years or older, with a laboratory-confirmed COVID-19 diagnosis. We compared, after propensity score matching, those who received chronic anticoagulation for atrial fibrillation with those who did not.
Results
Overall, 4697 COVID-19 patients fulfilled inclusion criteria, and the propensity score matching yielded 559 patients per arm. All-cause mortality rate ratio was significantly higher among non-anticoagulated patients (32.2% vs 26.5%, p = 0.036). On time to event analysis, all-cause mortality was found lower among anticoagulated patients, although the estimate was not statistically significant. (HR 0.81, 95%CI 0.65–1.01, p = 0.054).
Conclusion
Among elderly patients with COVID-19, those on chronic oral anticoagulant treatment for atrial fibrillation seem to be at lower risk of all-cause mortality compared to their propensity score matched non-anticoagulated counterpart. This finding needs to be confirmed in further studies.
Keywords: COVID-19, Anticoagulation, Mortality, Survival, Atrial fibrillation, VKA, DOAC
1. Introduction
Coronavirus disease 2019 (COVID-19) has become a global pandemic with a strikingly high mortality rate. The main disease's expression is in the respiratory tract. However, there is growing evidence that other organs and systems are involved, in particular the coagulation system may have a relevant pathogenic role [[1], [2], [3], [4], [5]]. There is growing body of evidence suggesting that hemostatic abnormalities resulting in a hypercoagulability state are part of the clinical picture in patients affected by COVID-19 [1,6]. The precise mechanism and extension of hypercoagulability of COVID-19 is poorly understood [7]. Postmortem examinations have identified both micro- and macro-embolism in COVID-19 casualties [8,9]. While increased D-dimer levels have been reported to be related with a high fatality rate [1,10] and prompt for anticoagulation, there are no sufficient data to back the routine use of empiric therapeutic anticoagulation among all patients with COVID-19. Available data are based on weak evidence mainly derived from small retrospective samples and lack of validation, even though a recent large retrospective study [11] reported that anticoagulation was associated with lower mortality and intubation among hospitalized COVID-19 patients.
Until data from rigorous trials on anticoagulation become available, collection of retrospective data of patients on oral anticoagulant treatment before hospitalization may offer a proof of concept towards the effect of anticoagulation on COVID-19 morbidity and mortality. Among patients on anticoagulant treatment, those with Atrial Fibrillation (AF) are at higher risk of death as they are older and with associated cardiovascular risk factors [12]. We performed a retrospective analysis of elderly patients with confirmed COVID-19 diagnosis, comparing outcomes among those who were receiving anticoagulation for AF and a propensity score matched group of patients who were not receiving anticoagulation.
2. Materials and methods
Patients were enrolled through the COVID-19 regional integrated surveillance system, containing data on all patients with a positive result on a reverse transcriptase polymerase chain reaction (RT-PCR). We included all residents in the Veneto Region (Region of the first COVID-19 outbreak in Italy), aged 65 years or older, with a diagnosis of COVID-19 in the period 23 February- 3 June 2020. The day of the diagnostic test, or index date, identified the date of enrollment in the cohort. Oral anticoagulation was searched through index prescription of VKAs (ATC B01AAxx) or NOACs (dabigatran B01AE07, rivaroxaban B01AF01, apixaban B01AF02) or edoxaban (B01AF03) at least 6 months prior to index date. Linkage with the regional inpatients register allowed the exclusion of patients with mechanical heart valves, diagnosed mitral stenosis, venous thromboembolism or other indications for anticoagulation [13].
Demographics were recorded at the time of enrollment in the cohort. By linkage of drug prescriptions, inpatients records, and co-payment exemptions, we identified patient comorbidities and drugs of interest. Comorbidities included diabetes, congestive heart failure, cerebrovascular disease, peripheral and carotid artery diseases, hypertension, myocardial infarction, coronary bypass graft and percutaneous coronary intervention, chronic renal disease, chronic liver disease, history of major bleeding, and diagnosis of cancer. Assessed drugs of interest included concomitant prescription of antiplatelet agents, non-steroidal anti-inflammatory drugs (NSAIDs), and antihypertensive drugs. Study outcomes were hospital admission, ICU admission and all-cause mortality.
All analyses were carried out on routinely collected health records submitted to an anonymization process allowing linkage of archives without any possibility of identification of individuals. There was no direct patient involvement in this study.
