ABSTRACT
Butyrate produced by gut microbiota has multiple beneficial effects on host health, and oligosaccharides derived from host diets and glycans originating from host mucus are major sources of its production. A significant reduction of butyrate-producing bacteria has been reported in patients with inflammatory bowel diseases and colorectal cancers. Although gut butyrate levels are important for host health, oligosaccharide metabolic properties in butyrate producers are poorly characterized. We studied the metabolic properties of fructooligosaccharides (FOSs) and other prebiotic oligosaccharides (i.e. raffinose and xylooligosaccharides; XOSs) in gut butyrate producers. 1-Kestose (kestose) and nystose, FOSs with degrees of polymerization of 3 and 4, respectively, were also included. Fourteen species of butyrate producers were divided into four groups based on their oligosaccharide metabolic properties, which are group A (two species) metabolizing all oligosaccharides tested, group F (four species) metabolizing FOSs but not raffinose and XOSs, group XR (four species) metabolizing XOSs and/or raffinose but not FOSs, and group N (four species) metabolizing none of the oligosaccharides tested. Species assigned to groups A and XR are rich glycoside hydrolase (GH) holders, whereas those in groups F and N are the opposite. In total, 17 enzymes assigned to GH32 were observed in nine of the 14 butyrate producers tested, and species that metabolized FOSs had at least one active GH32 enzyme. The GH32 enzymes were divided into four clusters by phylogenetic analysis. Heterologous gene expression analysis revealed that the GH32 enzymes in each cluster had similar FOS degradation properties within clusters, which may be linked to the conservation/substitution of amino acids to bind with substrates in GH32 enzymes. This study provides important knowledge to understand the impact of FOS supplementation on the activation of gut butyrate producers.
Abbreviations: SCFA, short chain fatty acid; FOS, fructooligosaccharide; XOS, xylooligosaccharide; CAZy, Carbohydrate Active Enzymes; CBM, carbohydrate-binding module; PUL, polysaccharide utilization locus; S6PH sucrose-6-phosphate hydrolase.
KEYWORDS: Butyrate producing bacteria, GH32, fructooligosaccharide, kestose, nystose, genome
Introduction
The human gut microbiota plays essential roles in host health, including food digestion, immunological homeostasis, protection against colonization by pathogens, and the extraction and storage of energy from food components. Recent studies also suggested that the impact of the gut microbiota on the host is not restricted to gut health, but also includes brain development and behavior (gut-brain axis),1 lung health (gut-lung axis),2 nephrolithiasis and renal phosphorus homeostasis (gut-kidney or gut-renal axis),3,4 physical exercise (gut-joint axis),5 and cardiovascular health (gut-heart axis).6 The microbiota excretes many metabolites, which are sometimes essential for host homeostasis. Short chain fatty acids (SCFAs) are among the essential metabolites. Most gut microbes produce SCFAs and/or organic acids in a species- or sometimes strain-specific manner,7 and these have marked impacts on host health. Butyrate, one of the SCFAs produced by the gut microbiota, is the major energy source for epithelial cells in the distal colon,8 induces differentiation of the colonic regulatory T cells,9 and functions as an inhibitor of histone deacetylase in the host.10 These activities are essential for the documented beneficial properties of butyrate, including anti-inflammation,11 gut immune homeostasis,9 inhibition of proliferation, and induction of apoptosis of colorectal cancer cells.12
When compared with other SCFAs or lactate, relatively limited bacteria are responsible for butyrate production in the human gut microbiota. They are mainly specific species in the Clostridium clusters IV and XIVa.13 Significant reduction of the gut butyrate producers has been reported in patients with Crohn’s disease,14 ulcerative colitis,15 diabetes,16 colorectal cancer,17 and infantile food allergy18 when compared with healthy subjects. These microbes usually produce butyrate by metabolizing carbohydrates through butyryl-CoA:acetate CoA-transferase (encoded by the but gene) or butyrate kinase (encoded by the buk gene), but some use lactate and acetate as sources for its production.19,20 Different animal species possess different profiles of gut butyrate producers and diet habits impact the profiles.21 The human gut butyrate producers are highly sensitive to oxygen and the use of organisms as probiotics is not easily applicable due to the difficulty in maintaining their viability. The application of prebiotic oligosaccharides is thus a reasonable tool for the proliferation of the gut organisms. Short-chain fructooligosaccharide (FOS) is one of the well commercialized and investigated prebiotics, and increased butyrate production and/or proliferation of butyrate-producing microbes has been reported in healthy adults, infants with atopic dermatitis, and several animals after the administration of short-chain FOS.22–25 Commercialized short-chain FOS is generally a mixture of FOSs with a degree of polymerization (DP) of 3 and 4, i.e. 1-kestose (kestose) and nystose, respectively, with trace DP5 FOS, fructosylnystose.26 The former study reported that the DP of FOS markedly affects the growth of bifidobacteria and lactic acid bacteria.7,26 Moreover, the DP significantly affected the growth of a human commensal butyrate producer, Anaerostipes caccae;7 however, FOS metabolic properties of most human commensal butyrate producers focusing on DP have been poorly characterized. FOSs are generally hydrolyzed by glycoside hydrolase (GH) family 32 enzymes,27 and the resultant fructose and glucose are metabolized through indigenous metabolic pathways in each microbe.28,29 GH32 enzymes thus play a key role in FOS metabolism, but few studies to date have characterized the prevalence and activity of GH32 enzymes in butyrate-producing gut microbes.
