Graphical abstract
Keywords: COVID-19, Head and neck cancer, Symptoms, Hospitalization, Mortality
Highlights
-
•
We note high 30-day all-cause mortality for HNC patients admitted with COVID-19.
-
•
ICU admission and residing in a LTC facility predicted poor outcomes.
-
•
Most deaths were in HNC survivors and not in those on active cancer therapy.
Abstract
Background
The impact of COVID-19 on patients with cancer is emerging, but data are urgently needed for head and neck cancer (HNC) patients or survivors who are inherently high-risk for severe illness and mortality with SARS-CoV-2 infection.
Methods
This multi-institution, academic cohort study collected comprehensive data on clinical risk factors, COVID-19 symptoms and viral testing patterns, information about hospitalization rates, and predictors of survival among HNC patients with active disease or in remission. The primary endpoint was 30-day all-cause mortality from the date of confirmed COVID-19. We performed multivariate analysis to understand the prognostic value of clinical and laboratory parameters on outcomes.
Results
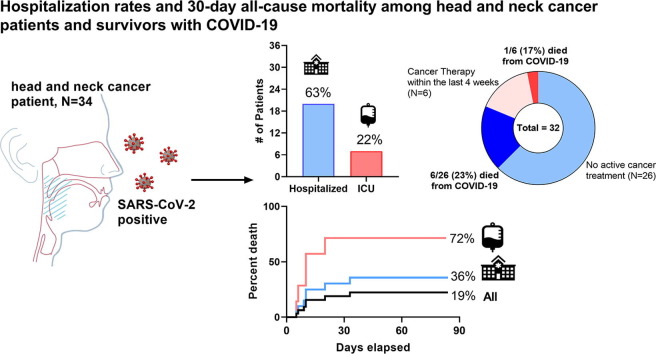
Thirty-two patients with COVID-19 and HNC were included. Median age was 70 (range: 38–91) with 38% aged 75+, and 34% resided in long-term care facilities (LTCF). Thirteen (41%) had active cancer, with 6 (19%) on cancer therapy within 4 weeks of COVID-19 diagnosis. New or worsening cough and fatigue were the most commonly reported presenting symptoms. More than 30% required >1 SARS-CoV-2 test before confirming a positive result. Twenty (63%) required hospitalization. At data cutoff, 7 (22%) had died (1 on active cancer treatment), with a 30-day all-cause mortality of 18.9% (95%CI: 11.4–33.6) among all patients, and 71.5% (95%CI: 38.2–92.3) among those requiring intensive care unit (ICU) admission. ICU admission and residing in a LTCF predicted worse outcomes (p < 0.01), while age, gender, and recent treatment did not.
Conclusions
We observed high 30-day all-cause mortality among HNC patients with COVID-19, but most were not on active cancer therapy.
Background
Emerging evidence suggests that patients with cancer are at increased risk of contracting COVID-19, the resulting illness linked to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and may develop more severe complications from the illness that has caused a global pandemic [1], [2]. The use of cytotoxic therapies and/or immunosuppressive medication in a population with often coexisting medical issues, coupled with frequent healthcare interactions, places cancer patients in a higher risk category [3]. Among patients with any cancer and COVID-19, a considerable 30-day all-cause mortality of 13% was observed in an international cohort study, and linked to risk factors such as age, male gender, smoking status, and comorbid medical conditions [4].
Among all cancer subtypes, individuals with head and neck cancer (HNC) may be at substantial risk for COVID-19 related complications. HNC patients are often elderly, frail, and carry comorbid medical diagnoses [5]. The aerosolized SARS-CoV-2 virus remains airborne for hours and replicates in the nasal cavity and nasopharynx [6], [7] which are frequent sites of disease, examination, and treatment in this unique population, theoretically increasing the risk of viral exposure and transmission to unaffected individuals. Given the global prevalence of both HNC and COVID-19, understanding clinical factors influencing survival and disease outcomes is crucial. In this cohort study, we aimed to capture symptoms and COVID-19 testing patterns, hospitalization rates, and predictors of 30-day mortality among COVID-19-positive HNC patients.
