Abstract
The aims of the study were to review the rapidly emerging COVID-19 literature to determine 1) the relationship between obstructive sleep apnoea (OSA) and adverse COVID-19 outcomes and, 2) potential causal mechanisms 3) what effect COVID-19 has had on OSA diagnosis and 4) what effect COVID-19 has had on treatment and management of OSA during this period. PubMed was systematically searched up to 020620. Studies were included if they had examined the relationship between COVID-19 and OSA. Studies were included that were in English and had the full text available. The findings from this study suggest that many of the risk factors and co-morbidities associated for OSA which include obesity, hypertension and diabetes mellitus are associated with poor COVID-19 outcomes. There are plausible mechanisms by which OSA may independently increase one's risk of morbidity and mortality associated with COVID-19 and data from the newly published CORONADO study suggests that OSA treated patients may be at increased risk of death from COVID-19. It is clear that the pandemic has had a major effect on the treatment management and diagnosis of OSA and moving forward it may be necessary to explore new diagnosis and treatment pathways for these individuals.
Keywords: OSA, CPAP, Sleep, Inflammation, Vitamin D, Melatonin
Abbreviations
- AASM
American Academy of Sleep Medicine
- ACE2
angiotensin converting enzyme 2
- AFSORL
French Association of Otorhinolaryngology and Sleep disorders
- AHI
apnoea hypopnea index
- ARDS
acute respiratory distress syndrome
- AST
aspartate transaminase
- AVAPS
average volume-assured pressure support
- COPD
chronic obstructive pulmonary disease
- CORONADO
Coronavirus SARS-CoV-2 and Diabetes Outcomes
- COVID-19
novel coronavirus disease
- CPAP
continuous positive airway pressure
- EDS
excessive daytime sleepiness
- EERS
enhanced expiratory rebreathing space
- GERD
gastroesophageal reflux disease
- IPF
idiopathic pulmonary fibrosis
- NIV
non-invasive ventilation
- NIPPV
non-invasive positive pressure ventilation
- OSA
obstructive sleep apnoea
- PPE
personal protection equipment
- PRISMA
preferred reporting items for systematic reviews
- RAAS
renin-angiotensin-aldosterone system
- ROS
reactive oxgen species
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SDB
sleep disordered breathing
- SFORL
French Society of Otorhinolaryngology
Introduction
The latter part of 2019 saw the emergence of a new coronavirus, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in Wuhan China. The virus has now reached global pandemic proportions. As of 26th June 2020, there have been 9,612,250 confirmed cases of Novel coronavirus disease 2019 (COVID-19) due to infection of SARS-CoV-2 in the world, with 489,372 fatalities globally [1]. Some individuals are at greater risk from adverse outcomes associated with the disease. These include older adults, and those with respiratory and cardiovascular risk factors like, obesity and diabetes mellitus [2]. Furthermore, a disproportionate number of black and minority ethnic individuals have been affected by COVID-19. In England a third of confirmed cases admitted to critical care are non-white [3].
The sleep disorder, Obstructive sleep apnoea (OSA) is characterized by repetitive partial or complete blockages of the airway during sleep, leading to interruptions in breathing, blood oxygen desaturation and arousals from sleep. In the UK there are approx. 1.5M adults with OSA. It is associated with increased prevalence of hypertension (39%), obesity (34%), depression (19%), gastroesophageal reflux disease (GERD) (18%), diabetes mellitus (15%), hypercholesterolemia (10%), asthma (4%) [4]. Many of these factors have also been identified as risk factors for poor COVID-19 outcomes [5]. It is unclear whether the virus might pose an increase risk for patients with OSA.
The aims of this study were to conduct a systematic review to examine the potential relationship between COVID-19 severity and outcomes, and OSA, and to examine possible mechanistic pathways, and to determine what effect the COVID-19 pandemic has had on the diagnosis and treatment of this condition.
Methods
Search strategy and selection criteria
Systematic searches using the Preferred reporting items for systematic reviews guidelines (PRISMA) [6] to identify studies that reported the association between COVID-19 and OSA were performed. Electronic searches were performed (from 1966 to 2nd June 2020) of PubMed. The following “COVID-19 terms” (coronavirus OR corona-virus OR COVID OR COVID-19 OR COVID-2019 OR severe acute respiratory syndrome coronavirus OR severe acute respiratory syndrome coronavirus 2 OR 2019-nCoV” OR SARS-CoV-2 OR 2019nCoV) in combination with Sleep terms” (sleep OR sleep wake disorders OR sleep disturbance OR (“sleep apnoea syndromes OR OSA OR OSAS OR sleep initiation and maintenance disorders OR insomnia OR bedtime OR “nightmare) were used. The articles identified by the searches were reviewed along with any relevant references cited within them.
Inclusion and exclusion criteria
Papers were included if they examined the relationship between COVID-19 outcomes and OSA, or were related to the effect of COVID-19 pandemic on the diagnosis and treatment of this condition.
Papers were excluded if the full paper was not available or if they were not published in English.