In the present study, FOS metabolic properties in human commensal butyrate-producing bacteria, taxonomically classified into Clostridium clusters IV and XIVa and the genus Butyricimonas (phylum Bacteroidetes), were characterized. A xylooligosaccharide mixture (XOSs) and raffinose, which are used as bifidogenic prebiotics,30,31 were also included in this study. Genomes of the butyrate producers were used to study the prevalence of GH32 enzymes. Activities of the GH32 enzymes were characterized by the Escherichia coli heterologous expression system.
Results
Oligosaccharide metabolic properties
Fourteen butyrate-producing bacteria used in the present study are shown in Table 1. They had different oligosaccharide metabolic properties. Roseburia intestinalis and Roseburia inulinivorans grew with all oligosaccharides tested (hereafter group A), although growth levels were slightly different among oligosaccharides for R. inulinivorans (Figure 1). Faecalibacterium prausnitzii and three species of the genus Anaerostipes actively grew on FOS-type oligosaccharides, i.e. kestose, nystose, and FOSs (hereafter group F), but growth of A. caccae was not observed with nystose (Figure 1). Their growth on raffinose or XOSs was similar level to that on sugar-free medium. On the other hand, Agathobacter rectalis, Coprococcus eutactus, Roseburia faecis, and Roseburia hominis did not metabolize the FOS-type oligosaccharides, and instead metabolized XOSs and/or raffinose (hereafter group XR). Anaerobutyricum hallii, Butyricicoccus faecihominis, and two species of Butyricimonas did not metabolize any of the oligosaccharides tested (hereafter group N). Levels of butyrate production from the metabolism of oligosaccharides after 72 h of incubation were generally consistent with their growth (Figure 2), except that marked butyrate production was not noted by Butyricimonas faecihominis.
Table 1.
Butyrate-producing bacteria used in the present study
| Phylogenetic group | Bacterial strains | Metabolic group* | Genomic accession number |
Genome level | Size (Mb) | Number of CDSs |
|---|---|---|---|---|---|---|
| Clostridium cluster IV | Faecalibacterium prausnitzii JCM 31915 | F | GCA_002734145.1 | Complete | 3.11 | 2790 |
| Clostridium cluster IV | Butyricicoccus faecihominis JCM 31056 T | N | BLYJ01000000 | Draft | 3.03 | 2857 |
| Clostridium cluster XIVa | Agathobacter rectalis JCM 17463 T | XR | GCA_000020605.1 | Complete | 3.45 | 3161 |
| Clostridium cluster XIVa | Anaerobutyricum hallii JCM 31263 | N | BLYK01000000 | Draft | 3.74 | 3261 |
| Clostridium cluster XIVa | Anaerostipes butyraticus JCM 17466 T | F | BLYI01000000 | Draft | 3.17 | 3183 |
| Clostridium cluster XIVa | Anaerostipes caccae JCM 13470 T | F | GCA_000154305.1 | Draft | 3.61 | 3347 |
| Clostridium cluster XIVa | Anaerostipes hadrus ATCC 29173 T | F | GCA_000332875.2 | Draft | 2.77 | 2589 |
| Clostridium cluster XIVa | Coprococcus eutactus JCM 31265 | XR | BLYL01000000 | Draft | 3.25 | 2865 |
| Clostridium cluster XIVa | Roseburia faecis JCM 17581 T | XR | GCA_001406815.1 | Draft | 3.33 | 3046 |
| Clostridium cluster XIVa | Roseburia hominis JCM 17582 T | XR | GCA_000225345.1 | Complete | 3.59 | 3148 |
| Clostridium cluster XIVa | Roseburia intestinalis JCM 17583 T | A | GCA_900537995.1 | Complete | 4.49 | 3928 |
| Clostridium cluster XIVa | Roseburia inulinivorans JCM 17584 T | A | GCA_000174195.1 | Draft | 4.05 | 3559 |
| Bacteroidetes | Butyricimonas faecihominis JCM 18676 T | N | 2830045573** | Draft | 4.79 | 4004 |
| Bacteroidetes | Butyricimonas paravirosa JCM 18677 T | N | JAATLI010000000 | Draft | 5.53 | 4394 |
*Determined based on oligosaccharide metabolic properties in Figure 1
**Obtained from the Integrated Microbial Genomes (IMG) database at the Department of Energy Joint Genome Institute (http://genome.jgi.doe.gov/)
Figure 1.
