Abstract
Objectives
This study investigated demographics, comorbidities, and death rate in hospitalized patients with confirmed COVID-19. In addition, we hypothesized that functional status, according to the Clinical Frailty Scale (CFS), in patients aged 65 years or older is a better predictor of poor outcome than age and comorbidities.
Methods
A total of 255 randomly selected COVID-19 patients admitted to a university hospital were included and followed up for 60 days. Patient data were extracted manually from the electronic health records with use of a standardized protocol.
Results
The age of the study population ranged between 20 and 103 years (mean age 66 years ± 17 years). Hypertension, diabetes mellitus, and obesity were the three most prevalent comorbidities. At the 60-day follow-up, 70 patients (27%) had died. In multivariate analyses, age, chronic kidney disease, and previous stroke were associated with death. Most fatal cases (90%) occurred in patients aged 65 years or older. Among such patients, CFS level was the only predictor of death in multivariate analyses.
Conclusions
This study shows that increasing age, chronic kidney disease, and previous stroke significantly contribute to a fatal outcome in hospitalized patients with COVID-19. In patients aged 65 years or older, CFS level was the strongest prognostic factor for death.
Keywords: COVID-19, SARS-CoV-2, Risk factors, Mortality, Clinical Frailty Scale, Frailty
Introduction
The COVID-19 pandemic has greatly challenged the health care systems of many countries because of rapidly increasing numbers of infected patients within a short period. Danderyd University Hospital in Stockholm was one of the first hospitals in Sweden to experience a surge of COVID-19 patients in early March 2020. Despite a fast expansion of inpatient beds and intensive care units dedicated to COVID-19 patients, the high number of patients with severe disease threatened to exceed the hospital capacity. For the numerous elderly and frail patients with rapid progress of respiratory failure, questions regarding optimal clinical management and limitations of life-sustaining treatment were raised.
In the light of the difficulty in making well-grounded decisions on advanced life support treatment for this new patient group, we initiated a retrospective study of demographics, comorbidities, clinical course, and case fatality rate in a group of randomly selected patients admitted to the hospital.
In addition, for patients aged 65 years or older, we investigated how physical and functional status, according to the Clinical Frailty Scale (CFS), correlated with clinical outcome. The CFS is a validated tool for prediction of future functional decline and death among patients aged 65 years or older (Rockwood et al., 2005), and we hypothesized that it would be a better predictor of poor outcome in patients aged 65 years or older than age and comorbidities.
Methods
Study population and data collection
A total of 255 patients admitted to Danderyd University Hospital between March 5 and April 28, 2020, were included. Patients were randomly selected from lists including all patients with confirmed COVID-19 who were discharged from the hospital during the period. These lists were extracted from the hospital discharge register by a registrar, and contained only the birth date and a four-digit identification code of the patients. To achieve a similar age distribution between study patients and the whole COVID-19 cohort of the hospital, the patients were stratified by age, and manual selection was subsequently performed at random within the different age strata. After inclusion, ten experienced physicians working at the hospital extracted the patient data manually from the electronic health records using a standardized protocol with detailed instructions. Data extraction was supervised by two senior clinicians. The final date of follow-up was July 10. At that time all patients included in the study had been discharged or had died, and all patients were followed up at 60 days after hospital admission. No antivirals or corticosteroids were used for the treatment of COVID-19 during the study period. Chloroquine derivatives were used only during the first few weeks of the study.
Data on demographics, comorbidities, laboratory and radiographical findings, treatments, need for respiratory support, including intensive care unit transfer and mechanical ventilation, complications, and discharge from hospital were collected.
The functional state and frailty level of patients aged 65 years or older, before their acute illness, was determined on the original, seven-point Clinical Frailty Scale (CFS) (Rockwood et al., 2005). Determination of CFS level was based on a description of activity and functional status as documented in electronic health records. The CFS ranged from very fit (level 1) to severely frail (level 7; see Table 1 ), and was categorized into four groups: fit (levels 1–3), vulnerable (level 4), mildly frail (level 5), and moderately to severely frail (level 6 or 7). The frailty level for all patients was decided by one specialty registrar, and all borderline cases were adjudicated by a specialist physician.
Table 1.