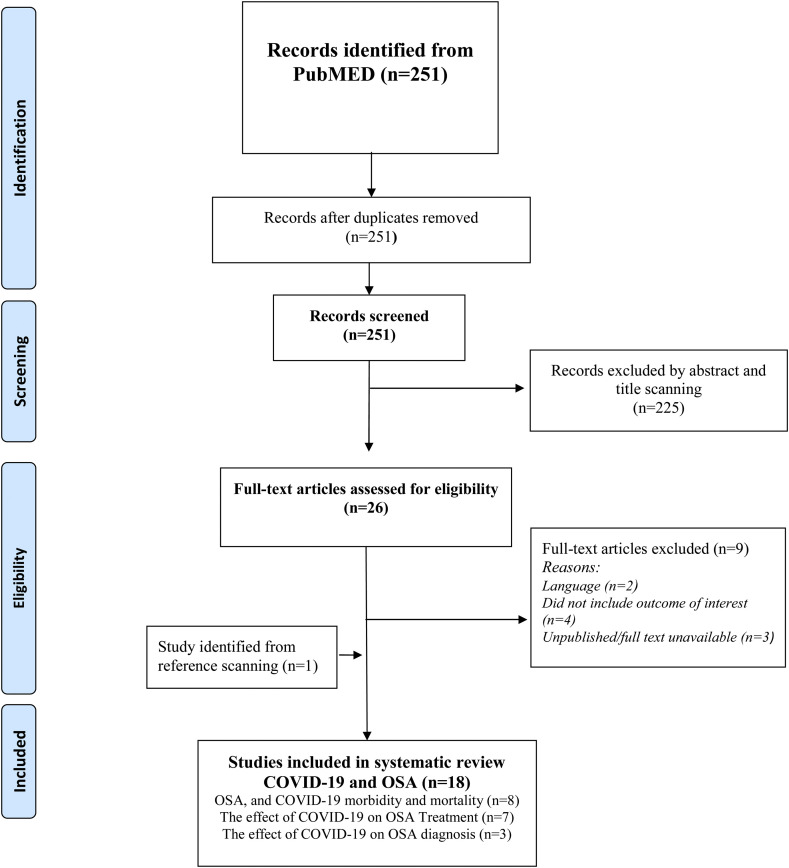
A total 251 potential studies were identified after removal of duplicates (see Fig. 1 ). Abstract scanning yielded 26 for full text evaluation from which 17 were included for the review [[7], [8], [9], [10], [11], [12], [13], [14], ∗[15], [16], ∗[17], ∗[18], ∗[19], ∗[20], ∗[21], ∗[22], [23]] a further study was identified by reference searching [24].
Fig. 1.
PRISMA flow chart.
Results
Of the 18 studies identified eight were mainly related to risk of COVID-19 mortality and 10 to diagnosis, treatment and management.
OSA and COVID-19 morbidity and mortality
Eight papers, seven from the original search [7,8,17,18,∗[20], ∗[21], ∗[22]] and one from reference searching [24] were examined. Of these six contained original data [17,18,∗[20], ∗[21], ∗[22],24] (see Table 1 ; part (a)); two were viewpoints [7,8]. Pazarlı et al. [7] noted that common risk factors for COVID-19 mortality were shared with OSA, and these included increased age, hypertension, cardiovascular diseases, lung diseases, and diabetes mellitus. Likewise, McSharry [8] also noted that morbidity and mortality worldwide were particularly high amongst those with these comorbidities but that obesity, which is also commonly associated with OSA, might also be important.
Table 1.
Summary of studies included in systematic review.
| Author | Study details | Main results and authors' conclusions | Strengths and limitations |
|---|---|---|---|
| Part (a) OSA and COVID-19 morbidity and mortality | |||
| Arentz et al., 2020 [24] | Case Series (n = 21) Patients with confirmed SARS-CoV-2 infection admitted to the ICU at Evergreen Hospital, Washington State. Feb 20th–5thMarch 2020 |
Mean age, 70 years [range, 43–92 years]. 52% men. Comorbidities were identified in 18 cases (86%), with chronic kidney disease and congestive heart failure being the most common. 28.6% of patients had obstructive sleep apnoea. Mechanical ventilation was initiated in 71% of patients. As of March 17, 2020, mortality was 67% and 24% of patients remained critically ill. Emphasizes the need to limit exposure of nursing home residents to SARS-CoV-2. |
Small number from a single center, the study population included older residents of nursing facilities |
| Bhatraju et al., 2020 [17] | Case series (n = 24) min 14 days follow up. Patients from nine Seattle-area hospitals, admitted to ICU with confirmed SARS-CoV-2. February 2020 | Mean age 64 ± 18years, BMI 33.2 ± 7.2 Kg 63% men. 58% had diabetes mellitus, 22% were current or former smokers, 21% had chronic kidney disease, 21% had obstructive sleep apnoea, 14% had asthma. All the patients were admitted for hypoxemic respiratory failure, 75% of the patients required mechanical ventilation. 50% died. The most common reason for admission to ICU was hypoxemic respiratory failure requiring mechanical ventilation. Mortality was high. |
Small sample size. |
| Cariou et al., 2020 [18] | The CORONADO Study. Observational Study Nationwide study in 53 French centers 10th −31st March 2020. |
The primary outcome was combined tracheal intubation for mechanical ventilation and/or death within 7 days of admission. 1317 participants: 64.9% men, mean age 69.8 ± 13.0 years, median BMI 28.4 (25th–75th percentile: 25.0–32.7) kg/m2; with a predominance of type 2 diabetes (88.5%) Age (OR 2.48 [1.74, 3.53]), treated obstructive sleep apnoea (OR 2.80 [1.46, 5.38]), and microvascular (OR 2.14 [1.16, 3.94]) and macrovascular complications (OR 2.54 [1.44, 4.50]) were independently associated with the risk of death on day 7. OSA is an independent risk factor for risk of death in COVID-19 patients. |
|
| Gupta et al. [20] | Case series (n = 21) of the first COVID-19 patients admitted to a tertiary care center in India. 1st Feb-19th March 2020 |
The mean age of the population was 40.3 years (range 16–73 years). There was a male preponderance (66.7%). All patients were Indian. Six patients (28.6%) had comorbidities. The most common comorbidity was hypertension (5 patients) and diabetes mellitus (3 patients) adequately controlled with drugs. One (4.8%) patient with hypertension and one with diabetes mellitus also showed anxiety disorder and hypothyroidism. Another patient had an underlying migraine and obstructive sleep apnoea. As of 19th March 19 of the patients had been discharged |
Small sample size. |
| Memtsoudis et al., 2020 [21] | Case series (n = 124) of whom 60 in ICU and 64 non-ICU | In ICU the average BMI was: males, 28.3 [SD 5.3] (n = 44) of whom five had OSA and females, 30 [6.3] (n = 16) of whom none had OSA. In patients severely ill with respiratory failure but not in ICU and therefore not requiring mechanical ventilation) the average BMI was: males, 29 [6.1] (n = 35) of whom two had OSA and females average BMI, 33.5 [12.1] (n = 21) of whom two had OSA. The authours hypothesise that the low incidence of diagnosed OSA in a high-risk obese patient population may reflect under-diagnosis. | |
| Mittal et al. 2020 [22] | Case study | 74 yrs old male with notable OSA and COVID-19. Successfully intubated and treated with AVAPS. | Small sample size |