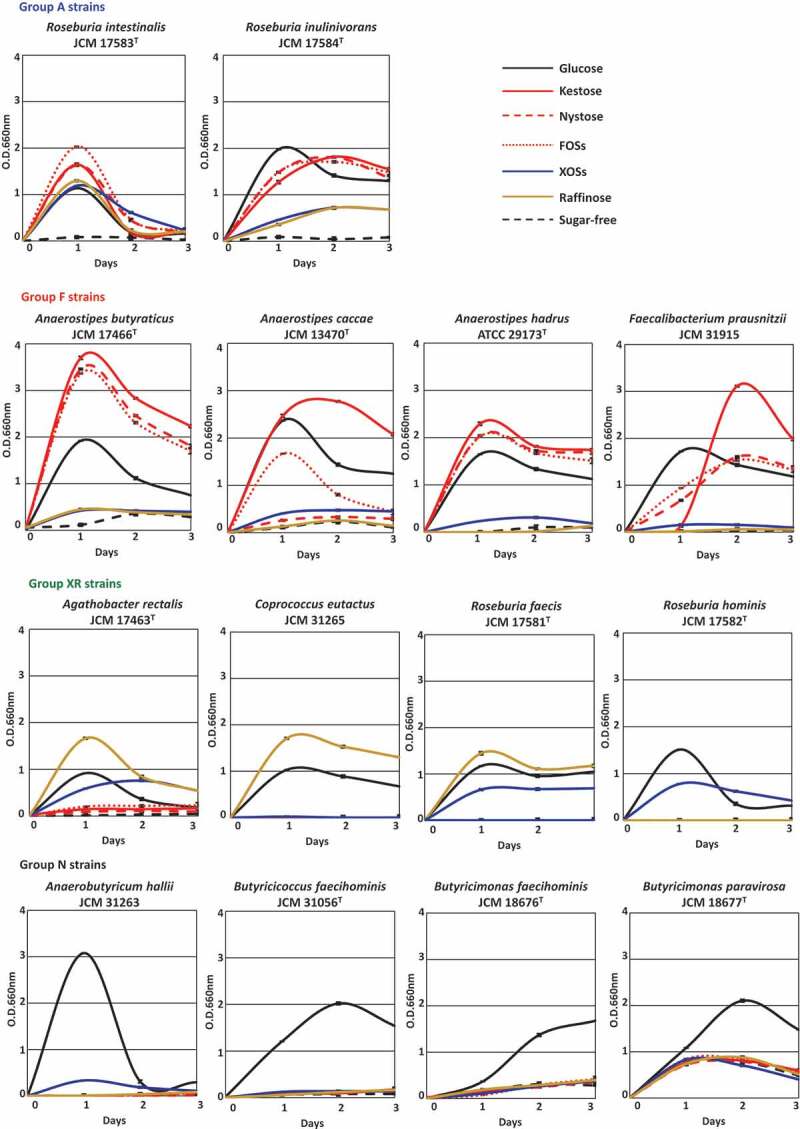
Growth of 14 butyrate-producing bacteria on several oligosaccharides. Growth was monitored at 660 nm in basal YCFA broth supplemented with 0.5% (w/v) oligosaccharides, including kestose, nystose, fructooligosaccharide mixture (FOSs), xylooligosaccharide mixture (XOSs), and raffinose, and glucose and sugar-free broths were included as controls. Data were obtained every 24 h until 72 h. Data plotted in graphs are the means ± standard deviations of triplicates of strains
Figure 2.
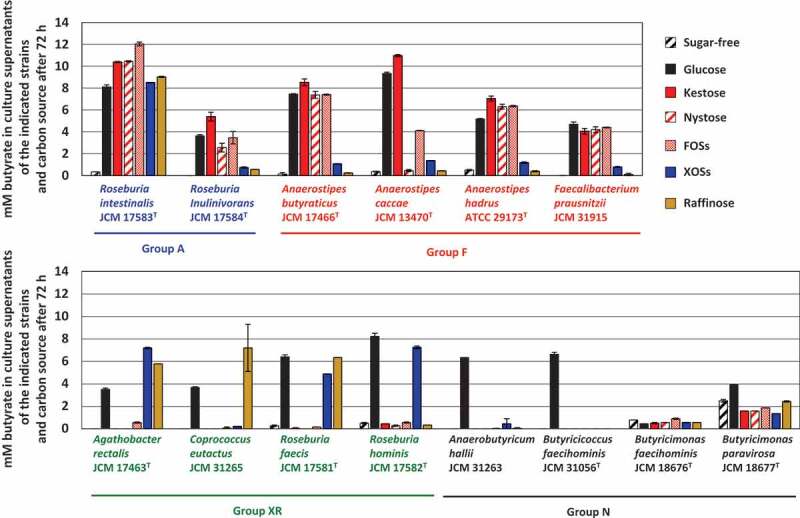
Concentration of butyrate in culture supernatants of 14 butyrate producers in the presence of each oligosaccharide (0.5%, w/v) after incubation of 72 h. Bars and error bars indicate means and standard deviations from triplicates, respectively. Groups A, F, XR, and N correspond to the metabolic groups shown in Figure 1
As FOSs and XOSs are mixtures of different DP oligosaccharides, oligosaccharide metabolic profiles were assessed by measuring the remaining oligosaccharides after culturing. Members in groups A and F metabolized all oligosaccharides in FOSs well, except A. caccae, which completely consumed kestose but left nystose and fructosylnystose (Figure 3(a)). Members in groups XR and N did not consume oligosaccharides in FOSs. Regarding XOSs, strains in group A consumed DP2, DP3, and DP5 oligosaccharides but left DP4 oligosaccharides (Figure 3(b)). Of the group XR strains, R. faecis and R. hominis mainly consumed DP2 and DP3 oligosaccharides, but A. rectalis consumed DP3 and DP4 oligosaccharides and left DP2 oligosaccharide. This suggests that even if butyrate producers grow on XOSs, metabolized oligosaccharides vary among the species. Species that did not actively grow on XOSs (Figure 1) did not exhibit marked consumption of oligosaccharides in XOSs.
Figure 3.