Clinical Frailty Scale.
| Level | Definition |
|---|---|
| 1 | Very fit. People who are robust, active, energetic, and motivated. These people commonly exercise regularly. They are among the fittest for their age |
| 2 | Well. People who have no active disease symptoms but are less fit than those with level 1. Often they exercise or are very active occasionally (e.g., seasonally) |
| 3 | Managing well. People whose medical problems are well controlled but who are not regularly active beyond routine walking |
| 4 | Vulnerable. Although these people are not dependent on others for daily help, often their symptoms limit activities. A common complaint is being “slowed up” and/or being tired during the day |
| 5 | Mildly frail. These people often have more evident slowing, and need help with high-order instrumental activities of daily living (e.g., finances, transportation, heavy housework, and medications) |
| 6 | Moderately frail. People who need help with all outside activities and with housekeeping. Inside, they often have problems with stairs and need help with bathing and might need minimal assistance with dressing |
| 7 | Severely frail. Completely dependent for personal care because of any cause (physical or cognitive). |
Definitions
Patients were considered to have a confirmed infection if SARS-CoV-2 was detected in respiratory specimens by a polymerase chain reaction assay. All patients were followed up for at least 60 days after hospital admission. The term “older patients” in this study refers to patients aged 65 years or older. The mention of limitation of life-sustaining therapy in the text refers to withholding mechanical ventilation, since respiratory failure is the predominant complication of COVID-19.
Obesity was defined as body mass index (BMI) greater than 30 kg/m2. Chronic kidney disease (CKD) was defined as stage 3 or higher with reduced estimated glomerular filtration rate, below 60 mL/min/1.73 m2. Only progressive neurological conditions with disabilities (e.g., multiple sclerosis or Parkinson’s disease) were diagnosed as neurological disease in the study. Dementia was defined by a registered dementia diagnosis before the study. History of stroke did not include previous transient ischemic attacks. During hospital stay, acute kidney failure was defined as a 50% increase in serum creatinine levels from baseline levels. Pulmonary embolism was diagnosed through computed tomography pulmonary angiography (CTPA). Acute heart failure was documented from the health records as defined by attending clinicians on the basis of clinical, laboratory, or ultrasound findings. Septic shock was defined according to the Sepsis-3 definition (Singer et al., 2016).
Statistical analysis
Categorical and continuous variables were presented as the number of patients, the percentage of patients, the mean ± standard deviation, or the median with the interquartile range (IQR). Independent t tests, the Mann–Whitney U test, or Fisher’s exact test was used to evaluate differences between groups, as appropriate. Survival of older patients within different CFS categories was estimated by the Kaplan–Meier method and compared with use of the log rank test. To explore the risk factors associated with death, univariate and multivariate binomial logistic regression models were used with 60-day mortality as the dependent variable. P values less than 0.05 were considered statistically significant. All statistical analyses were performed with Statistica version 13 (TIBCO Software Inc.).
Results
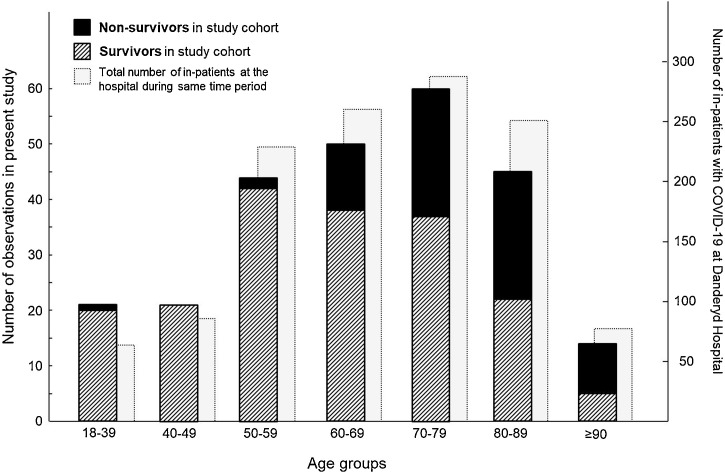
Between March 5 and April 28, 832 patients with confirmed COVID-19 were admitted to Danderyd University Hospital. Of these, 255 patients were randomly selected and included in the study cohort. The age distributions for all inpatients and the 255 patients included in our study during the same period are presented in Figure 1 .
Figure 1.
Age distribution of inpatients with COVID-19 in the study cohort and at Danderyd University Hospital between March 5 and April 28, 2020. The data show the number of survivors (striped bars) and nonsurvivors (black bars) in the study cohort (left y-axis) and the total number of inpatients at Danderyd University Hospital (light gray bars) during the same period (right y-axis).
The most common criterion for hospital admission for COVID-19 patients was room air hypoxemia. Intensive care unit admissions were most commonly reserved for patients with acute respiratory failure that required mechanical ventilation.