Methods
Subjects
We queried the electronic medical record (EMR) using diagnosis codes to identify patients aged 18 years or older carrying a diagnosis of head and neck cancer (aerodigestive or cutaneous squamous cell carcinoma (SCC) of the head and neck) and salivary gland carcinoma having one or more positive SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) results obtained by nasopharyngeal swab with data capture covering March 11, 2020 to June 1, 2020. Contributing institutions included two formal members of the Dana-Farber/Harvard Cancer Center (DF/HCC) research consortium, DF/Brigham & Women’s Hospital and Massachusetts General Hospital, which share an EMR. The study received expedited institutional review board (IRB) approval (DFCI #20-348).
Procedures
De-identified patient data was collected with over 60 continuous and categorical variables which included clinical and demographic information, HNC diagnosis, disease status (in remission or active, requiring cancer-directed therapy within 30 days of confirmed infection), active treatment(s) for their HNC malignancy (including surgery, radiotherapy, and systemic therapies), and whether a tracheostomy tube, laryngectomy stoma, and/or percutaneous endoscopic gastrostomy (PEG) was present at viral diagnosis. Time from last cancer-directed therapy was recorded (in months) and whether patients were living or working in a long-term healthcare facility at the time of viral diagnosis was noted. The number of SARS-CoV-2 tests obtained per patient, dates of first and last positive results were documented, along with time from symptom development (if any) to a first confirmed positive test result – and time from a first positive result to the end of symptoms or recovery. Self-reported presenting symptoms, comorbid medical conditions, and any admission to the hospital along with length of stay, intensive care unit (ICU) admission, and COVID-19 specific treatments were recorded. Ferritin, D-dimer, CRP, and ESR peak values were collected for each patient during their hospitalization period, if obtained.
Outcomes
The primary endpoint of the cohort analysis was 30-day all-cause mortality from the time of confirmed COVID-19 infection among the entire HNC population, and among those hospitalized and requiring ICU level care. Various factors were evaluated for their independent association with overall mortality including: clinical features, the impact of peak inflammatory markers, comorbid medical conditions, the presence of a surgically modified airway or PEG tube, and disease status and cancer treatment within 30 days of viral infection.
Statistical analysis
Descriptive statistics were utilized to report clinical characteristics and demographics, and to summarize the relationship between symptom timing and patterns of testing results. Fisher’s exact and Wilcoxon rank sum tests were used to evaluate the association between categorical and continuous variables by treatment setting, respectively. Mann-Whitney testing (non-parametric) compared peak inflammatory markers among hospitalized and ICU level care patients. The Kaplan-Meier method was applied using log-rank testing to evaluate survival. Overall survival (OS) was determined from the date of initial COVID-19 diagnosis by laboratory testing to death or censored at last follow-up, and 30-day all-cause mortality estimates were generated. The Cox proportional-hazards model was used to investigate the association between survival and clinicopathologic, diagnostic, and laboratory parameters (unadjusted). We partially adjusted covariates for age, gender, and smoking status. All statistical tests were two-sided, and a p-value < 0.05 was considered significant. Stata 14.2 (College Station, TX) was used as the software for analysis.
Results
Study population
From March 11th, 2020 to the data cutoff of June 1st, 2020 we identified 32 patients managed through the DF/HCC consortium at two partnering academic institutions with a concurrent diagnosis of COVID-19 and active or previously diagnosed HNC. Median age of the cohort was 70 (range: 38–91) with 38% aged 75 years or older at viral diagnosis (Table 1 ). Patients were more often male, former or current smokers, with a favorable performance status, but 34% had 3 or more significant medical comorbid conditions (diabetes or organ dysfunction). While most were living at home, 34% of patients resided (one was an employee) in a nursing or long-term care facility at the time of COVID-19 diagnosis.
Table 1.