| Part (b) The effect of COVID-19 pandemic on treatment and management of OSA | |||
| Attias et al. 2020 [19] | In a cohort of 7485 patients with OSA the impact of the coronavirus disease (COVID-19) national lockdown in France on objective adherence to CPAP was assessed by telemonitoring. Data was compared from the pre-COVID-19 period (one month before 15 March 2020, the date of the national lockdown announcement) to data post-lockdown. There was a 3.9% (p < 0.001) increase in adherence from a mean value of 386 min per night pre-COVID-19 to 401 min per night during lockdown. These data were confirmed by comparing the CPAP adherence rate during the first month of lockdown (15th March 2020 to 15th April 2020). The proportion of very low adherers (less than 10 min of CPAP use per night) dropped by 18.5% (p < 0.001) between the similar periods in 2019 and 2020). The authours hypothesise that fear of being hospitalized may have motivated patients to increase CPAP adherence and decreased occupational stress and increased opportunity for sleep may be important. |
Large sample size Prospective cohort. |
|
| Baker and Sovani 2020 [11] | Opinion piece | Summary of main points: NIV: This is primarily used for those with previous, or at risk from, hypercapnic respiratory failure).
CPAP: This is primarily used for those with OSA.
|
Non-systematic |
| Barker et al., 2020 [10] | Opinion piece | The authors summarise existing evidence which suggests that:
|
Non-systematic |
| Bastier et al., 2020 [23] | The French Association of Otorhinolaryngology and Sleep disorders (AFSORL) and the French Society of Otorhinolaryngology (SFORL) put forward a summary of the measures for continuing the treatment of sleep apnoea syndrome in these new practice conditions. Emphasis is placed on teleconsultation, the conditions for treatment by CPAP, and the postponement of more invasive treatments | ||
| Kryger and Thomas 2020 [12] | The authors describe a new method to reducing viral shedding in COVID-19 patients on PAP devices. The new circuit elements are designed to vent exhaled air away from the patient and impose a filter before the air can exit the system As at home, it may be difficult protect others, it may be better to treat patients with severe OSA and COVID-19 infection in a healthcare facility. |
Non-systematic | |
| Lance et al., 2020 [9] | Review | Summary main points: PAP therapy may increase the risk of transmission of COVID-19 to others in the environment. PAP therapy user should sleep in a separate bedroom and use a separate bathroom where possible. PAP equipment should be diligently cleaned. For infected patients it may be reasonable to discontinue PAP or to maintain strict quarantine. AASM recommendations include: Postponing in-laboratory sleep studies for all patients. Postponing home sleep apnea testing unless using disposable testing devices. Sleep clinics should remain open for phone calls, telemedicine visits, and emergency in-person visits only. Basic continuous PAP and bilevel PAP devices are not a substitute for the use of a ventilator in the setting of acute respiratory failure. Should the need arise to use PAP/NIV devices to assist with mild hypoxemia and hypercapnia pending endotracheal intubation, adaptations will need to be made to the mask and filtration systems. |
Non-systematic |
| Lavigne et al., 2020 [14] | Whilst oral appliance treatments are normally low-risk procedures, dental and sleep medicine professionals may have to review the required levels of protection required, for patients, families, and staff, with respect to COVID-19. There are a number of issues that need to be addressed including whether mouth-breathing sleepers, patients with positive disease history, and “healthy”
carriers pose risks for their sleep partners. And, are greater precautions now needed when cleaning oral sleep appliances? It is hoped that the AASM may add recommendations for oral devices to any sleep related COVID treatment guidance. |
Non-systematic | |
| Part (c) The effect of COVID-19 on OSA diagnosis | |||
| Grote et al., 2020 [15] | Questionnaire study 25 of 29 sleep centers from the European Sleep Apnoea Database (ESADA) and 15 of 283 accredited sleep centres of the German Sleep Society (DGSM). |
Prior to the pandemic, laboratory-based polysomnography was performed in 92.5% of centres v 20% during the pandemic (p < 0.001). Telemedicine- based sleep apnoea diagnosis was used in 30.0% of centres prior to the pandemic v 27.5% during the pandemic. Prior to the pandemic, laboratory-based CPAP or bi-level PAP titration and initiation were practiced in almost all centres but less than one fifth of centres continued this routine during the pandemic (p < 0.001). Prior to the pandemic, 39 centres report regular follow-up procedures in patients with SDB. This service continued in 36 centres during the pandemic but the mode of follow up had changed. The majority of centers provided follow-up by phone or video-calls. Staffing levels in the sleep medicine service was reduced to 25% (Interquartile range (IQR) 40) for physicians and to 19% (IQR 28) for nurses/technicians compared to pre-pandemic levels.