Of the 255 randomly selected inpatients with COVID-19, 70 patients (27%) had died at the 60-day follow-up. All but one death occurred within the first 30 days of hospital admission, and 64 of the 70 patients died while in hospital. The median time from hospital admission to death was 6 days (IQR 3–9 days). The length of hospital stay for patients who were discharged alive was 7 days (IQR 3–13 days). Six patients died after discharge, of whom five died during palliative care.
The baseline characteristics of the study cohort, including demographics, comorbidities, ongoing medication, and laboratory findings, are listed in Table 2 . The mean age was 66 years ± 17 years. Nonsurvivors were significantly older than survivors. Among the nonsurvivors, 63 of 70 patients (90%) were aged 65 years or older. A male predominance of about 60% was observed in the study cohort, with similar male-to-female ratios among survivors and nonsurvivors. Most patients in the study were overweight (median BMI 27.4 kg/m2, IQR 24.3–31.1 kg/m2). BMI was not significantly different between survivors and nonsurvivors. Hypertension, diabetes, and obesity were the three most prevalent comorbidities. Among nonsurvivors, the frequencies of hypertension, chronic heart failure, previous stroke, dementia, and chronic kidney disease were higher than in survivors. About 82% of the patients in the study had one or several comorbidities. Of the 70 patients who died, only three (4%) had no relevant comorbidities.
Table 2.
Patient characteristics on admission.
| All patients (n = 255) | Nonsurvivors (n = 70) | Survivors (n = 185) | P | |
|---|---|---|---|---|
| Age (years) | 66 ± 17 | 78 ± 11 | 62 ± 17 | <0.0001 |
| Sex (n, %) | ||||
| - Female | 105 (41) | 28 (40) | 77 (42) | 0.89 |
| - Male | 150 (59) | 42 (60) | 108 (58) | |
| Current smoker (n, %) | 9 (4) | 3 (4) | 6 (3) | 0.71 |
| Previous smoker (n, %) | 103 (40) | 38 (54) | 65 (35) | 0.07 |
| BMI (kg/m2) | 27.4 (24.3–31.1) | 27.2 (23.9–30.0) | 27.5 (24.6–31.6) | 0.15 |
| Clinical Frailty Scale category of patients aged ≥65 years (n, %) | n = 143 | n = 63 | n = 80 | |
| - Fit (levels 1–3) | 38 (27) | 5 (8) | 33 (41) | <0.0001 |
| - Vulnerable (level 4) | 34 (24) | 15 (24) | 19 (24) | 1.0 |
| - Mildly frail (level 5) | 38 (27) | 17 (27) | 21 (26) | 1.0 |
| - Moderately to severely frail (level 6 or 7) | 33 (23) | 26 (41) | 7 (9) | <0.0001 |
| Comorbidity (n, %) | ||||
| - Hypertension | 137 (54) | 50 (71) | 87 (47) | 0.0007 |
| - Diabetes mellitus | 78 (31) | 25 (36) | 53 (29) | 0.29 |
| - Obesity (BMI > 30 kg/m2) | 63 (25) | 15 (21) | 48 (26) | 0.52 |
| - Chronic kidney disease | 49 (19) | 28 (40) | 21 (11) | <0.0001 |
| - Ischemic heart disease | 34 (13) | 14 (20) | 20 (11) | 0.06 |
| - Chronic heart failure | 31 (12) | 15 (21) | 16 (9) | 0.009 |
| - Previous stroke | 23 (9) | 13 (19) | 10 (5) | 0.002 |
| - Dementia | 15 (6) | 10 (14) | 5 (3) | 0.001 |
| - Neurological disease | 7 (3) | 2 (3) | 5 (3) | 1.0 |
| - Asthma | 33 (13) | 6 (9) | 27 (15) | 0.30 |
| - COPD | 14 (5) | 6 (9) | 8 (4) | 0.22 |
| - Obstructive sleep apnea syndrome | 5 (2) | 2 (3) | 3 (2) | 0.62 |
| - Rheumatic disease | 11 (4) | 4 (6) | 7 (4) | 0.50 |
| - Active cancer | 12 (5) | 5 (7) | 7 (4) | 0.32 |
| - None of above comorbidities | 47 (18) | 3 (4) | 44 (24) | 0.0001 |
| - ≥3 of above comorbidities | 89 (35) | 37 (53) | 52 (28) | 0.0004 |
| Ongoing medication (n, %) | ||||
| - ACEi/ARB | 89 (35) | 28 (40) | 61 (33) | 0.31 |
| - Anticoagulant treatment | 49 (19) | 20 (29) | 29 (16) | 0.03 |
| - Statin therapy | 69 (27) | 22 (31) | 47 (25) | 0.35 |
| Immunosuppressive drugs | 13 (5) | 7 (10) | 6 (3) | 0.049 |
| Blood biochemistry on admission | ||||
| - C-reactive protein (mg/L) | 76 (35–132) | 71 (27–129) | 76 (36–133) | 0.95 |
| - White blood cell count (×109/L) | 6.5 (4.9–8.4) | 7.0 (5.1–9.3) | 6.2 (4.9–7.7) | 0.04 |
| - Lymphocyte count (×109/L) | 0.9 (0.6–1.2) | 0.7 (0.5–1.0) | 0.9 (0.7–1.2) | 0.003 |
| - Platelet count (×109/L) | 195 (154–247) | 172 (135–238) | 200 (167–254) | 0.01 |
| - Creatinine (μmol/L) | 81 (65–105) | 103 (80–161) | 76 (61–92) | <0.0001 |
Data are presented as the number and percentage in parentheses, the mean ± standard deviation, or the median and the interquartile range in parentheses. P values show comparison of data between survivors and nonsurvivors.