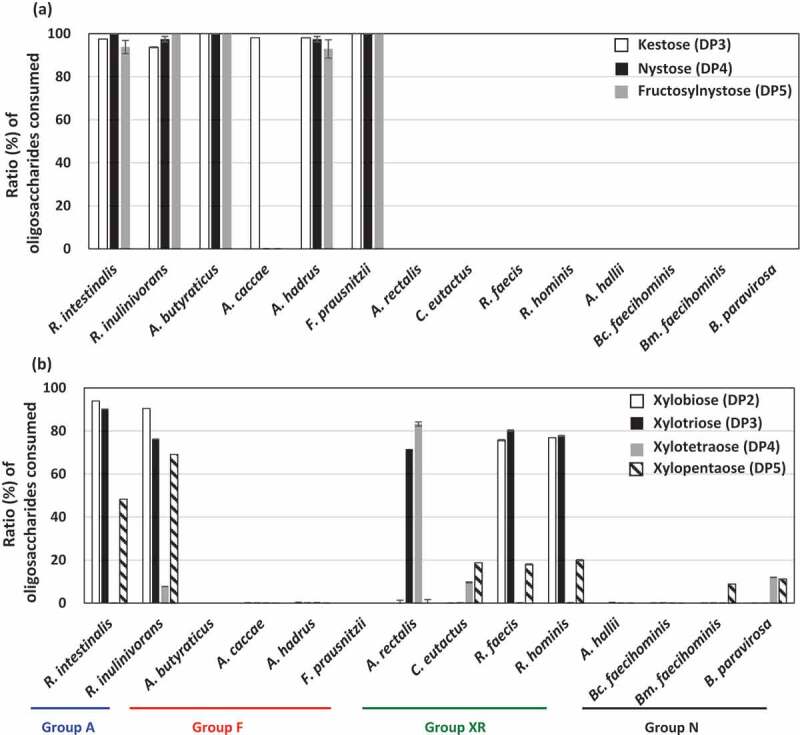
Ratio of each oligosaccharide consumed (%) after metabolism of FOSs (a) and XOSs (b) in butyrate producers. Ratios of oligosaccharides consumed were measured in culture supernatants of YCFA broth supplemented with 0.5% (w/v) FOSs (a) or 0.5% (w/v) XOSs (b) after incubation for 72 h. Kestose, nystose, and fructosylnystose were the major components of FOSs (>95% components in total), whereas xylobiose, xylotriose, xylotetraose, and xylopentaose were the major components of XOSs (>90% components in total). Bars and error bars indicate means and standard deviations from triplicates, respectively. Bars are absent when the strains did not metabolize the oligosaccharides. Groups A, F, XR, and N correspond to the metabolic groups shown in Figure 1. Bc. faecihominis, Butyricicoccus faecihominis; Bm. faecihominis, Butyricimonas faecihominis.
Identification of GH family proteins
Complete or draft genomes of the 14 strains (Table 1) were used to search for GH family proteins using dbCAN2 in the Carbohydrate Active Enzymes (CAZy) database. In total, 61 GH families were found in genomes of the butyrate producers and strains often possessed multiple proteins in a single GH family (Table 2 and Supplemental Table S1). Members in groups A and XR possessed 53–124 and 50–66 GH family proteins, respectively, and these numbers were much larger than those of members in groups F (18–32 proteins) and N (10–25 proteins). Of the 61 GH families found, GH3 and GH13 were conserved in all strains tested, whereas 13 families were unique to a specific strain (Table 2). GH57, GH63, GH84, GH92, and GH109 proteins were only found in Butyricimonas. One or two proteins assigned to GH4, which were annotated as 6-phospho-α-glucosidase and 6-phospho-β-glucosidase, were common in Anaerostipes spp. (Supplemental Table S1), but rare in other strains. GH5, GH8, GH31, GH42, GH43, GH51, and GH94 are unique GH families in groups A and XR with few exceptions (Table 2). All proteins assigned to GH8 found in groups A and XR were described as possible XOS degradation proteins (Supplemental Table S1). GH32 proteins, which are possibly involved in FOS degradation, were found in all members of groups A and F, and three of the four strains in group XR but not in group N. Hierarchical clustering analysis based on the number of proteins in each GH family produced two major clusters (Figure 4). One of the two consisted of members in groups F and N, and the other was composed of group XR and R. inulinivorans. Roseburia intestinalis was distantly positioned from other species, which was due to the presence of a larger number of GH proteins and several unique proteins. The phylogenetic position of the species was not related to this clustering.
Table 2.
Numbers of GH family proteins found in each butyrate producing bacteria
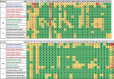 |
Figure 4.