|
Large European-wide study. Descriptive report from questionnaire findings |
| Drummond 2020 [16] | Review | Summary points include:
Clinical routines need to be adapted. Non-emergency diagnostic or therapeutic procedures should be postponed. |
Non-systematic |
| Zhang and Xiao 2020 [13] | Findings overview from task force Chinese sleep centers Task force commissioned by the Chinese Thoracic Society |
The aim of the task force was to develop a consensus on sleep study and NIPAP treatment during the epidemic of COVID-19 in China. Recommendations include:
|
Task force of sleep medicine specialists and pulmonologists |
NIPAP, non-invasive positive airway pressure; PAP, positive airway pressure; BMI, body mass index.
Of the six studies with original data, Arentz et al. demonstrated that OSA was present in 28% of the 21 patients with coronavirus disease who presented to their intensive care unit [24]. Likewise, Bhatraju et al. found that 21% of patients with severe COVID pneumonia had OSA [17]. Gupta noted that of the first twenty-one patients admitted to a hospital in India with COVID-19 one had OSA [20]. Memsoudis et al. noted that of sixty ICU patients 8.3% had OSA and of the non-ICU patients 6.3% had OSA [21]. He reflected that as nearly all the patients were obese the number of expected OSA patients was low and might reflect under-diagnosis of this disease, See Table 1; part (a). The Coronavirus SARS-CoV-2 and Diabetes Outcomes (CORONADO) study, aims to identify the clinical and biological features associated with risk of poor COVID-19 outcomes. In this study of people with diabetes hospitalized for COVID-19, Cariou et al. reported that treated obstructive sleep apnoea was independently associated with an increased risk of death on day 7 (OR 2.80 [1.46, 5.38]) [18]. In a single case study a 74-year old male, with notable OSA, presented to hospital with hypoxia and dyspnea. He tested positive for COVID-19 but was successfully extubated and treated with average volume-assured pressure support (AVAPS) [22].
What are the potential mechanistic pathways by which COVID-19 may affect OSA patients?
A number of different biochemical and inflammatory mechanisms are associated with the pathophysiology and progression of OSA and its associated poor health outcomes, some of which have also been associated with COVID-19 disease. The sleep-time disruptions in breathing that accompany OSA are associated with intermittent blood gas disturbances (hypercapnia and hypoxemia) and surges of sympathetic activation. Increased inflammatory markers have been reported in OSA but it remains to be determined if this is a result of the associated comorbidities [25]. Increased underlying inflammation however may be of particular importance in obese patients as potentially it may contribute to worsening hypoxemia and the cytokine storm [26] that occurs in COVID-19 pneumonia patients and in those with subsequent multi-organ failure [26]. Results from a French study of 124 COVID patients indicated that obesity (BMI >30 kg/m2) was a risk factor for invasive mechanical ventilation independent of age, diabetes mellitus and hypertension but frequency of OSA was not reported [27].
Recent studies have suggested that the sleep hormone melatonin may be beneficial for the treatment of COVID-19 [[28], [29], [30]]. Melatonin may decrease oxidative stress, inflammation and the immune response, which may be particularly important in patients with OSA in whom these pathways are already activated. Activation of these pathways leads to a cytokine storm and subsequent progression to acute lung injury/acute respiratory distress syndrome (ARDS) and often death. Melatonin may also improve sleeping quality, which might also be beneficial for better clinical outcomes for COVID-19 patients [28].
There is increasing evidence to suggest that vitamin D deficiency may be a risk factor for CVD [31]. Vitamin D may play an important role in the suppression of harmful reactive oxygen species (ROS) and inflammatory markers and may stimulate protective endothelial nitric oxide production [31]. In a study of 176 children, Kheinrandish-Gozal et al. demonstrated that OSA was independently associated with higher hsCRP and lipids, and lower vitamin D [32]. Furthermore, multiple logistic regressions identified a strong effect of African American race on lower vitamin D levels, and showed the severity of OSA as measured by the apnoea hypopnea index (AHI) correlated with lower vitamin D [32]. Vitamin D levels may be influenced by both diet and ethnicity. Vitamin D also enhances cellular immunity, in part by reducing the cytokine storm induced by the innate immune system and it has been suggested that low levels of vitamin D may in part be associated with poor COVID-19 outcomes [33]. A recent study from the UK Biobank, however, did not find any evidence to support this [34]. They examined the data from 348,598 UK Biobank participants of whom 449 had confirmed COVID-19 infection. They found that whilst vitamin D was associated with COVID-19 infection this did not persist after adjustment for confounders. They concluded that ‘their findings did not support a potential link between vitamin D concentrations and risk of COVID-19 infection, nor that vitamin D concentration may explain ethnic differences in COVID-19 infection’ [34].