2.1. Statistical analysis
We assessed all-cause mortality in the anticoagulated (AC) AF patients and non-anticoagulated (non-AC) patients. To adjust for differences in baseline characteristics between the cohorts, propensity scores were calculated using a logistic regression model, adjusting for: age, sex, CHF, hypertension, cancer, diabetes, history of stroke/TIA, previous bleeding, history of myocardial infarction, peripheral artery disease, abnormal renal function, abnormal hepatic function, use of antiplatelet drugs, NSAIDs and statin use. AC patients were matched by propensity score to non- AC patients in a one-to-one ratio by means of greedy nearest neighbor matching without replacement within specified caliper (0.01). The risk ratio of COVID-related outcomes (hospitalization, intensive-care admission, all cause-mortality) was estimated in AC patients compared to the matched cohort of non-AC patients.
Furthermore, time-to-event analysis was performed for all-cause mortality and analyses were expressed as Kaplan-Meier curves, with significance indicated using a log-rank P value. Cox regression was used to compare event rates between treatment groups with results expressed as hazard ratios (HR) with 95% confidence intervals (CI).
All analyses were performed using SAS ver. 9.3 for data management and STATA ver.15.
3. Results
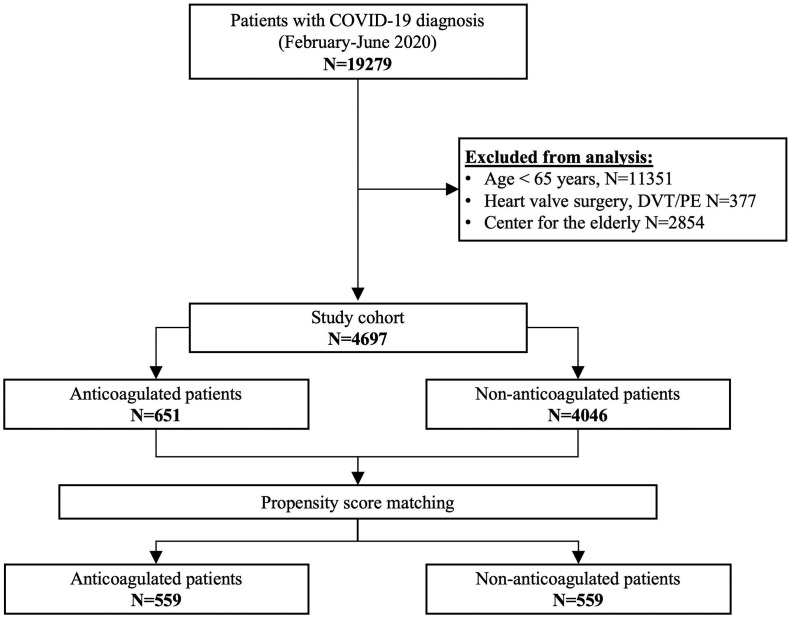
During the study period (23 February- 3 June 2020), 19279 patients were diagnosed COVID-19 infection. Of these, 14582 patients were excluded for: a) being below 65 years old; b) for heart valve surgery; c) for venous thromboembolism; d) for being confined in a retirement home (Fig. 1 ). The study population included 4697 patients: 651 AC patients and 4046 non-AC patients. Of the 651 AC patients, 269 were on VKAs, 138 on rivaroxaban, 116 on apixaban, 70 on edoxaban, and 58 on dabigatran. Baseline and clinical characteristics of the cohort are depicted in Table 1 . AC patients were older and had more comorbidities, including CHF, hypertension, diabetes, history of stroke/TIA, myocardial infarction, PAD, chronic kidney disease (Table 1). AC patients required more frequently hospital admission (68% vs 60.5%; p = 0.001). However, there were no significant differences in AC and non-AC patients admitted to ICU (respectively 8.8% vs 10.5%, p = 0.2). All-cause mortality was higher among AC patients (29% vs 21.4%, p = 0.001) mainly driven by higher in-hospital mortality (26.4% vs 18.8%, p = 0.001).
Fig. 1.
Study cohort.
Table 1.
