Klebsiella pneumoniae carbapenemase-producing K. pneumoniae (KPC-Kp) is an emerging multidrug-resistant organism (MDRO) with serious clinical and therapeutic implications [1]. In our 400-bed tertiary acute-care hospital, a targeted infection prevention and control (IPC) programme on KPC-Kp was implemented in 2019 due to the high prevalence of this bacterium. Here we report the impact of the COVID-19 pandemic on our anti-KPC programme.
In April 2019 an ‘anti-KPC-Kp bundle’ was adapted from an Italian reference centre with a low KPC burden (Infectious Diseases Unit of Modena Polyclinic) and implemented by a dedicated multidisciplinary working group [2]. The bundle comprised 10 elements: hand hygiene; contact precautions; participative healthcare worker (HCW) training; proper use of devices (especially central venous catheters); notification of KPC-Kp isolates; communication of colonization status at discharge; environmental cleaning; antibiotic stewardship; staff cohorting; and active surveillance using rectal swabs.
The programme was established in the intensive care unit (ICU) and contiguous sub-intensive emergency medicine (EM), with the intention to extend it to other hospital areas. Active control by participative focus groups among HCWs ensured the continuous implementation of the bundle. By January 2020 we had observed statistically significant negative trends of KPC-Kp colonization prevalence in ICU (from 71.4% to 0%) and EM (from 42.9% to 11.1%) (P<0.001). A statistically significant negative trend was also found for incidence density rates in ICU (P=0.007). This improvement was associated with improvements in measures of IPC performance. Alcoholic hand rub consumption rose from 19.8 to 51.1 L/1000 patient-days in ICU and from 17.2 to 34.6 L/1000 patient-days in the EM; hand hygiene compliance rates increased from 59.3% to 81% in ICU, and from 41% to 63% in the EM; meropenem defined daily dose decreased from 38.4 to 18.5/100 patient-days in ICU and from 14.7 to 6.9/100 patient-days in the EM.
By the beginning of March 2020, SARS-CoV-2 had spread to all regions of Italy [3]. Our hospital was identified as a COVID-19 reference centre. ICU was dedicated to COVID invasive mechanical ventilation dependant patients and the EM to spontaneously breathing patients requiring high intensity of care. After the pandemic, the anti-KPC-Kp training programme switched to being primarily focused on personal protective equipment (PPE) extended to all the HCWs of the hospital, and was increased the hydroalcoholic solution for hand hygiene.
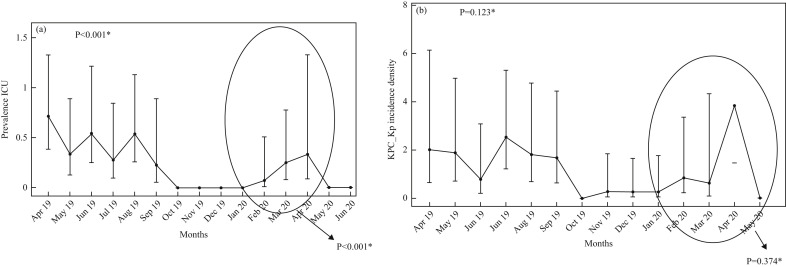
In the, ICU the prevalence of KPC-Kp faecal carriage increased significantly in the COVID-19 period (February–April 2020), against the previous decreasing trend (P<0.001) (Figure 1 a). Figure 1b shows that the incidence density rate in the ICU maintained a statistically significant trend in decrease (P=0.0123), although April 2020 was a non-significant outlier in the encircled period of January–May 2020 (P=0.374). Thereafter, the positive impact of the anti-KPC bundle began to be seen again.
Figure 1.
Indexes of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae (KPC-Kp) colonization in the intensive care unit (ICU). (a) Point prevalence: a Poisson regression model shows an overall trend of decrease since the beginning of the programme in April 2019 to last observations in June 2020 (P<0.001), with a relative statistically significant increase in the early COVID-19 pandemic period (encircled in the figure) (P<0.001) which did not affect this trend. (b) Incidence density rate: a Poisson regression model (considering the total hospitalization days as an offset in the model) shows that the multimodal prevention programme maintains its objective: a statistically significant overall decrease in trend was observed since the beginning to the last month of observation in June 2020 (P=0.0123), with the only exception of April 2020 as a non-significant outlier in the encircled period January–May 2020 (P=0.374).