OSA is observed in a large proportion of individuals with resistant hypertension. A recent meta-analysis demonstrated that OSA is associated with higher Angiotensin II and aldosterone levels, especially in hypertensive patients. It has been suggested therefore that OSA may lead to an increase in blood pressure through stimulation of the renin-angiotensin-aldosterone system (RAAS) [35]. The Angiotensin converting enzyme 2 (ACE2) has been identified as the entry receptor of SARS-CoV-2 [36] but unlike ACE, ACE2 does not convert Angiotensin I to Angiotensin II. Further investigation is therefore warranted to determine whether individuals, in whom there is an increased stimulation of the RAAS from OSA, are at increased risk of the cardiovascular complications and comorbidities seen in COVID-19 patients.
Idiopathic pulmonary fibrosis (IPF) is a chronic lung disease that is associated with oxygen desaturations and pulmonary arterial hypertension. OSA is often reported in patients with IPF and recent IPF guidelines have recognised OSA as an important associated comorbidity that can affect patient's survival [37]. It is recommended that newly diagnosed IPF patients should be referred to sleep centers for the diagnosis and treatment of OSA [38]. Fibrotic changes have also been seen in patients after COVID-19 [39]. It is therefore important that patients who have had COVID-19 and who have fibrotic changes are screened for potential OSA. It is as yet unclear whether OSA patients with existing fibrotic changes may be at increased risk from the effects of COVID-19.
The effect of COVID-19 pandemic on treatment and management of OSA
According to the available evidence, SARS-CoV-2 transmission occurs through droplets. Exhaled aerosol size depends on a number of factors including the characteristics of the fluid, the force and pressure at the moment of emission, and the environmental conditions [40]. Treatment of OSA with Continuous positive airway pressure (CPAP) improves health and wellbeing of patients and improves hypertension management and control in those with moderate-to-severe disease. CPAP is included in the non-invasive ventilation (NIV) category is included in the WHO list of high-risk aerosol-generating procedures [40]. The use of CPAP may therefore put those in its vicinity at increased risk of viral exposure and at a high risk of contagion [9]. It is for this reason that Barker et al. has argued that community CPAP and NIV should be stopped unless it is medically necessary for life support [10]. They acknowledge that evidence is limited but suggest that continued CPAP use might put other members of the household/carers at increased risk. In response to this Baker and Sovani have argued that there are risks from stopping NIV and CPAP for patients [11]. See Table 1; part 2) for more details.
Cessation of CPAP treatment may be associated with a return of symptoms. These may include effects on daytime sleepiness, concentration, memory and mood. A return of symptoms, in particular, excessive sleepiness, may also be harmful to the general public as a result of poor concentration and increased risk of performance errors and road traffic accidents [41],
OSA is a relatively common disorder with many of the 1.5 million individuals diagnosed in the UK alone being treated with CPAP. Given that the pandemic is affecting millions of people globally it is likely that many OSA CPAP–treated patients will be affected. Ways to reduce viral shedding from positive airway devices are therefore important. Bacterial/viral filters are being designed to trap particles and recently Kryger et al. described how the use of Enhanced Expiratory Rebreathing Space (EERS) might help reduce viral shedding [12]. They suggest, however, that an individual with severe OSA who contracts COVID-19 might be best managed in a healthcare facility where staff might be able to take necessary precautions and use Personal Protection Equipment (PPE).
In the UK, the British Sleep Society with the OSA Alliance (incorporating British Thoracic Society, British Sleep Society, Association for Respiratory Technology and Physiology, Sleep Apnoea Trust Association) has just released guidelines with regards to the use of CPAP during the pandemic [42]. The guidance suggests that people with OSA should continue to use their CPAP at home as normal but suggests that individuals might wish to consider taking steps to distance themselves from vulnerable household members by changing bedrooms or stopping CPAP for a short time. It encourages patients to persist with CPAP when experiencing symptoms of respiratory infection. The current National Health Service guidance states, for patients, who remain at home during the coronavirus pandemic, to continue with their usual method of ventilation.
In France, the French Association of Otorhinolaryngology and Sleep disorders (AFSORL) and the French Society of Otorhinolaryngology (SFORL) put forward a summary of the measures for continuing the treatment of sleep apnoea syndrome in these new practice conditions [23] see Table 1; part (b). Interestingly, in a separate study of 7485 patients it was noted that compliance to CPAP improved during lockdown compared with data one month before and when compared with the same period the preceding year. It was suggested that this may be the result of publicity regarding COVID-9, which has described as a disease that affects the airways and, a fear of hospitalization may have motivated patients to adhere to treatment. Spending more time at home might have also led to Increased opportunity to sleep and to use the CPAP treatment [23].
Oral devices may also be used for the treatment for OSA. Given that many dental procedures generate aerosols there is a concern that viral droplets may remain suspended in the air. Thus whilst oral appliance treatments may be low-risk procedures there may be risk to sleep patients from other procedures conducted at dental practices. Likewise, due to the transmission risk, the procedures for cleaning oral sleep appliances, which include mandibular advancement appliance for snoring and sleep apnea, may need to be reviewed [14].