Clinical characteristics and demographics among head and neck cancer patients with COVID-19.
| Parameter | All N = 32, (%)A |
Hospitalized N = 20 |
Outpatient N = 12 |
p-Value |
|---|---|---|---|---|
| Age, years | 70 (38–91) | 70 (47–91) | 61 (38–86) | 0.02 |
| <65 years | 13 (41) | 6 (30) | 7 (58) | |
| ≥75 years | 12 (38) | 8 (40) | 4 (33) | |
| Gender | 0.19 | |||
| Male | 20 (63) | 14 (70) | 6 (50) | |
| Female | 12 (38) | 6 (30) | 6 (50) | |
| Race and ethnicity | 0.11 | |||
| Non-Hispanic white | 16 (50) | 13 (65) | 4 (33) | |
| Non-Hispanic black | 5 (16) | 2 (10) | 3 (25) | |
| Hispanic | 5 (16) | 3 (15) | 2 (17) | |
| Other or unknown | 6 (19) | 2 (10) | 3 (25) | |
| Smoking status | 0.45 | |||
| Never | 12 (38) | 7 (35) | 5 (42) | |
| Former | 18 (56) | 12 (60) | 6 (50) | |
| Current | 2 (6) | 1 (5) | 1 (8) | |
| ECOG performance status | 0.14 | |||
| 0–1 | 25 (78) | 14 (70) | 11 (92) | |
| 2+ | 7 (22) | 6 (30) | 1 (8) | |
| Tracheostomy or laryngectomy stoma in place | 5 (16) | 3 (15) | 2 (17) | – |
| Percutaneous endoscopic gastrostomy (PEG) tube in place | 3 (9) | 3 (15) | 0 | |
| Comorbid medical conditions | 0.32 | |||
| None | 6 (19) | 2 (10) | 4 (33) | |
| 1–2 | 15 (47) | 10 (50) | 5 (42) | |
| 3 or more | 11 (34) | 8 (40) | 3 (25) | |
| Hypertension | 19 (59) | 16 (80) | 3 (25) | |
| Cardiovascular disease | 21 (66) | 16 (80) | 5 (42) | |
| Diabetes mellitus | 8 (25) | 6 (30) | 2 (17) | |
| Chronic kidney disease | 9 (28) | 4 (20) | 5 (42) | |
| Chronic liver disease | 3 (9) | 2 (10) | 1 (8) | |
| Primary Residence at the time of testing | <0.01 | |||
| Home | 21 (66) | 10 (50) | 11 (92) | |
| Nursing home or long-term care facilityB | 11 (34) | 10 (50) | 1 (7) | |
| Primary malignancy | 0.76 | |||
| Oral cavity | 5 (16) | 3 (15) | 2 (17) | |
| OropharynxC | 6 (19) | 4 (20) | 2 (17) | |
| Larynx/hypopharynx | 9 (28) | 6 (30) | 3 (25) | |
| Salivary gland cancer | 5 (16) | 2 (10) | 3 (25) | |
| Cutaneous (head & neck) | 7 (22) | 5 (25) | 2 (17) | |
| Cancer staging at diagnosisD | 0.52 | |||
| Stage I, II | 9 (28) | 5 (25) | 4 (33) | |
| Stage III, IV | 23 (72) | 15 (75) | 8 (67) | |
| Cancer status | 0.22 | |||
| Remission or no evidence of disease | 19 (59) | 13 (65) | 6 (50) | |
| Present, stable or responding to therapy | 8 (25) | 2 (10) | 6 (50) | |
| Present, progressive | 5 (16) | 5 (25) | 0 | |
| Recurrent or metastaticE | 9 (28) | 5 (25) | 4 (33) | |
| Anti-cancer therapyF | 0.69 | |||
| None within 4 weeks of COVID-19 diagnosis | 26 (81) | 14 (70) | 12 (100) | |
| Cytotoxic chemotherapy | 3 (9) | 3 (15) | 0 | |
| Targeted therapy | 0 | 0 | 0 | |
| Immunotherapy | 1 (3) | 1 (5) | 0 | |
| Surgery within 4 weeks of COVID-19 diagnosis | 4 (13) | 3 (15) | 1 (8) | |
| Radiation within 4 weeks of COVID-19 diagnosis | 1 (3) | 1 (5) | 0 | |
| Time from last cancer treatment to COVID-19 diagnosis (in months) | 16.0 (0–160) | 16.0 (0–160) | 19.0 (3–138) | 0.83 |
except age and time from last cancer treatment with median reported and range in parentheses.
one participant lived at home but worked in a skilled nursing facility.
5/6 (83%) tested positive for p16 by immunohistochemistry and/or by confirmatory ISH/PCR testing.