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor antagonist; BMI, body mass index; COPD, chronic obstructive pulmonary disease.
Clinical outcomes of all patients in the study are summarized in Table 3 . About one-third of the inpatients with COVID-19 in the study did not require any respiratory support, while 47% were treated with oxygen supply administered through a nasal cannula, an OxyMask, or a reservoir mask (see Table 2). Thirty patients (12%) were treated with invasive mechanical ventilation, of whom 20 survived. The median number of days with invasive ventilation was 14.
Table 3.
Treatment and clinical outcomes of inpatients with COVID-19.
| All patients (n = 255) | Nonsurvivors (n = 70) | Survivors (n = 185) | P | |
|---|---|---|---|---|
| Respiratory failure | ||||
| - No respiratory support | 94 (37) | 13 (19) | 81 (44) | 0.0002 |
| - Oxygen supply | 121 (47) | 41 (59) | 80 (43) | 0.04 |
| - Noninvasive ventilation/HFNO | 11 (4) | 6 (9) | 5 (3) | 0.15 |
| - Invasive mechanical ventilation | 30 (12) | 10 (14) | 20 (11) | 0.51 |
| - Days with invasive ventilation | 14 (9–21) | 13 (4–23) | 14 (10–17) | 0.66 |
| Circulatory failure | ||||
| - Acute heart failure | 6 (2) | 5 (7) | 1 (1) | 0.007 |
| - Peak troponin I level (ng/L) | 13 (7–34) | 57 (26–144) | 9 (6–17) | <0.0001 |
| Renal failure | ||||
| - Acute kidney failure | 37 (15) | 22 (31) | 14 (8) | <0.0001 |
| - Renal replacement therapy (new onset) | 4 (2) | 2 (3) | 2 (1) | 0.30 |
| - Peak serum creatinine level (μmol/L) | 78 (60–120) | 106 (77–165) | 73 (58–92) | <0.0001 |
| Thromboembolism | ||||
| - Pulmonary embolism | 3 (1) | 0 | 3 (2) | 0.56 |
| - Peak D-dimer level (mg/L) | 1.9 (0.7–4.3) | 2.5 (2.2–6.9) | 1.0 (0.6–3.2) | 0.03 |
| - No anticoagulant treatment | 119 (47) | 27 (39) | 92 (50) | 0.12 |
| - LMWH, single prophylactic dose | 90 (35) | 30 (43) | 60 (32) | 0.14 |
| - LMWH, double prophylactic dose | 9 (4) | 1 (1) | 8 (4) | 0.45 |
| - Full-dose LMWH or OAC | 36 (14) | 12 (17) | 24 (13) | 0.42 |
| Inflammation and infections | ||||
| - Peak C-reactive protein level (mg/L) | 120 (68–233) | 165 (92–265) | 107 (63–215) | 0.006 |
| - Positive blood culture | 12 (5) | 2 (3) | 10 (5) | 0.52 |
| - Antibiotic treatment >4 days | 66 (26) | 27 (39) | 39 (21) | 0.006 |
| - Hydroxychloroquine treatment (μg/L) | 65 (25) | 16 (23) | 49 (26) | 0.63 |
| Length of hospital stay (days) | 7 (4–12) | 7 (4–10) | 7 (3–13) | 0.84 |
Data are presented as the number and percentage in parentheses or the median and the interquartile range in parentheses. P values show comparison of data between survivors and nonsurvivors.