The effect of COVID-19 on OSA diagnosis
OSA is very prevalent worldwide, and given that the comorbidities of OSA patients are shared with those of COVID-19 patients who experience adverse outcomes it may be of great importance to ensure that OSA patients receive effective CPAP therapy if confronted with COVID-19 infection. Despite this, COVID-19 has had a major effect on sleep services both in the UK and elsewhere (see Table 1; part (c)). A recent study by Grote et al. assessed the impact of the COVID-19 pandemic on the management of sleep disordered breathing (SDB) in patients in nineteen European countries [15]. In 31 of the total 40 participating centers patients were unable to physically attend because of travel restrictions. It was found that major changes to the services provided occurred during the pandemic. For the diagnosis of OSA it was reported that, prior to COVID-19 pandemic, 92.5% used in lab Polysomnography, 87.5% used Polygraphy at home and 30% used Telemedicine. During the pandemic, in lab polysomnography had significantly reduced to 20%, at home Polygraphy to 32.5% and Telemedicine based diagnosis was used in 27.5%. They also reported that commencement of treatment for SDB by various types of positive airway pressure therapy were equally reduced in the vast majority of centres and countries. Furthermore, staffing levels in the sleep medicine service was reduced to 25% for physicians and to 19% for nurses/technicians compared to pre-pandemic levels [15].
In China, in high epidemic areas, such as Wuhan and the nearby cities in Hubei province, sleep study and non-invasive positive pressure ventilation (NIPPV) were suspended except in case of emergency whereas for areas with low COVID-19 it was decided that in lab sleep studies could be performed in ‘at risk’ individuals providing the possibility of COVID-19 infection could be excluded and with the proviso that appropriate PPE is available and virus control procedure are followed. Other sleep services and follow up appointments are provided via online services [13].
In the US, the American Academy of Sleep Medicine (AASM) issued revised recommendations on 27th April 2020 that include the postponement of in-laboratory sleep studies for all patients [43]. For necessary testing, it recommends that consideration is given to using single-use, fully disposable devices and/or components. If reusable devices are used these must be cleaned and sanitized according to CDC disinfection standards and manufacturer's instructions and that personnel should have appropriate PPE. It advises that a reusable device should be removed from service for at least 72 h in addition to disinfection before its next use [43]. Similar procedures should be followed for home testing and the use of a mail delivery service would ensure that patients do not have to leave their home to receive or return the device [16,43]. Telemedicine may play a more important role in the diagnosis and management of these conditions. For individuals who do need to be seen in sleep clinics it is clear that measures to screen individuals arriving at the clinics as well as increased personal protection measures and sanitization measures need to be implemented. New procedures for the treatment of patients requiring ventilation who become infected with COVID-19 may be required to prevent spread to staff, carers or family members [16].
Discussion
This comprehensive systematic literature review is consistent with a recent study of 1099 COVID-19 patients, which did not specifically look at OSA, but which showed higher rates of chronic obstructive pulmonary disease (COPD), diabetes and hypertension in patients who were admitted to intensive care units, required mechanical ventilation or died [44]. In the majority of the reviewed studies it has not been possible to establish whether OSA is simply a co-morbidity that is associated with Covid-19 morbidity and mortality or whether is it potentially and independent risk factor for poor COVID-19 outcomes. However, the CORONADO study has examined this in more detail [18]. Data published online on May 29th, documents the clinic characteristics of the 1317 individuals with diabetes, in the study prior to admission to hospital with coronavirus symptoms; of these 1189 individuals had treated OSA. The study demonstrated that dyspnea, lymphocyte count, C-reactive protein and AST measured on admission were independent predictors of the primary outcome of tracheal intubation and/or death at day 7. Age, treated obstructive apnoea and microvascular and macrovascular complications were also independently associated with risk of death at day 7 [18]. Further studies are therefor warranted to establish this potentially causal relationship between OSA and COVID-19 outcomes, both in individuals with and in those without diabetes.
In the UK it has been estimated that more than 85% of potential cases of OSA remain undetected [45], and so potentially there could be a large number of unidentified individuals who may be at increased risk from COVID-19.
There are a number of plausible pathways by which COVID-19 might have an adverse effect on OSA patients and further research is warranted to investigate these pathways. Furthermore, given that recent studies suggest that melatonin may be beneficial for the treatment of COVID-19 [[28], [29], [30]] it remains to be seen if sleep and treatment for sleep disorders may have beneficial effects on COVID-19 outcomes.
Many COVID-19 patients suffer pulmonary fibrosis which itself is a risk factor for future development of OSA. Careful monitoring of discharged patients for thrombotic and fibrotic effects and subsequent OSA development are warranted.
Moving forward it is necessary that guidelines are reviewed so that as new knowledge is acquired the best practices for the diagnosis and treatment of sleep disorders under these restrictive pandemic conditions may be developed. Many sleep laboratory teams have been assisting with the respiratory effects suffered by COVID-19 patients and it is clear for ‘normal‘ sleep services to resume, all sleep center stuff including physicians, sleep technicians and respiratory physiotherapists or respiratory nurses will need ongoing training in updated clinical knowledge of the COVID-19 pandemic. It will be necessary to continually review the practices and to develop new safe operating procedures with reference to the guidelines from the government, scientific societies and national authorities.
The emergence of disposable kits for overnight sleep studies is important as they may provide a new and safe way for the diagnosis of sleep conditions, under these restrictive conditions.
Conclusions
The findings from this study suggest that many of the risk factors for OSA are associated with poor COVID-19 outcomes. There are plausible mechanisms by which OSA may be associated with poor outcomes and further studies are required to corroborate the data from the CORONADO study which suggests that treatment for OSA is independently associated with risk of death from COVID-19. The pandemic has had a major effect on the treatment management and diagnosis of OSA and moving forward it may be necessary to explore new diagnosis and treatment pathways for these individuals. This may include the increased telemedicine and the use of disposable diagnostic tools and non-contact sleep surveillance for sleep apnoea diagnosis. Those already diagnosed but awaiting treatment may need priority at this time to mitigate any potential increase in risk.