The fight against infection and colonization with MDROs is a common objective in hospitals' care standard improvement plans, but the best results from multimodal approaches are seen with continuous efforts [4]. An emergency such as COVID-19 can alter the balance reached and constitute an obstacle to optimal adherence to all measures proposed for MDROs hospital infection control programmes [5]. Moreover, some aspects of management of COVID-19 patients may have paradoxically promoted the spread of KPC-Kp, e.g., use of PPE for droplet and airborne precautions, cohorting of possible or confirmed COVID-19 cases, and the high workload for HCWs. Gloves and gowns have been of paramount importance for the personal protection of HCWs during the pandemic, but can hinder hand hygiene [6].
In our experience, as part of a multimodal infection control strategy, the HCWs education training with a comprehensive approach to integrating personal protection and correct adherence to hand rub practice is crucial and should be implemented to better manage future epidemic emergencies.
One lesson learned from the current pandemic is that vigilance is required with regard to other infections, and that established infection prevention and control programmes must be maintained, or reinstated as soon as possible.
Acknowledgements
The authors would like to acknowledge Hospital Health Direction, medical and nursing staff for their work. The authors also wish to acknowledge all members of the Hospital Infection Control Committee for their continuous contribution to the Anti KPC-Kp programme. Finally, the basis for this work would be impossible to create without Prof. Cristina Mussini and Dr. Marianna Meschiari (Infectious Diseases Unit of Modena Polyclinic): the feasibility of the project in our hospital and many suggestions ‘to ameliorate’ are the result of their precious collaboration.
Contributor Information
IPC Program Working Group:
C. Cosentino, L. Alibardi, L. De Marchis, M. Aiuti, A. Carraturo, S. Parrocchia, A. Mecozzi, and A. De Meo
Conflict of interest statement
All authors report no conflicts of interest relevant to this article.
Funding sources
No financial support was provided relevant to this article.
References
- 1.Gasink L.B., Edelstein P.H., Lautenbach E., Synnestvedt M., Fishman N.O. Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase–producing K. pneumoniae. Infect Control Hosp Epidemiol. 2009;30:1180–1185. doi: 10.1086/648451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gentile B., Grottola A., Orlando G., Serpini G.F., Venturelli C., Meschiari M. A retrospective whole-genome sequencing analysis of carbapenem and colistin-resistant Klebsiella pneumoniae nosocomial strains isolated during an MDR surveillance program antibiotics. Basel) 2020;9:246. doi: 10.3390/antibiotics9050246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Distante C., Piscitelli P., Miani A. Covid-19 outbreak progression in Italian regions: approaching the peak by the end of March in Northern Italy and first week of April in Southern Italy. Int J Environ Res Public Health. 2020;17:3025. doi: 10.3390/ijerph17093025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel J.D., Rhinehart E., Jackson M., Chiarello L. Management of multidrug-resistant organisms in health care settings. Am J Infect Control. 2007;35:165–193. doi: 10.1016/j.ajic.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Friedman N.D., Carmeli Y., Walton A.L., Schwaber M.J. Carbapenem-resistant Enterobacteriaceae: a strategic roadmap for infection control. Infect Control Hosp Epidemiol. 2017;38:580–594. doi: 10.1017/ice.2017.42. [DOI] [PubMed] [Google Scholar]
- 6.Morgan D.J., Rogawski E., Thom K.A., Johnson J.K., Perencevich E.N., Shardell N. Transfer of multidrug-resistant bacteria to healthcare workers’ gloves and gowns after patient contact increases with environmental contamination. Crit Care Med. 2012;40:1045–1051. doi: 10.1097/CCM.0b013e31823bc7c8. [DOI] [PMC free article] [PubMed] [Google Scholar]