HFNO, high-flow nasal oxygen support; LMWH, low molecular weight heparin; OAC, oral anticoagulant.
Acute renal failure, defined as 50% increase in creatinine levels, was more common among nonsurvivors. Of the 49 patients with known chronic kidney disease, 12 patients had an acute-on-chronic kidney failure. Renal replacement therapy was started for four patients during the study.
Nonsurvivors had higher troponin levels than survivors, (median 57 ng/L versus 9 ng/L). The highest troponin level measured in a patient during hospitalization was 642 ng/L. Acute heart failure was detected in six patients. Ten patients had new onset of arrhythmia.
Pulmonary embolism was found in three patients. However, CTPA was performed in only 24 patients (9%) in the study population. Patients with severe respiratory failure who were considered too unstable to undergo CTPA were treated with full-dose low molecular weight heparin in the absence of contraindications. Treatment with full-dose anticoagulants (oral anticoagulants or full-dose low molecular weight heparin) during the hospital stay was not associated with reduced mortality.
Secondary bacterial infections were diagnosed on the basis of clinical and laboratory findings in 66 patients (26%). Two patients experienced septic shock, and both survived. Peak levels of C-reactive protein were higher in nonsurvivors than in survivors. Treatment with hydroxychloroquine was neither associated with increased mortality nor associated with reduced mortality. During the study period, corticosteroid therapy was started in none of the patients.
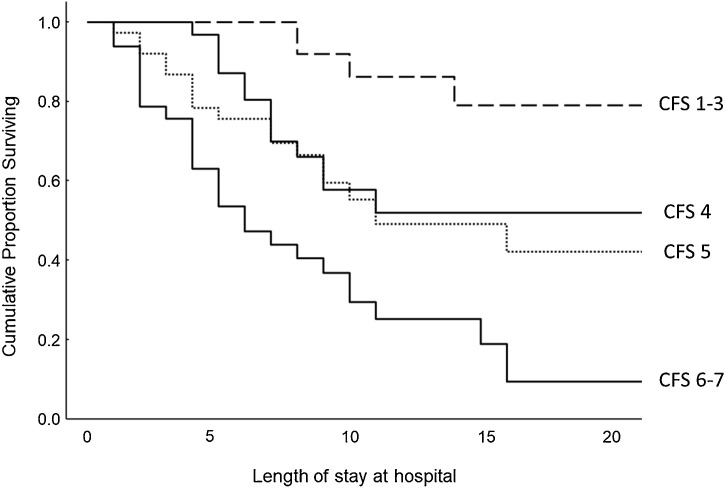
Of the 255 patients included in the study, 143 were aged 65 years or older. The frailty of these older patients before their first presentation of symptoms was determined retrospectively with use of the CFS. CFS category was significantly correlated to survival (log rank test, P < 0.0001; Figure 2 ). Limitation of life-sustaining therapy was common in older and vulnerable/frail patients (CFS levels 4–7). Table 4 demonstrates the number of patients for whom mechanical ventilation therapy was withheld among survivors and nonsurvivors within the different CFS categories. Death events were not significantly different in patients for whom mechanical ventilation was withheld compared with patients without such limitation of life-sustaining therapy among the vulnerable and frail older patients.
Figure 2.
Kaplan–Meier survival analyses in relation to Clinical Frailty Scale (CFS) category in patients aged 65 years or older.
Table 4.
Survivors and nonsurvivors with limitation of treatment and mechanical ventilation therapy based on Clinical Frailty Scale (CFS) category among patients aged 65 years or older.
| CFS category | Survivors |
Nonsurvivors |
||||
|---|---|---|---|---|---|---|
| n | Limitation of treatmenta | Mechanical ventilation | n | Limitation of treatmenta | Mechanical ventilation | |
| Fit (levels 1–3) | 33 | 4 | 7 | 5 | 3 | 3 |
| Vulnerable (level 4) | 19 | 15 | 0 | 15 | 11 | 2 |
| Mildly frail (level 5) | 21 | 17 | 0 | 17 | 16 | 0 |
| Moderately to severely frail (level 6 or 7) | 7 | 7 | 0 | 26 | 26 | 0 |
Data are presented as the number of patients.
Refers to patients for whom intubation and mechanical ventilation was withheld.