Baseline demographics and clinical characteristics of anticoagulated and non-anticoagulated COVID-19 patients (propensity score 1:1 match).⁎
| All study subjects |
Propensity score-matched |
|||||
|---|---|---|---|---|---|---|
| Non-AC (n = 4046) | AC (n = 651) | P Value⁎⁎ | Non-AC (n = 559) | AC (n = 559) | P Value⁎ | |
| Gender | ||||||
| Male | 50.1% | 53.9% | 0.139 | 54.7% | 54.2% | 0.857 |
| Female | 49.2% | 46.1% | 45.3% | 45.8% | ||
| Age groups | ||||||
| 65–74 yrs | 41.1% | 17.4% | <0.001 | 17.2% | 19.3% | 0.559 |
| 75–84 yrs | 36.7% | 45.3% | 47.6% | 44.9% | ||
| ≥ 85 yrs | 22.2% | 37.3% | 35.2% | 35.8% | ||
| Comorbidities | ||||||
| Congestive heart failure | 3.4% | 25.3% | <0.001 | 16.3% | 16.6% | 0.873 |
| Hypertension | 60.0% | 89.4% | <0.001 | 86.8% | 87.7% | 0.654 |
| Stroke/TIA/systemic thromboembolism | 7.3% | 17.4% | <0.001 | 16.3% | 14.0% | 0.278 |
| Myocardial infarction | 2.2% | 5.1% | <0.001 | 4.7% | 4.5% | 0.886 |
| Peripheral artery disease | 1.4% | 3.4% | <0.001 | 2.1% | 2.7% | 0.559 |
| Diabetes | 18.8% | 26.4% | <0.001 | 24.5% | 23.6% | 0.726 |
| Cancer | 10.7% | 13.1% | 0.072 | 12.5% | 13.4% | 0.656 |
| Chronic renal disease | 3.7% | 11.7% | <0.001 | 8.8% | 8.4% | 0.831 |
| Chronic liver disease | 1.1% | 1.8% | 0.173 | 0.9% | 1.1% | 0.763 |
| History of bleeding | 3.2% | 3.8% | 0.386 | 3.9% | 3.8% | 0.876 |
| Medications | ||||||
| Aspirin | 21.0% | 10.9% | <0.001 | 13.2% | 11.6% | 0.415 |
| Clopidogrel | 1.8% | 1.8% | 0.909 | 1.4% | 2.0% | 0.488 |
| NSAIDs | 11.8% | 7.8% | 0.003 | 6.6% | 7.3% | 0.637 |
| Statin | 36.2% | 45.8% | <0.001 | 44.7% | 44.2% | 0.854 |
| Outcomes | ||||||
| Hospital admission | 60.5% | 68.0% | 0.001 | 64.9% | 65.7% | 0.802 |
| ICU admission | 10.5% | 8.8% | 0.178 | 8.2% | 8.8% | 0.748 |
| All-cause mortality | 21.4% | 29.0% | 0.001 | 32.2% | 26.5% | 0.036 |
| In hospital mortality | 18.8% | 26.4% | 0.001 | 28.3% | 24.2% | 0.118 |
| Out of hospital mortality | 2.6% | 2.6% | 0.981 | 3.9% | 2.3% | 0.112 |
Standardized difference for all variables included in the propensity score and all values were less than 0.10.
Chi Square P-Values.
After matching by propensity score, the two cohorts were comparable, with 559 patients in each arm (Table 1). Study outcomes were similar in the two cohorts with regard to hospital admission (risk ratio 1.01, 95%CI 0.92–1.10) and intensive care unit admission (risk ratio 1.06, 95%CI 0.72–1.56), whereas all-cause mortality was significantly lower among AC patients (26.5% vs. 32.2%; risk ratio 0.82, 95%CI 0.68–0.99).
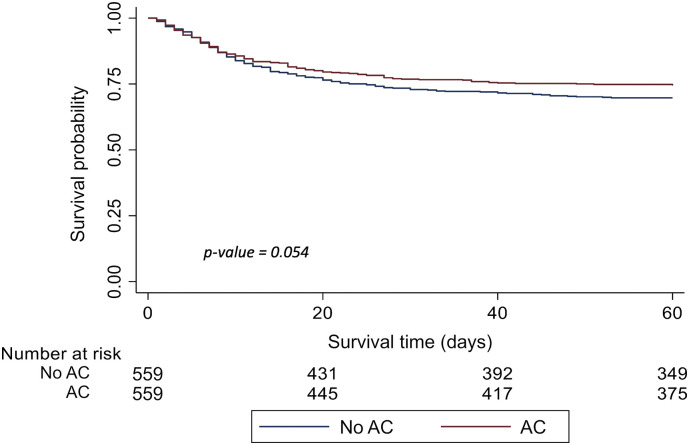
On time to event analysis, HR for all-cause mortality was lower among AC patients, despite missing statistical significance (HR 0.81, 95%CI 0.65–1.01, p = 0.054) (Fig. 2 ).