Practice points.
A review of the current diagnosis, treatment and management protocols for OSA may be required in light of the COVID-19 pandemic.
-
1.
Common risk factors and co-morbidities for OSA are shared with those individuals who have poor COVID-19 outcomes;
-
2.
There are plausible mechanistic pathways by which COVID-19 may have adverse effects on OSA and treated OSA patients may be at increased risk of death from COVID-19;
-
3.
COVID-19 has had major effects on the treatment, management and diagnosis of OSA during the pandemic;
-
4.
Moving forward sleep centres may need to establish new ways to diagnose patients with OSA, potentially using robust decontamination procedures for reusable equipment or disposable sleep study kits;
-
5.
The management and treatment of existing patients will need to be changed, possibly using more telemedicine.
Research agenda.
In the future we need to be able to safely and effectively diagnose and manage OSA and to:
-
1.
Determine whether questionnaire based screening programmes or disposable sleep tests can be used for OSA detection;
-
2.
Corroborate the data that suggest that individuals treated for OSA may be at increased risk of death from COVID-19;
-
3.
Determine whether any adverse risk from COVID-19 for OSA patients can be modified by effective treatment compliance.
Financial statement
The study is part of the Sleep, Health & Society Programme of The University of Warwick.
Conflicts of interest
The authors do not have any conflicts of interest to disclose.
Footnotes
The most important references are denoted by an asterisk.
References∗
- 1.John Hopkins Coronavirus Research Centre. https://coronavirus.jhu.edu/map.html [accessed 26.06.2020].
- 2.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pareek M., Bangash M.N., Pareek N., Pan D. Ethnicity and COVID-19: an urgent public health research priority. Lancet. 2020 doi: 10.1016/S0140-6736(20)30922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinto J.A., Ribeiro D.K., da Silva Cavallini A.F., Duarte C., Freitas G.S. Comorbidities associated with obstructive sleep apnea: a retrospective study. Int Arch Otorhinolaryngol. 2016;20(2):145–150. doi: 10.1055/s-0036-1579546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cappuccio F.P., Siani A. Covid-19 and cardiovascular risk: susceptibility to infection to SARS-CoV-2, severity and prognosis of Covid-19 and blockade of the renin-angiotensin-aldosterone system. An evidence-based viewpoint. Nutr Metab Cardiovasc Dis. 2020 Jul 24;30(8):1227–1235. doi: 10.1016/j.numecd.2020.05.013. Published online 2020 May 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pazarlı A.C., Ekiz T., İlik F. Coronavirus disease 2019 and obstructive sleep apnea syndrome. Sleep Breath. 2020;1 doi: 10.1007/s11325-020-02087-0. [published online ahead of print, 2020 Apr 28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McSharry D., Malhotra A. Potential influences of obstructive sleep apnea and obesity on COVID-19 severity. J Clin Sleep Med. 2020 doi: 10.5664/jcsm.8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lance C.G. PAP therapy increases the risk of transmission of COVID-19. Cleve Clin J Med. 2020 May 5 doi: 10.3949/ccjm.87a.ccc003. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Barker J., Oyefeso O., Koeckerling D., Mudalige N.L., Pan D. COVID-19: community CPAP and NIV should be stopped unless medically necessary to support life. Thorax. 2020;75:367. doi: 10.1136/thoraxjnl-2020-214890. [DOI] [PubMed] [Google Scholar]
- 11.Baker J.G., Sovani M. Case for continuing community NIV and CPAP during the COVID-19 epidemic. Thorax. 2020;75:368. doi: 10.1136/thoraxjnl-2020-214913. [DOI] [PubMed] [Google Scholar]
- 12.Kryger M., Thomas R. Home PAP devices in COVID-19 infected patients. J Clin Sleep Med. April 9, 2020 doi: 10.5664/jcsm.8490. Published Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X.L., Xiao Y. Sleep health service in China during the COVID-19 outbreak. J Clin Sleep Med. 2020 Apr 6;16(7):1221–1222. doi: 10.5664/jcsm.8472. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavigne G., Fabbro C.D., Babiloni A.H., Huynh N., Gauthier L., Arcache P., et al. Dental sleep medicine perspectives post-COVID-19: interprofessional adaptation and directions. J Clin Sleep Med. 2020 May 4 doi: 10.5664/jcsm.8546. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote L., McNicholas W.T., Hedner J., ESADA Collaborators Sleep apnoea management in Europe during the COVID-19 pandemic: data from the European sleep apnoea database (ESADA) Eur Respir J. 2020 May 4:2001323. doi: 10.1183/13993003.01323-2020. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drummond M. Sleep labs, lung function tests and COVID-19 pandemic – only emergencies allowed! Pulmonology. 2020 Apr 27 doi: 10.1016/j.pulmoe.2020.04.002. S2531-0437(20)30089-1. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Keith R., Jerome K.R., et al. Covid-19 in critically ill patients in the Seattle region – case series. N Engl J Med. 2020 Mar 30 doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-Salameh A., Allix I., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020 doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attias D., Pepin J.L., Pathak A. Impact of COVID-19 lockdown on adherence to continuous positive airway pressure (CPAP) by obstructive sleep apnoea patients. Eur Respir J. 2020 May 19:2001607. doi: 10.1183/13993003.01607-2020. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N., Agrawal S., Ish P., Mishra S., Gaind R., Usha G., et al. Clinical and epidemiologic profile of the initial COVID-19 patients at a tertiary care centre in India. Monaldi Arch Chest Dis. 2020 Apr 10;90(1) doi: 10.4081/monaldi.2020.1294. [DOI] [PubMed] [Google Scholar]