We performed univariate binomial logistic regression analyses to investigate possible risk factors associated with death in patients with COVID-19. Age, sex, and comorbidities that were prevalent in at least 20 patients in the study cohort were included in the univariate analyses. For older patients, CFS level (range 1-7) was also included as a possible predictor of death. Variables significantly associated with death in the univariate analyses were included in a multivariate logistic analysis model (Table 5 ). In the univariate analyses of patients of all ages, patients with chronic kidney disease had the highest odds ratio for death. Increasing age, hypertension, chronic heart disease, and previous stroke were also associated with death. In the multivariate model, increasing age, chronic kidney disease, and previous stroke remained as significant predictors of death. Among the older patients, CFS level was the strongest predictor of death in the univariate analyses, and the only predictor with significant impact on death in the multivariate analyses.
Table 5.
Risk factors associated with death.
| Univariate regression analyses |
Multivariate regression analyses |
|||
|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
| All patients Age Male sex |
1.08 (1.05–1.11) | <0.0001 | 1.07 (1.04–1.10) | <0.0001 |
| Obesity | 1.07 (0.61–1.87) | 0.81 | – | – |
| Diabetes mellitus | 1.35 (0.68–2.66) | 0.39 | – | – |
| Hypertension | 1.37 (0.77–2.46) | 0.29 | – | – |
| Chronic kidney disease | 2.79 (1.54–5.05) | 0.0007 | 0.92 (0.45–1.92) | 0.83 |
| Chronic heart failure | 5.17 (2.68–10.01) | <0.0001 | 3.30 (1.54–7.10) | 0.002 |
| Previous stroke | 2.86 (1.33–6.17) | 0.007 | 1.01 (0.42–2.42) | 0.98 |
| Ischemic heart disease | 3.97 (1.65–9.54) | 0.002 | 2.82 (1.06–7.51) | 0.04 |
| Asthma | 2.05 (0.97–4.33) | 0.06 | – | – |
| Patients aged ≥65 years | 0.55 (0.21–1.38) | 0.20 | – | – |
| Age | 1.05 (1.01–1.09) | 0.03 | 1.00 (0.96–1.06) | 0.83 |
| Sex (male) | 1.29 (0.66–2.51) | 0.46 | – | – |
| Male sex | 1.06 (0.45–2.51) | 0.89 | – | – |
| CFS level (anrange 1–7) | 2.18 (1.59–2.99) | <0.0001 | 2.07 (1.47–2.92) | <0.0001 |
| Hypertension | 1.25 (0.60–2.60) | 0.55 | – | – |
| Chronic kidney disease | 2.73 (1.31–5.69) | 0.007 | 2.07 (0.94–4.57) | 0.07 |
| Chronic heart failure | 1.22 (0.54–2.76) | 0.64 | – | – |
| Previous stroke | 2.02 (0.80–5.10) | 0.14 | – | – |
| Ischemic heart disease | 1.04 (0.47–2.32) | 0.92 | – | – |
| Diabetes mellitus | 1.05 (0.53–2.09) | 0.90 | – | – |
| Asthma | 0.42 (0.13–1.39) | 0.15 | – | – |
CFS, Clinical Frailty Scale; CI, confidence interval.
Discussion
This report presents baseline characteristics and clinical outcomes of 255 inpatients with COVID-19 admitted to a university hospital in Stockholm between early March and late April, 2020. At the 60-day follow-up, 27% of the patients (70 of 255 patients) in the study cohort had died. Most deaths occurred in hospital and within 30 days of admission. The high fatality rate in our study could in part be explained by the fact that COVID-19 patients at that time were not treated with corticosteroids or other specific therapies, which have since proven to be beneficial. Our survival data are, however, comparable to those of a study of 191 inpatients with COVID-19 hospitalized in Wuhan showing a death rate of 28% (Zhou et al., 2020), and to a large-scale UK study of 20,133 inpatients with COVID-19 showing a death rate of 26% (Docherty et al., 2020). We found that 90% of fatal cases occurred among patients aged 65 years or older, where the death rate was as high as 44% (63 of 143 patients).
Decisions on limitations of life-sustaining therapy, which are common for older patients with multimorbidity due to medical futility, may, of course, have contributed to the higher fatality rate among older patients in our study. Indeed, we found that intubation and mechanical ventilation was withheld in 69% of the older patients (99 of 143 patients) in the study. However, decisions to withhold mechanical ventilation therapy were based on futility and not because of exhausted capacity at the hospital. In addition, vulnerable and frail older patients had similar death rates, irrespective of whether mechanical ventilation was withheld (Table 4). We therefore believe that the overall fatality rate in our study was not affected by decisions on limitations of life-sustaining therapy among older patients.