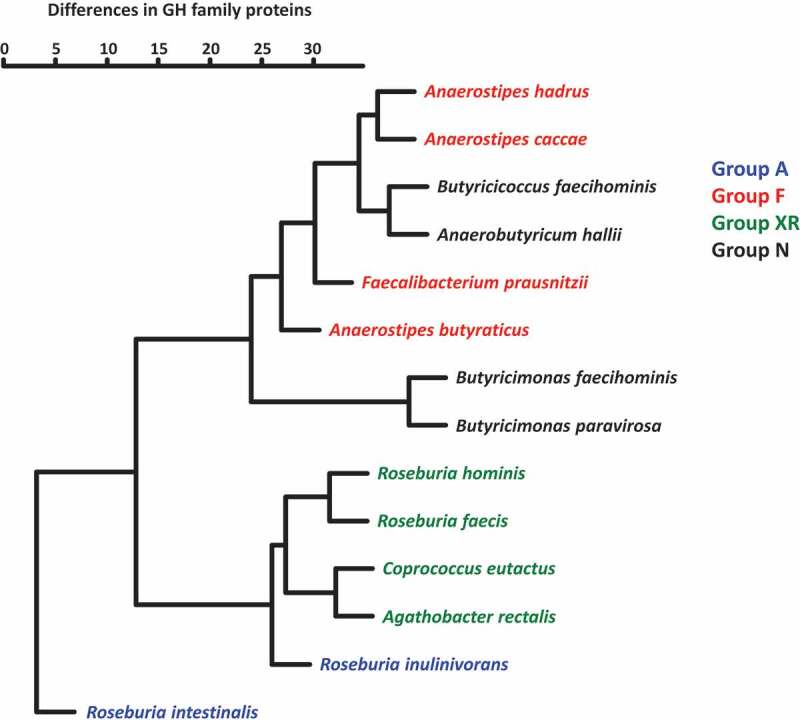
Hierarchical clustering of 14 butyrate-producing bacteria based on the presence of GH family proteins. Numbers of estimated proteins in each GH family were used to prepare a dendrogram using the hclust function with the Ward.D2 algorithm in the R package (version 3.6.2)
Phylogenetic analysis and activities of GH32 proteins
Phylogenetic analysis was conducted based on amino acid sequences of the 17 proteins assigned to GH32 found in the butyrate producers, and two reference GH32 enzymes originating from Bifidobacterium longum strain KN29.1 and Lactobacillus gasseri strain 224–1, whose crystal structures have been determined, were also included in this analysis. The phylogenetic tree produced four major clusters (Figure 5). Cluster 1 included A.rectalis-GH32-1, C.eutactus-GH32-1, C.eutactus-GH32-2, F.prausnitzii-GH32, R.faecis-GH32, R.inulinivorans-GH32, and a reference, B.longum-GH32. The genes encoding these GH32 proteins were adjacent to genes encoding ABC transporters, except that possible transporters were not found adjacent to C.eutactus-GH32-1 and C.eutactus-GH32-2. Genes encoding the two proteins in C. eutactus were adjacent to genes encoding conjugal transfer protein or integrase TN1549-like. The genome of the strain B. longum KN29.1 has not been published and a possible transporter was unable to be identified for B.longum-GH32. Cluster 2 consisted of five GH32 proteins found in three species of Anaerostipes and a reference enzyme, L.gasseri-GH32. All genes encoding these proteins were located adjacent to genes encoding the phosphotransferase system (PTS). Cluster 3 included R.intestinalis-GH32, A.butyraticus-GH32-3, and A.hadrus-GH32-4, and the first was adjacent to ABC transporter and the latter two were adjacent to the PTS. Cluster 4 contained GH32 proteins of A.hadrus-GH32-3, A.rectalis-GH32-2, and C.eutactus-GH32-3, and the first was adjacent to the PTS and the latter two were adjacent to ABC transporters. Of these 17 GH32 proteins found in butyrate producers, signal sequences and family 66 carbohydrate-binding modules (CBM) were identified only from A.butyraticus-GH32-3 and A.hadrus-GH32-4 in cluster 3. The gene encoding A.butyraticus-GH32-3 (4140 bp in total) contains a stop codon at the 200th codon, and the gene is separated into two open reading frames (Locus_Tag ANBU17_10420 and ANBU17_10430). One encodes a 199-amino acid residue protein containing 32 amino acid residues of a signal sequence (Locus_Tag ANBU17_10430), and the other produces a 1179-amino acid residue protein with a catalytic domain (ANBU17_10420). The presence of the stop codon was re-confirmed by Sanger sequencing (data not shown).
Figure 5.
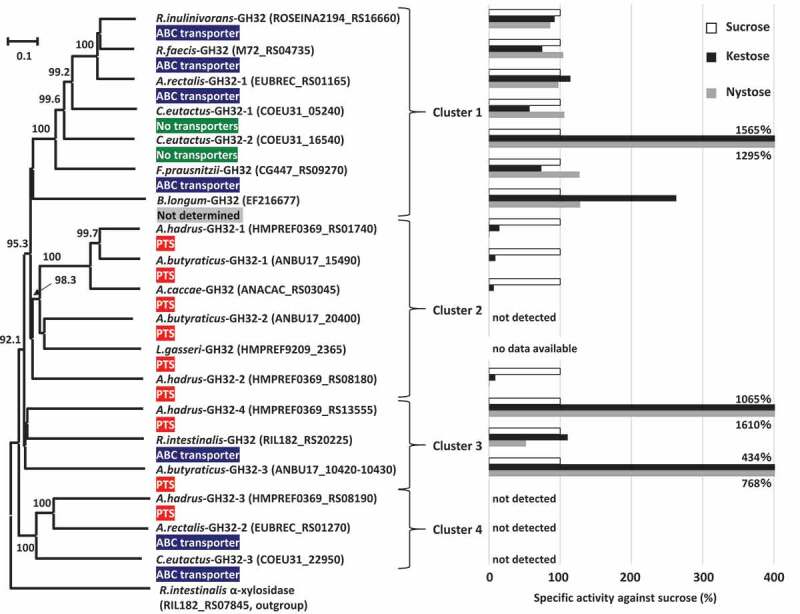
Phylogenetic relationships of GH32 enzymes found in butyrate-producing bacteria, and their relative specific activities with kestose and nystose against sucrose. Locus tags of each protein are shown in parenthesis and transporters adjacent to each GH32 protein are supplied. Extracellular GH32 enzymes (A.hadrus-GH32-4 and A.butyraticus-GH32-3) were expressed using a surface display system on E. coli cells, and whole cells of the transformed E. coli were included to assess the activities of the GH32 enzymes on sucrose, kestose, and nystose. For intracellular GH32 enzymes, cell-free extracts prepared from the transformed E. coli strains were used to assess the activities. Relative specific activities (%) of recombinant GH32 enzymes with kestose and nystose against sucrose are shown. For A.butyraticus-GH32-3, specific activities by the fused enzyme (ANBU17_10420 and ANBU17_10430) are shown, and those by the partial A.butyraticus-GH32-3 (ANBU17_10420) were unable to be assessed because of the lack of activity with sucrose. B.longum-GH32 and L.gasseri-GH32, originating from B. longum KN29.1 and L. gasseri 224–1, respectively, were included as reference proteins, and a possible transporter for B.longum-GH32 was unable to be identified due to the unavailability of genome data for B. longum KN29.1. Specific activities for B.longum-GH32 and A.caccae-GH32 were obtained from previous reports.32,33 α-Xylosidase in Roseburia intestinalis (RIL182_RS07845) was used as an outgroup. Bootstrap percentages above 70% are given at branching points