American Joint Committee on Cancer (AJCC) 2017 8th edition staging reported.
includes patients with disease present either stable, responding, or progressing while on current therapy.
section does not total n = 32 as some patients fit more than one category. ECOG = Eastern Cooperative Oncology Group
The most common HNC diagnosis was aerodigestive SCC (20, 63%), and most patients had no evidence of cancer (19, 59%) at the time of COVID-19 infection. Thirteen (41%) had stable or progressive cancer (4 receiving curative therapy and 9 receiving treatment for recurrence or metastatic HNC). Most patients (26, 81%) had not received cancer-directed therapy within 4 weeks of their confirmed COVID-19 diagnosis, with a median of 16 months (range: 0–160) between their last cancer-directed therapy and viral infection. Four (13%) and 1 (3%) had undergone surgery or received radiation within 4 weeks of COVID-19 diagnosis, respectively.
Of the 7 patients with cutaneous SCC almost all were age 80 or older (86%) and 71% resided in nursing facilities at the time of diagnosis, but few were on active cancer therapy in the month prior to COVID-19 diagnosis (21%).
Symptoms and COVID-19 testing
The most commonly reported presenting symptom of COVID-19 among the cohort was new or worsening cough (18, 56%), followed by fatigue (17, 53%), and new or worsening shortness of breath (13, 41%) (Table 2 ). Only 1 patient reported anosmia, and 3 (9%) were asymptomatic by self-report; with a median of 2 symptoms reported per patient (range: 0–6).
Table 2.
Symptoms, hospitalization rates, and treatment among head and neck cancer patients with COVID-19.
| Parameter | N = 32, (%)A |
|---|---|
| Patient reported presenting symptomsA | |
| Asymptomatic or none | 3 (9) |
| New or worsening cough | 18 (56) |
| Generalized malaise or fatigue | 17 (53) |
| New or worsening shortness of breath | 13 (41) |
| Fever or chills | 10 (31) |
| Congestion | 6 (19) |
| Sore throat | 5 (16) |
| Nausea, emesis, or diarrhea | 3 (9) |
| Myalgias | 3 (9) |
| Change or loss of sense of smell | 1 (3) |
| Hospitalization rate | 20 (63) |
| In-hospital days (median, range)C | 8 (1–32+) |
| Intensive care unit (ICU) stay required | 7 (22) |
| In-ICU days (median, range) | 4 (1–16) |
| Supplemental oxygenation needsB | 15 (47) |
| Nasal cannula | 11 (34) |
| Non-invasive positive pressure ventilation | 4 (13) |
| Mechanical ventilation | 3 (9) |
| Treatment of COVID-19D | |
| Antibiotics (cephalosporins, vancomycin, macrolides) | 16 (50) |
| Antivirals | 2 (6) |
| Corticosteroids | 5 (16) |
| Hydroxychloroquine | 6 (19) |
| Tocilizumab (anti-IL6) | 1 (3) |
Patient reported symptoms could be multiple and thus total exceeds N = 32.
Total may exceed N = 32 because patients may have required supplemental oxygen via nasal cannula and progressed to greater breathing support.
‘+’ in the range indicates patients who remain hospitalized at the time of analysis and follow-up.
Treatment approaches are not mutually exclusive and patients may have received more than one therapy in combination or sequentially.
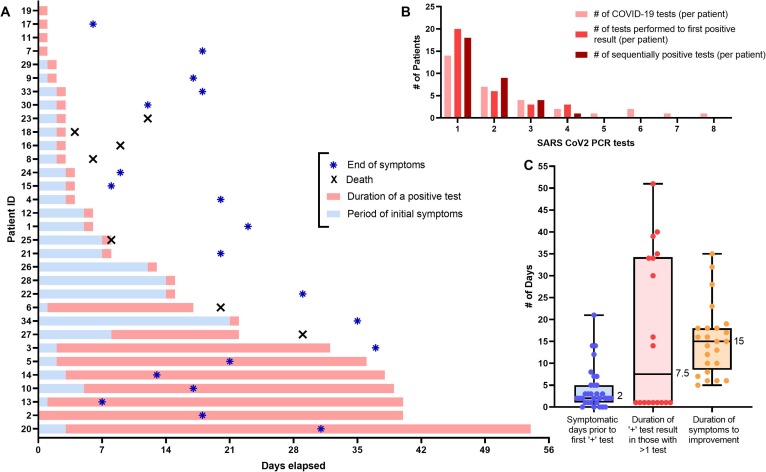
Fig. 1 correlates the timing and duration of symptoms with a first positive, and the duration of a positive, COVID-19 test result. The median time from first reported symptoms to a first positive COVID-19 test was 2 days (range: 0–21). The number of COVID-19 RT-PCR tests performed per patient was variable, ranging from 0 to 8 with a median of 2 tests per patient. While most (23, 72%) had a single positive test result, 9 patients had multiple positive test results which spanned a period of 14–51 days (median: 34 days) – including 4 patients who tested negative after their first positive result, and later retested positive (range from negative to second positive result: 1–22 days). The median duration of time from first symptoms to full self-reported recovery was 15 days (range: 5–35).