The two most prevalent comorbidities found in patients with COVID-19 were hypertension (54%) and diabetes mellitus (31%). We found that hypertension, but not diabetes, was associated with a fatal outcome in the univariate analysis. Potential overrepresentation of hypertension among patients with COVID-19 has been discussed by several investigators, as reviewed by Sardu et al. (2020), and hypertension has also been reported as an independent risk factor for severe COVID-19 (Guan et al., 2020). However, hypertension is a common condition worldwide, with a prevalence of about 78% in individuals aged between 65 and 74 years in Sweden (Wolf-Maier et al., 2003), and after adjustment for age, we no longer found an association between hypertension and death. Furthermore, among the older patients in our study, hypertension was not associated with death in the univariate analysis. Thus, our results indicate that hypertension is not an independent risk factor for death in hospitalized patients with COVID-19.
Overweight was also common among the patients in our study, and obesity was the third most prevalent comorbidity. High prevalence of obesity among inpatients with COVID-19 has also been reported by others (de Siqueira et al., 2020). The association between obesity and severe COVID-19 may be due to a defective immune system and/or higher mass of adipose tissue with angiotensin-converting enzyme 2 (ACE2) receptors (Fortis et al., 2012, Hussain et al., 2020, Kassir, 2020). Furthermore, obesity may have a detrimental effect on lung volume and respiratory function. Notably though, our data showed no association between BMI and disease severity in terms of survival or need for respiratory support (i.e., oxygen supply, noninvasive ventilation, or mechanical ventilation; data not shown).
The only two comorbidities that were identified as independent risk factors for death in our multivariate analyses were chronic kidney disease and previous stroke. The association between chronic kidney disease and an increased risk of severe pneumonia was shown previously (Chou et al., 2014). For patients with COVID-19, the association between kidney failure and a fatal outcome has also been demonstrated and discussed by other authors (Guan et al., 2020, Cheng et al., 2020, Henry and Lippi, 2020). The association between previous stroke and death in our study may in part be due to lasting disabilities in this patient group. Another plausible explanation is that patients with previous stroke carry a predisposition that, under the influence of COVID-19-induced coagulopathy, may have led to vascular events contributing to a fatal outcome.
Age was highly associated with the risk of death in our univariate analyses (odds ratio 1.08, 95% confidence interval 1.05–1.11, P < 0.0001). This finding is in accordance with several large-scale studies that presented increasing age as a risk factor for death in hospitalized patients with COVID-19 (Docherty et al., 2020, Argenziano et al., 2020, Petrilli et al., 2020). However, those studies investigated age as a prognostic factor in a general population with COVID-19, and not specifically among older patients. In our multivariate analyses among older patients, age was not associated with death when data were adjusted for relevant comorbidities and frailty (Table 5). This distinction is important, as activity levels and comorbidities differ substantially between individuals, even at a high chronological age.
As a consequence of decline in multiple physiological functions, older and frail individuals are at increased risk of adverse outcomes when they have an acute illness. It seems that the number of dysfunctional organ systems is more predictive of clinical frailty than abnormalities in any particular organ system (Fried et al., 2009). This is in line with our findings, where multimorbidity was strongly associated with a fatal outcome (Table 2). Although frailty increases with age and is related to multimorbidity, it has been shown to be an independent predictor of adverse health effects (Clegg et al., 2013). In this study, we used the original, seven-point CFS for assessment of frailty among older patients, since this tool is easy to use and has been validated for prediction of future functional decline and death among patients aged 65 years or older (Rockwood et al., 2005, Cardona et al., 2018).
We found that the CFS was strongly associated with death in older patients with COVID-19 (Figure 2 and Table 5). The CFS level was in fact the only independent predictor of fatal outcome in our multivariate analyses, with a hazard ratio of 2.13 (95% confidence interval 1.49–3.03). Thus, our data indicate that frailty is a stronger predictor of death than age and comorbidities in older COVID-19 patients requiring hospitalization. Accordingly, a recent study of 81 older patients with COVID-19 in a geriatric department in Belgium demonstrated that the CFS level had a stronger association with death than age in bivariate logistic regression analyses (De Smet et al., 2020). In addition, a large-scale European multicenter study showed that the CFS level was a better predictor of outcomes in adult hospitalized patients with COVID-19 than age and comorbidities (Hewitt et al., 2020). That study determined the CFS level in all patients aged 18 years or older admitted to the hospital. However, since the CFS has not been validated for younger adults, we determined the CFS level only in patients aged 65 years or older. On the basis of our results and the results in the above-mentioned studies, we suggest that CFS grading should be part of a holistic approach in the decision-making process for older patients.