In addition to the genes encoding carbohydrate transport function proteins, transcriptional regulators and carbohydrate kinases were also located with most of the genes encoding GH32 proteins and formed polysaccharide utilization loci (PULs, Figure 6), as described previously.34,35 The PUL of R.intestinalis-GH32 contains five more GH family proteins, including GH10, two GH13 proteins, GH36, and GH53, of which the GH10 and GH53 proteins are extracellular enzymes (Supplemental Table S1). Genes encoding A.hadrus-GH32-2 and A.hadrus-GH32-3 formed a single PUL, and sandwiched a gene encoding the PTS. Carbohydrate kinases were located with nine of 17 GH32 proteins and transcriptional regulators were with 12 of the GH32 proteins.
Figure 6.
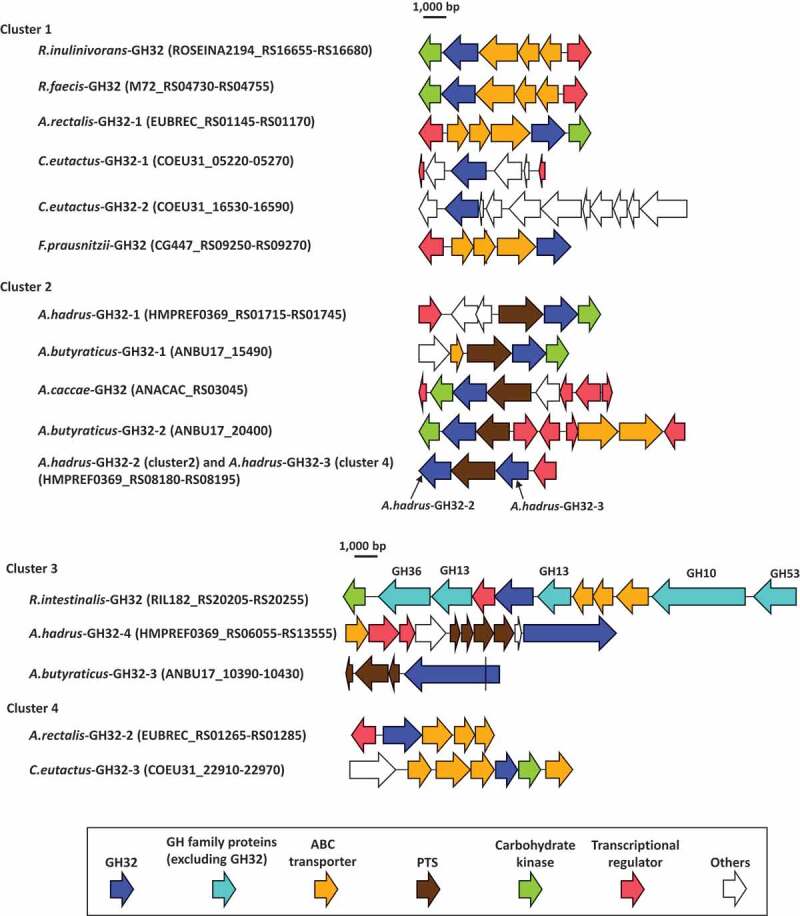
Gene arrangements surrounding GH32 proteins found in butyrate-producing bacteria. Range of locus tags of the genes shown are indicated in parenthesis. A.hadrus-GH32-2 of cluster 2 and A.hadrus-GH32-3 of cluster 4 formed a single PUL. The gene encoding A.butyraticus-GH32-3 contains a stop codon (shown as a black line in the gene) and is separated into two coding sequences based on genomic data. The presence of the stop codon was re-confirmed by Sanger-sequencing
Genes encoding the GH32 enzymes found in butyrate producers were cloned into a plasmid and transferred to Escherichia coli. For intracellular GH32 enzymes, cell-free extracts prepared from the transformed E. coli strains were used to study the activities of the GH32 enzymes on sucrose, kestose, and nystose. Extracellular GH32 enzymes were expressed using a surface display system on E. coli cells, and whole cells of the transformed E. coli were included to assess the activity. Activities of A.butyraticus-GH32-3 containing a stop codon were evaluated by preparation of the two recombinants, one intracellularly expressing the catalytic domain of A.butyraticus-GH32-3 (Locus_Tag ANBU17_10420) and the another expressing the entire A.butyraticus-GH32-3 (Locus_Tag ANBU17_10420 and ANBU17_10430) fused by amino acid replacement of the stop codon with a glutamine codon using the surface display system. The cell-free extracts of E. coli harboring pET28a plasmid or intact E. coli cells harboring pCDF-PgsA plasmid degraded none of the tested substrates. Cell-free extracts containing any one of the six GH32 enzymes in cluster 1 degraded all tested substrates well (Figure 5). These enzymes generally degraded the three substrates at similar levels, except that degradation activity in C.eutactus-GH32-2 was more than 10-times higher with kestose and nystose than with sucrose. Cell-free extracts containing three (A.butyraticus-GH32-1, A.hadrus-GH32-1, and A.hadrus-GH32-2) of the five GH32 enzymes in cluster 2 actively degraded sucrose, but exhibited lower relative degradation activity with kestose (ranging from 9 to 15%) than with sucrose. Nystose was not degraded (relative degradation activity below 1%). These characteristics are consistent with those of A.caccae-GH32 reported previously.32 Degradation of the three substrates was not detected by cell-free extracts containing A.butyraticus-GH32-2. In cluster 3, cell-free extracts containing R.intestinalis-GH32 and E. coli cells displaying extracellular A.hadrus-GH32-4 degraded the three substrates well, and the latter degraded kestose and nystose markedly more than sucrose (relative degradation activity over 1,000%). Cell-free extracts containing a