- Memtsoudis S.G., Ivascu N.S., Pryor K.O., Goldstein P.A. Obesity as a risk factor for poor outcome in COVID-19-induced lung injury: the potential role of undiagnosed obstructive sleep apnoea. Br J Anaesth. 2020 May 1 doi: 10.1016/j.bja.2020.04.078. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal A., Forte M., Leonard R., Sangani R., Sharma S. Refractory acute respiratory distress syndrome secondary to COVID-19 successfully extubated to average volume-assured pressure support non-invasive ventilator. Cureus. 2020;12(4) doi: 10.7759/cureus.7849. Published 2020 Apr 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bastier P.L., Aisenberg N., Durandd F., Lestange P., Abedipourf D., Gallet de Santerreh O., et al. Treatment of sleep apnea by ENT specialists during the COVID-19 pandemic. Eur Ann Otorhinolaryngol Head Neck Dis. 2020 Sep;137(4):319–321. doi: 10.1016/j.anorl.2020.05.001. Published online 2020 May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M., et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. J Am Med Assoc. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unnikrishnan D., Jun J., Polotsky V. Inflammation in sleep apnea: an update. Rev Endocr Metab Disord. 2015;16(1):25–34. doi: 10.1007/s11154-014-9304-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jose R.J., Ari Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30216-2. S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., et al. High prevalence of obesity in severe acute respiratory syndrome corona-virus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020 Apr 9 doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang R., Wang X., Ni L., Di X., Ma B., Niu S., et al. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020 Jun 1;250:117583. doi: 10.1016/j.lfs.2020.117583. [Epub 2020 Mar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shneider A., Kudriavtsev A., Vakhrusheva A. Can melatonin reduce the severity of COVID-19 pandemic? Int Rev Immunol. 2020 Apr 29:1–10. doi: 10.1080/08830185.2020.1756284. [DOI] [PubMed] [Google Scholar]
- 30.Zambrelli E., Canevini M., Gambini O., D'Agostino A. Delirium and sleep disturbances in COVID-19: a possible role for melatonin in hospitalized patients? Sleep Med. 2020 Apr 17;70:111. doi: 10.1016/j.sleep.2020.04.006. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kassi E., Adamopoulos C., Basdra E.K., Papavassiliou A.G. Role of vitamin D in atherosclerosis. Circulation. 2013;128(23):2517–2531. doi: 10.1161/CIRCULATIONAHA.113.002654. [DOI] [PubMed] [Google Scholar]
- 32.Kheirandish-Gozal L., Peris E., Gozal D. Vitamin D levels and obstructive sleep apnoea in children. Sleep Med. 2014;15(4):459–463. doi: 10.1016/j.sleep.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988. Published 2020 Apr 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hastie C.E., Mackay D.F., Ho F., Celis-Morales C.A., Katikireddi S.V., Niedzwiedz C.L., et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab Syndr. 2020;14(4):561–565. doi: 10.1016/j.dsx.2020.04.050. [published online ahead of print, 2020 May 7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin Z.-N., Wei Y.-X. Meta-analysis of effects of obstructive sleep apnea on the renin-angiotensin-aldosterone system. J Geriatr Cardiol. 2016 May;13(4):333–343. doi: 10.11909/j.issn.1671-5411.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.South A.M., Diz D.I., Chappell M.C. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318(5):H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiza S., Mermigkis C., Margaritopoulos G.A., Daniil Z., Harari S., Poletti V., et al. Idiopathic pulmonary fibrosis and sleep disorders: no longer strangers in the night. Eur Respir Rev. 2015;24:327–339. doi: 10.1183/16000617.00009114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lancaster L.H., Mason W.R., Parnell J.A., Rice T.W., Loyd J.E., Milstone A.P., et al. Obstructive sleep apnea is common in idiopathic pulmonary fibrosis. Chest. 2009;136(3):772–778. doi: 10.1378/chest.08-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye Z., Zhang Y., Wang, Huang Z., Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020 doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization . 27 February 2020. Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19) Interim guidance.https://apps.who.int/iris/bitstream/handle/10665/331215/WHO-2019-nCov-IPCPPE_use-2020.1-eng.pdf [Google Scholar]
- 41.Garbarino S., Durando P., Guglielmi O., Dini G., Bersi F., Fornarino S. Sleep apnea, sleep debt and daytime sleepiness are independently associated with road accidents. A cross-sectional study on truck drivers. PLoS One. 2016;11(11) doi: 10.1371/journal.pone.0166262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://brit-thoracic.org.uk/media/455098/osa-alliance-cpap-covid-19-advice-20-3-20-v10.pdf
- https://aasm.org/covid-19-resources/covid-19-mitigation-strategies-sleep-clinics-labs
- 44.Guan W.J., Ni Z.Y., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mackay T. British Lung Foundation Tool Kit; 2010. OSA working towards the development of minimal standards for referral, investigation and treatment in Scotland.https://www.blf.org.uk/sites/default/files/OSA_Toolkit_2015_BLF_0.pdf [Google Scholar]