Fig. 1.
(A) Swimmer plot showing N = 32 head and neck cancer patients with positive SARS-CoV2 PCR testing results in the context of symptomatic presentation. Blue bars represent the duration of patient reported symptoms (in days), while the pink bars represent the duration of a confirmed positive COVID-19 test result (>1 day represents the period between sequential positive test results among an individual patient). Blue asterisks represent the end of patient reported symptoms, and a black 'X' indicates when/if the patient died. (B) Bar graph showing the number of COVID-19 tests obtained, the number of tests to a first positive result, and the total number of sequentially positive test results per patient. (C) Box and whisker plot showing median # of days from symptoms to a positive test, duration of a positive result (in those with >1 result), and the duration of patient reported symptoms with ranges identified (median values shown, in days). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Hospitalization rates and COVID-19 treatments
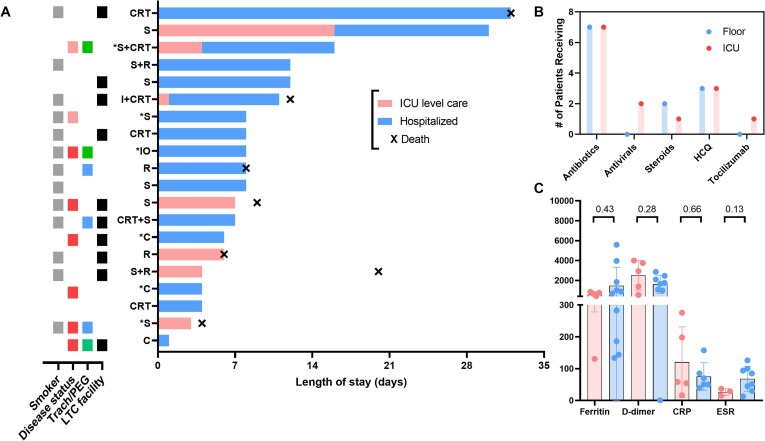
Of the 32 patients in the cohort, 20 (63%) required hospital admission for 1 or more days due to COVID-19 infection, with 7 (22%) of those necessitating ICU level care (Table 2). The hospitalization rate was 71% among the 7 cutaneous SCC patients. Hospitalized patients were more often older (p = 0.01) and were often residing in long-term care facilities (p < 0.01) as shown in Table 1. Fig. 2 shows that among admitted HNC cancer patients with COVID-19, 65% were former or current smokers, 30% received cancer-directed therapies in the last 30 days, and 50% resided (or worked) in long-term care facilities prior to admission. The median number of days in the hospital was 8 (range: 1–32+) and 4 (range: 1–16) for days in the ICU. Peak median inflammatory markers (ESR, CRP, D-dimer, ferritin) among hospitalized and ICU patients appeared similar, but often markedly elevated.
Fig. 2.