partial A.butyraticus-GH32-3 excluding the N-terminal sequence and signal sequence region (expressing LocusTag ANBU17_10420) degraded kestose and nystose but not sucrose, whereas E. coli cells with the fused whole A.butyraticus-GH32-3 actively degraded all three substrates. Kestose and nystose were degraded more than sucrose by the fused A.butyraticus-GH32-3 (relative degradation activity ranging from 434 to 768%). Cell-free extracts containing GH32 enzymes in cluster 4 did not degrade sucrose, kestose, or nystose. Arabinan, inulin, and levan were also included in this assay, but none of these newly included substrates were degraded by the enzymes in cluster 4.
Eight amino acid residues each in B.longum-GH32 (β-fructofuranosidase) and L.gasseri-GH32 (sucrose-6-phosphate hydrolase, S6PH) form hydrogen bonds with β-fructofuranose at subsite −1. Among the eight amino acid residues, Trp78 in B.longum-GH32 is substituted by His71 in L.gasseri-GH32 and Lys74 in L.gasseri-GH32 is substituted by Met81 in B.longum-GH32 (Supplemental Fig. S1, Table 3), resulting in the nine total amino acid residues listed in Table 3 being essential for the binding to β-fructofuranose at subsite −1. The corresponding amino acid residues of the GH32 enzymes in butyrate producers were determined based on multiple sequence alignment. The eight amino acid residues in B.longum-GH32 were fully conserved in GH32 enzymes belonging to cluster 1, but one (Trp78), one (Ser114), and two/three (Asn53, Gln70, and Trp78) amino acid substitution(s) were found in clusters 2, 3, and 4, respectively (Supplemental Fig. S1, Table 3). GH32 enzymes of cluster 2 had no amino acid substitutions when compared with the eight amino acid residues in L.gasseri-GH32, and one (Lys74), two (Lys74 and Ser107), and two/three (Asn46, Gln63, and Lys74) amino acid substitution(s) were found in clusters 1, 3, and 4, respectively. These substitutions were not noted in catalytic triads or the RDP motif. The NDPNG motif, which is characteristic of GH32 enzymes,36 was conserved in GH32 enzymes of clusters 1, 2, and 3, but not in those of cluster 4 (Supplemental Fig. S1).
Table 3.
Amino acid residues at positions involved in hydrogen bonds with β-fructofuranose in B.longum-GH32 and L.gasseri-GH32
| B.longum-GH32* | L.gasseri-GH32** | Cluster 1 (n = 6) |
Cluster 2 (n = 5) |
Cluster 3 (n = 3) |
Cluster 4 (N = 3) |
|
|---|---|---|---|---|---|---|
| Asn53 | Asn46 | Asn | Asn | Asn | Gly | NDPNG motif Catalytic triad, NDPNG motif |
| Asp54 | Asp47 | Asp | Asp | Asp | Asp | |
| Gln70 | Gln63 | Gln | Gln | Gln | Gln, Leu | |
| Trp78 | (His71)*** | Trp | Gly, His | Trp | Gly, Ala, Arg | |
| (Met81)*** | Lys74 | Met | Lys | Met, Leu | Thr, Phe | |
| Ser114 | Ser107 | Ser | Ser | Thr, Ala | Ser |
RDP motif Catalytic triad, RDP motif Catalytic triad, ECP motif |
| Arg180 | Arg166 | Arg | Arg | Arg | Arg | |
| Asp181 | Asp167 | Asp | Asp | Asp | Asp | |
| Glu235 | Glu222 | Glu | Glu | Glu | Glu |
Amino acids identical with those of the two reference enzymes are written in bold. Clusters 1 to 4 correspond to clusters shown in Fig. 5.
*Eight amino acid residues involved in hydrogen bonds with β-fructofuranose in B.longum-GH32
**Eight amino acid residues involved in hydrogen bonds with β-fructofuranose in L.gasseri-GH32
***(Met81) in B.longum-GH32 and (His71) in L.gasseri-GH32 are not involved in forming a hydrogen bond with β-fructofuranose but are equivalent loci to Lys74 in L.gasseri-GH32 and Trp78 in B.longum-GH32, respectively
Discussion
Although gut butyrate levels are important for host health, oligosaccharide metabolic properties in butyrate producers are poorly characterized. Although certain oligosaccharides, e.g. human milk oligosaccharides, have multiple functions in host health,37 proliferation of beneficial microbes is one of the most important characteristics in dietary oligosaccharides.