Fig. 2.
Time-to-event analysis on overall all-cause mortality of the cohort after propensity score matching (p-value calculated with the log-rank test).
4. Discussion
Our results suggest that among elderly patients with COVID-19, those on chronic oral anticoagulant treatment for AF seem to be at lower risk of all-cause mortality compared to their propensity score matched counterpart not on anticoagulant treatment. The two groups are similar for the presence cardiovascular risk factors but differ because the tested group was on oral anticoagulant treatment for stroke prevention in AF. Because AF is per se a risk factor for mortality in both general [12], and COVID-19 patients [14], our results show that chronic anticoagulation might have some role in reducing the mortality in this group of patients. Although some reports [15] show that acute phase anticoagulation may be beneficial in selected patients [1], the role of chronic anticoagulation was not found to be protective for COVID-19–related morbidity and mortality [16]. Our study differs from the study from Tremblay et al., [16] because of the more rigorous propensity score matching, including more factors, and the final number of included patients. The latter might have tipped the point towards a benefit of chronic anticoagulation on mortality of COVID-19 patients.
This study has some limitations. We do not have data on the anticoagulation dose patients received while in the intensive care unit. We cannot draw direct conclusions on the effect of acute phase anticoagulation, since probably all patients in both groups were switched to LMWH during hospitalization. Further, we did not assess the influence of in-hospital interventionswhich could have affected the outcomes. Despite the relevant number of factors included in the propensity score, we might have missed some factors that could impact mortality such as illness severity metrics. Strengths of our study include a very large population of COVID-19 patients, a consolidated methodology and a validated statistical analysis. Furthermore, due to the method of identification of anticoagulated patients, there is little chance of anticoagulation indication bias.
In conclusion, study outcomes were similar between the two groups. The tendency of a lower mortality in anticoagulated patients needs to be confirmed in further studies.
Author contribution
G. Denas, V. Pengo and Iliceto S conceived and designed the study, interpreted the data, and drafted the manuscript. N. Gennaro, E. Ferroni, U. Fedeli created the database, performed the statistical analysis, and contributed in the drafting of the manuscript. G. Lorenzoni and D. Gregori provided critical input to the interpretation of data. All authors critically revised the manuscript and gave their approval to the final version.
Author Agreement Form – International Journal of Cardiology
This statement is to certify that all authors have seen and approved the manuscript.
being submitted, have contributed significantly to the work, attest to the validity and legitimacy of the data and its interpretation, and agree to its submission to the International Journal of Cardiology.
We attest that the article is the Authors' original work, has not received prior publication and is not under consideration for publication elsewhere. We adhere to the statement of ethical publishing as appears in the International of Cardiology (citable as: Shewan LG, Rosano GMC, Henein MY, Coats AJS. A statement on ethical standards in publishing scientific articles in the International Journal of Cardiology family of journals. Int. J. Cardiol. 170 (2014) 253–254 DOI:https://doi.org/10.1016/j.ijcard.2013.11).
On behalf of all Co-Authors, the corresponding Author shall bear full responsibility for the submission. Any changes to the list of authors, including changes in order, additions or removals will require the submission of a new author agreement form approved and signed by all the original and added submitting authors.
All authors are requested to disclose any actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the submitted work that could inappropriately influence, or be perceived to influence, their work. If there are no conflicts of interest, the COI should read: “The authors report no relationships that could be construed as a conflict of interest”.
Funding
None.
Declaration of Competing Interest
None.