(A) Swimmer plot showing N = 20 COVID-19 positive head and neck cancer patients (y-axis indicates their current or prior cancer treatment while an asterisk indicates they received treatment <30 days from COVID-19 diagnosis) hospitalized and/or requiring intensive care unit (ICU) care organized by length of stay (in days). To the left is a color grid with tiles showing key clinical and disease characteristics (grey: former or current smoker, red: recurrent/metastatic disease, pink: curative intent disease, blue: tracheostomy or laryngectomy stoma, green: PEG in place, black: at a long-term care [LTC] facility at the time of admission). CRT = chemoradiation, S = surgery, R = radiotherapy, I = induction chemotherapy, IO = immunotherapy, C = chemotherapy. (B) Bar graph showing the number of patients receiving COVID-19 therapies during admission to hospital floor vs. the ICU. HCQ = hydroxychloroquine. (C) Box and whisker plot showing peak median inflammatory markers for hospitalized patients with COVID-19 separated by hospital floor and ICU stay (*) p < 0.05, Mann-Whitney U test, two-sided. Normal reference ranges: ESR (0–20 mm/h), CRP (0–10 mg/L), D-dimer (<500 ng/dL), ferritin (30–400 ug/L). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fifteen patients (47%) required supplemental oxygenation with nasal cannula (range: 2–10 L, L), while 7 (22%) required non-invasive positive pressure ventilation (NIPPV) or mechanical ventilation (MV) during the period of COVID-19 illness. Nineteen (59%) received COVID-19 directed therapies during illness including antibiotics (16), antivirals (2), corticosteroids (5), hydroxychloroquine (HCQ) (6), and tocilizumab (1).
Factors impacting survival and 30-day mortality
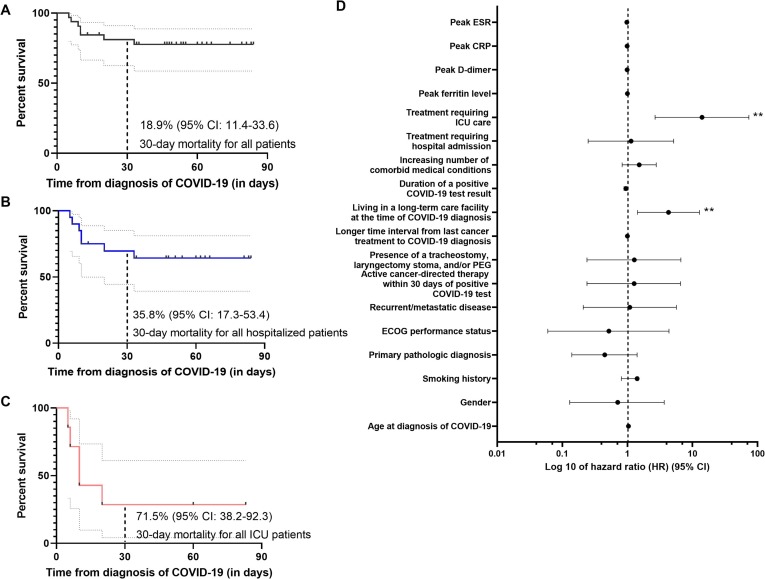
As of the data cutoff of June 1, 2020, 7 patients (22%) had died, only 1 of which had received active cancer therapy (surgery) in the last 4 weeks. Figure 3 shows an all-cause 30-day mortality of 18.9% (95% CI: 11.4–33.6) from the time of confirmed COVID-19 diagnosis among our HNC cancer cohort. Thirty-day mortality was further increased for those patients who required hospital admission during their illness (35.8%, 95% CI: 17.3–53.4), but overall the highest for patients admitted to the ICU (71.5%, 95% CI: 38.2–92.3). When considering a broad range of clinical, laboratory, and treatment parameters, treatment requiring ICU care during illness (hazard ratio [HR] 14.00, 95% CI: 2.66–73.60, p < 0.01), and residing (or working) at a long-term care facility (HR 4.28, 95% CI: 1.43–12.80, p < 0.01) were independently associated with poor survival (Supplemental Table 1). Advanced age, gender, smoking history, initial cancer staging, primary pathologic diagnosis, increasing comorbid medical conditions, the presence of a surgical airway or PEG, and recent anti-cancer therapy did not appear to significantly impact survival at this cohort size, but its worth noting that 6/7 (86%) deaths occurred in individuals 65 years and older and two had cutaneous SCC.
Fig. 3.