Our study has some important limitations. Because of the retrospective study design, evaluation of the CFS level had to rely on information obtained from the electronic health records. However, since most primary care units, outpatient clinics, and hospitals in Stockholm County share the same electronic health record system, data from various health care providers, including physiotherapist evaluations, were considered when we were estimating the CFS level. Also, we were not able to include all patients with COVID-19 admitted to the hospital, and the patients were not consecutively included in the study, which could result in a less representative sample. However, we believe that this risk is minimal since the patients were randomly selected and no information on their clinical course and outcome was available at the time of inclusion. Furthermore, the age distribution of our study population agreed with that of the complete population of COVID-19 patients admitted to the hospital during the same period. We therefore believe that our results can be generalized to all patients with COVID-19 who were admitted to the hospital.
In conclusion, in this study of COVID-19 patients admitted to a university hospital in Stockholm, we found that increasing age, chronic kidney disease, and previous stroke contributed significantly to a fatal outcome. However, among patients aged 65 years or older, the physical and functional status, as assessed by the CFS, was the strongest prognostic factor for death.
Conflict of interest
The authors have no conflict of interest to report for this study. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The protocol of this trial was approved by the Swedish Ethical Review Authority (record number 2020-0177).
Acknowledgments
We acknowledge the physicians who extracted patient data from the electronic health records for this study: Carl-Johan Tornberg, Marcus Dahlquist, Emma Warholm, Lisa Knutsson, Martin Strömdahl, Sofia Tornvall, Lena Rennerfelt, Julia Åström, and Carolin Lindholm.
References
- Argenziano M.G., Bruce S.L., Slater C.L., Tiao J.R., Baldwin M.R., Barr R.G. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona M., Lewis E.T., Kristensen M.R., Skjot-Arkil H., Ekmann A.A., Nygaard H.H. Predictive validity of the CriSTAL tool for short-term mortality in older people presenting at emergency departments: a prospective study. Eur Geriatr Med. 2018;9:891–901. doi: 10.1007/s41999-018-0123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C.Y., Wang S.M., Liang C.C., Chang C.T., Liu J.H., Wang I.K. Risk of pneumonia among patients with chronic kidney disease in outpatient and inpatient settings. Medicine (Baltimore) 2014;93:e174. doi: 10.1097/MD.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg A., Young J., Iliffe S., Rikkert M.O., Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Siqueira J.V.V., Almeida L.G., Zica B.O., Brum I.B., Barceló A., de Siqueira Galil A.G. Impact of obesity on hospitalizations and mortality, due to COVID-19: a systematic review. Obes Res Clin Pract. 2020:S1871. doi: 10.1016/j.orcp.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet R., Mellaerts B., Vandewinckele H., Lybeert P., Frans E., Ombelet S. Frailty and mortality in hospitalized older adults with COVID-19: retrospective observational study. J Am Med Dir Assoc. 2020;21:928–932. doi: 10.1016/j.jamda.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty A.B., Harrison E.M., Green C.A., Harwick H.E., Pius R., Norman L. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortis A., Garcia-Macedo R., Maldonado-Bernal C., Alarcon-Aguilar F., Cruz M. The role of innate immunity in obesity. Salud Publica Mex. 2012;54:171–177. doi: 10.1590/s0036-36342012000200014. [DOI] [PubMed] [Google Scholar]
- Fried L.P., Xue Q.L., Cappola A.R., Ferrucci L., Chaves P., Varadhan R. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64:1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B.M., Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol. 2020;52:1193–1194. doi: 10.1007/s11255-020-02451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt J., Carter B., Vilches-Moraga A., Quinn T.J., Braude P., Verduri A. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5:e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A., Mahawar K., Xia Z., Yang W., El-Hasani S. Obesity and mortality of COVID-19. Meta-analysis. Obes Res Clin Pract. 2020;9:S1871. doi: 10.1016/j.orcp.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kassir R. Risk of COVID‐19 for patients with obesity. Obes Rev. 2020;21:e13034. doi: 10.1111/obr.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O´Donnell L., Chernyak Y. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K., Song X., MacKnight C., Bergman H., Hogan D.B., McDowell I. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardu C., Gambardella J., Morelli M.B., Wang X., Marfella R., Santulli G. Hypertension, thrombosis, kidney failure, and diabetes: Is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J Clin Med. 2020;9:1417. doi: 10.3390/jcm9051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf-Maier K., Cooper R.S., Banegas J.R., Giampaoli S., Hense H.W., Joffres M. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003;289:2363–2369. doi: 10.1001/jama.289.18.2363. [DOI] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]