References
- 1.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M., Clark C., Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020 doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levi M., Thachil J., Iba T., Levy J.H. 2020. Coagulation Abnormalities and Thrombosis in Patients with COVID-19, Lancet Haematol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., Der Nigoghossian C., Ageno W., Madjid M., Guo Y., Tang L.V., Hu Y., Giri J., Cushman M., Quéré I., Dimakakos E.P., Gibson C.M., Lippi G., Favaloro E.J., Fareed J., Caprini J.A., Tafur A.J., Burton J.R., Francese D.P., Wang E.Y., Falanga A., McLintock C., Hunt B.J., Spyropoulos A.C., Barnes G.D., Eikelboom J.W., Weinberg I., Schulman S., Carrier M., Piazza G., Beckman J.A., Steg P.G., Stone G.W., Rosenkranz S., Goldhaber S.Z., Parikh S.A., Monreal M., Krumholz H.M., Konstantinides S.V., Weitz J.I., Lip G.Y.H. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J. Am. Coll. Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan B.E., Chong V.C.L., Chan S.S.W., Lim G.H., Lim K.G.E., Tan G.B., Mucheli S.S., Kuperan P., Ong K.H. Hematologic parameters in patients with COVID-19 infection. Am. J. Hematol. 2020 doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 7.Rico-Mesa J.S., Rosas D., Ahmadian-Tehrani A., White A., Anderson A.S., Chilton R. The role of anticoagulation in COVID-19-induced hypercoagulability. Curr. Cardiol. Rep. 2020 doi: 10.1007/s11886-020-01328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wichmann D., Sperhake J.-P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., Heinrich F., Mushumba H., Kniep I., Schröder A.S., Burdelski C., de Heer G., Nierhaus A., Frings D., Pfefferle S., Becker H., Bredereke-Wiedling H., de Weerth A., Paschen H.-R., Sheikhzadeh-Eggers S., Stang A., Schmiedel S., Bokemeyer C., Addo M.M., Aepfelbacher M., Püschel K., Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann. Intern. Med. 2020 doi: 10.7326/m20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolhnikoff M., Duarte-Neto A.N., Almeida Monteiro R.A., Silva L.F.F., Oliveira E.P., Saldiva P.H.N., Mauad T., Negri E.M. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J. Thromb. Haemost. 2020;18:1517–1519. doi: 10.1111/jth.14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C., Du B., Li L., Zeng G., Yuen K.Y., Chen R., Tang C., Wang T., Chen P., Xiang J., Li S., Wang J.L., Liang Z., Peng Y., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J., Chen Z., Li G., Zheng Z., Qiu S., Luo J., Ye C., Zhu S., Zhong N. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadkarni G.N., Lala A., Bagiella E., Chang H.L., Moreno P., Pujadas E., Arvind V., Bose S., Charney A.W., Chen M.D., Cordon-Cardo C., Dunn A.S., Farkouh M.E., Glicksberg B., Kia A., Kohli-Seth R., Levin M.A., Timsina P., Zhao S., Fayad Z.A., Fuster V. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: a single health system study. J. Am. Coll. Cardiol. 2020 doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denas G., Fedeli U., Gennaro N., Ferroni E., Corti M.C., Pengo V. Death rates and causes in anticoagulated atrial fibrillation patients. J. Cardiovasc. Med. 2020;21:415–419. doi: 10.2459/JCM.0000000000000987. [DOI] [PubMed] [Google Scholar]
- 13.Denas G., Gennaro N., Ferroni E., Fedeli U., Saugo M., Zoppellaro G., Padayattil Jose S., Costa G., Corti M.C., Andretta M., Pengo V. Effectiveness and safety of oral anticoagulation with non-vitamin K antagonists compared to well-managed vitamin K antagonists in naïve patients with non-valvular atrial fibrillation: propensity score matched cohort study. Int. J. Cardiol. 2017;249:198–203. doi: 10.1016/j.ijcard.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 14.Inciardi R.M., Adamo M., Lupi L., Metra M. Atrial fibrillation in the COVID-19 era: simple bystander or marker of increased risk? Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tremblay D., van Gerwen M., Alsen M., Thibaud S., Kessler A., Venugopal S., Makki I., Qin Q., Dharmapuri S., Jun T., Bhalla S., Berwick S., Feld J., Mascarenhas J., Troy K., Cromwell C., Dunn A., Oh W.K., Naymagon L. Impact of anticoagulation prior to COVID-19 infection: a propensity score–matched cohort study. Blood. 2020;136:144–147. doi: 10.1182/blood.2020006941. [DOI] [PMC free article] [PubMed] [Google Scholar]