Kaplan Meier survival curves showing overall survival from the time of COVID-19 diagnosis among (A) all head and neck cancer patients, (B) those requiring hospital admission for 1 day or more, and (C) those requiring ICU admission at any point, censored at the time of death or last known follow-up. 30-day mortality estimates are shown with 95% confidence intervals. (D) Forest plot of factors associated with mortality or survival among COVID-19 positive head and neck cancer patients. Cox proportional hazard modeling was performed with values shown in log 10 scale. (*) p < 0.05 and (**) p < 0.01.
Discussion
This academic consortium data is the first to summarize important observations regarding symptom and testing patterns, hospitalization rates, and 30-day all-cause mortality among HNC patients (with active disease and among those in remission) with confirmed COVID-19. Perhaps the most striking observation was the 22% death rate among HNC patients with a concurrent COVID-19 diagnosis, with a doubling of 30-day all-cause mortality among those requiring hospitalization which exceeded 70% among those requiring ICU level care. This compared with a death rate of 11–13% among previously reported aggregate cancer populations with COVID-19 in the United States [4], [8] and increasing case-fatality rates among non-cancer elderly subgroups (2.7–4.9% among those 65–74, and 4.3–10.5% among those 75–84) [9]. Only 1/7 (14%) deaths occurred in patients on active cancer therapy within the last month prior to COVID-19 and only a third of the cohort in general had recently received active cancer treatments which supports the notion of uninterrupted cancer care for most. We acknowledge some bias whereby more severe cases may be referred to academic centers. Nonetheless, these findings have important implications regarding the need for expanded testing, and minimizing unnecessary healthcare exposures or aerosolizing procedures in this high-risk cancer subpopulation when possible.
Among this cancer subpopulation, individuals residing in (or working in) long-term care facilities and those patients requiring ICU level care were at highest risk for poor outcomes. While advanced age and male gender have been previously reported as risk factors for worse outcomes among the general population with COVID-19 [10], we did not observe a significant impact on outcomes among HNC patients based on these clinical factors although we acknowledge a modest number of events in our multivariate model. Emerging data has also has suggested worse outcomes for COVID-19-positive cancer patients in general with poor ECOG performance status and among those on active cancer therapy (treated within the last 4 weeks) [4], but this was not observed in our HNC focused population. For context, 2 patients in our cohort with an ECOG performance score of 2 were on active cancer-directed therapy.
The rate of hospital admission for COVID-19-positive cancer patients has been reported around 50% [4] and we observed a similar rate (63%) among our head and neck-specific cancer subpopulation, but overall our ICU admission rate was higher (22 vs. 14%), comparatively. This increased rate could be explained by the composite adverse risk factors among these 7 patients: all but 1 was over age 65, 2 (29%) had received cancer-directed therapy in the last month, 4 (57%) resided in long-term care facilities, and 1 (14%) had a tracheostomy or laryngectomy stoma. The impact of treatments aimed at COVID-19 infection among hospitalized and ICU level care patients was not discernable due to small numbers and variable patterns of use as the pandemic evolved, but rates of antibiotic (54 vs. 100%) and HCQ (23 vs. 43%) use varied somewhat among these subgroups, respectively. Of note, 50% (3/6) of patients receiving HCQ later died. This trend seems consistent with recent reports suggesting that treatment with HCQ might be linked with worse outcomes among COVID-19 positive patients with cancer [4], but early observational data has shown no clear harm or benefit among non-cancer COVID-19 patients treated with HCQ [11].
Our results offer important preliminary observations regarding presenting symptoms as they relate to SARS-CoV-2 testing results among HNC cancer patients. Common reported symptoms included new or worsening cough, shortness of breath, and fatigue – important to keep in mind as these are common among HNC patients in general due to chronic competing issues like dysphagia and aspiration risk, the presence of complex surgical or modified airways, and coexisting medical issues (such as hypothyroidism and nutritional compromise). It is also important to recognize that >30% of our HNC patients required >1 SARS-CoV-2 PCR test before confirming a positive result. Recent pooled RT-PCR interpretation data suggests the false-negative rate (FNR) approaches 20% and is lowest within the first 3 days of symptom onset (8 days post-exposure) [12]. Nearly fifty percent of our cohort had their first positive test 3 days or more after the onset of symptoms, which explains our higher than expected FNR. Additionally, we expect the 4 individuals who were positive, then tested negative, and had a subsequent repeat positive test were not repeat infections but rather testing was falsely negative or represents residual viral RNA while monitoring for viral clearance. The development of COVID-19 serologic assays to detect antibodies may further clarify active vs. prior infection in the future.
Our study has important limitations, and thus these observations should be regarded as preliminary as further data about the impact of COVID-19 among head and neck cancer patients evolves. This was primarily a retrospective cohort study comprised of a modest number of patients but designed for rapid data collection during an unfolding global pandemic. Selection bias towards more symptomatic patients and those referred to academic centers is noteworthy, and we did not utilize a control subgroup of COVID-19-negative HNC cancer patients.
In conclusion, we present the first report of COVID-19 symptoms and testing patterns, hospitalization rates, and predictors of 30-day mortality among COVID-19-positive HNC patients within an academic consortium. We observed higher overall rates of death and escalating 30-day all-cause mortality for those COVID-19-positive HNC patients requiring hospitalization and ICU care. Residing at a long-term care facility and needing ICU level care were independent predictors of poor outcomes, but most of these patients were off active cancer treatment and therefore we advocate that continued standard of care management should be encouraged. These data collectively confirm the vulnerability and fragility of HNC cancer patients when considering their risk of complications from COVID-19. Long-term follow-up and broader sampling will help to further understand the effect of SARS-CoV-2 among this high-risk cancer population.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: ‘G.J.H. is funded by the American Society of Clinical Oncology, Conquer Cancer Foundation, V Foundation, and Gateway for Cancer Research. He receives institutional support from BMS, Exicure, GSK, NantKwest/Altor BioScience, Kite, Regeneron, Kartos, Sanofi/Genzyme. Consulting and honoraria from BMS, Maverick, Merck, Kura, Sanofi/Genzyme, Bio- Rads, Prelude, and Bicara. J.H.L. receives research support from Novartis, Bayer, BMS and Takeda; consulting and honoraria: Bayer, Genentech. J.D.S. receives research support from ACCRF, Merck, BMS, and Regeneron; consulting and advisory board: Debiopharm, BMS, ACI Clinical, Tilos, LEK, Catenion, Nanobiotix, and AZ. SAB/equity from Immunitas and expert witness fees. R.I.H. receives research support from Pfizer, Genentech, Merck, BMS, Kura, AZ, and GSK; consulting for Merck, GSK, BMS, Genentech, Bayer, Pfizer, Immunomic, Nanobiotix, ISA, Glenmark, AZ. R.B.T. is on the data safety monitoring board for PSI/Oragenics, advisory board for Regeneron. The remaining authors have no relevant disclosures or conflicts of interest.’.
Acknowledgments
We are always grateful to our patients and their families for their participation in cancer research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.oraloncology.2020.105087.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Liang W., Guan W., Chen R., Wang W., Li J., Xu K. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai M., Liu D., Liu M., Zhou F., Li G., Chen Z. Patients with cancer appear more vulnerable to SARS-CoV-2: A multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyashita H., Mikami T., Chopra N., Yamada T., Chernyavsky S., Rizk D. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol. 2020;(20):39303. doi: 10.1016/j.annonc.2020.04.006. S0923-7534(20)39303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R. COVID-19 and cancer consortium, clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;(20):31187–31189. doi: 10.1016/S0140-6736(20)31187-9. S0140-6736(20)31187-9. [DOI] [Google Scholar]
- 5.Day A.T., Sher D.J., Lee R.C., Truelson J.M., Myers L.L., Sumer B.D. Head and neck oncology during the COVID-19 pandemic: Reconsidering traditional treatment paradigms in light of new surgical and other multilevel risks. Oral Oncol. 2020;105:104684. doi: 10.1016/j.oraloncology.2020.104684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta V., Goel S., Kabarriti R., Cole D., Goldfinger M., Acuna-Villaorduna A. Case fatality rate of cancer patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020 doi: 10.1158/2159-8290.CD-20-0516. CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19)-United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson S., Hirsh J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G. Observational study of hydroxychloroquine in hospitalized patients with covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2012410. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kucirka L.M., Lauer S.A., Laeyendecker O., Boon D., Lessler J. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med. 2020 doi: 10.7326/M20-1495. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.