Based on the growth properties in the presence of oligosaccharides, 14 butyrate producers were divided into four groups, i.e. group A, group F, group XR, and group N. FOS-type oligosaccharides were metabolized by only six strains in groups A and F, and eight of the 14 strains did not, suggesting that FOS-type oligosaccharides are an energy source for some butyrate producers. Faecalibacterium prausnitzii, which is the most abundant butyrate producer in the healthy human gut and produces an anti-inflammatory molecule,38 metabolized only FOS-type oligosaccharides among the tested oligosaccharides. Anaerostipes spp. exhibited a similar pattern, except that A. caccae metabolized kestose but not nystose. This is consistent with a previous report.7 This suggests that the impact of the DP of FOSs on growth is not a common characteristic in the genus Anaerostipes, but rather a species- or strain-specific trait. The four strains in group F possess one to four genes encoding GH32 enzymes in their genomes. Among the group F strains, F. prausnitzii and A. hadrus possess GH32 enzymes that actively degrade nystose, but GH32 enzyme in A. caccae (A.caccae-GH32) did not. This may be the reason for the different nystose metabolic properties among the strains. A previous study revealed that genes encoding A.caccae-GH32 and clustered PTS and fructokinase were highly transcribed in cells cultured with kestose, but poorly transcribed in cells with nystose.32 Anaerostipes butyraticus, which metabolizes nystose, possesses three GH32 enzymes. Two of the three are A.butyraticus-GH32-1 degrading sucrose and kestose but not nystose, and A.butyraticus-GH32-2, exhibiting no degradation activity in the present study. The last is A.butyraticus-GH32-3, containing a stop codon. Fused complete A.butyraticus-GH32-3 by replacement of the stop codon with a glutamine codon and partial A.butyraticus-GH32-3 had similar characteristics, and degraded kestose and nystose with trace or no degradation of sucrose. The partial A.butyraticus-GH32-3 may therefore be responsible for the degradation of nystose by this strain. Another possibility is amino acid replacement at the stop codon in A.butyraticus-GH32-3 by a suppressor tRNA, leading to an intact extracellular A.butyraticus-GH32-3 being produced in A. butyraticus cells, as described in other microbes.39,40 All four strains in group F lacked GH8 proteins, which are potentially involved in the hydrolysis of XOSs.41 This is consistent with the results obtained by the in vitro culture study. Metabolism of raffinose requires the combination of a few enzymes, including GH36 α-galactosidase, GH32 β-fructosidase/GH13 sucrose phosphorylase, and melibiose/raffinose transporters.42–44 GH36 α-galactosidase and GH32 β-fructosidase are present in the genomes of three of the four strains (excluding A. hadrus) in group F (Table 3), but these strains did not metabolize raffinose, suggesting that transporters for melibiose or raffinose are missing.
Roseburia intestinalis and R. inulinivorans in group A metabolized all oligosaccharides tested. These strains possess GH32, GH36, and GH8 proteins, which may be involved in the metabolism of FOSs, raffinose, and XOSs, respectively, except that R. inulinivorans lacks GH8 proteins. The reason for this discrepancy is unclear, but GH30 protein, which is a unique protein in the strain, may function in the degradation of XOS, as described for other microbes.45,46 Roseburia intestinalis and R. inulinivorans possess a single GH32 enzyme. The enzymes shared similar degradation activities to FOSs, but they were located in different clusters in the phylogenetic tree (Figure 5). GH32 enzyme in R. inulinivorans is the most well examined GH32 enzyme in gut butyrate-producing bacteria, and was previously characterized to degrade sucrose, kestose, and nystose.27 The enzyme also degrades inulin and is strongly induced in its presence.27 Roseburia intestinalis has the best repertoire of GH proteins and possesses 124 GH proteins. Proteins assigned to GH35, GH38, GH74, GH95, GH125, and GH148 are unique to the strain. This organism has been linked to the degradation of several non-digestible carbohydrates, including β-mannan, xylan, and dietary plant polysaccharides.47–49 This species also plays a role in the deacetylation of hemicellulose, which leads to efficient utilization of dietary fiber by gut microbiota.50 These may be an advantage in the organism to survive in the complex and competitive gut microbiota.
Four organisms in group XR did not metabolize FOS-type oligosaccharides, whereas GH32 proteins were conserved in three of the four strains, i.e. A. rectalis, C. eutactus, and R. faecis. Although two of the GH32 proteins found in this group did not exhibit degradation activity against sucrose, kestose or nystose, the three strains possessed at least one GH32 enzyme degrading the FOS-type oligosaccharides. A possible reason for these conflicting results is the inactive induction of genes encoding the GH32 enzymes in the three strains. Bifidobacterium longum JCM 1217 T, which actively metabolizes kestose but not nystose, possesses β-fructofuranosidase, having degradation activity against both kestose and nystose, but the gene encoding β-fructofuranosidase was only transcribed in cells cultured with kestose.32 This should be noted because recent metagenomic approaches are sometimes included to understand the metabolic potential of microbiota without in vitro tests.51 The present study suggested that even if functional genes are present in microbes, they are sometimes unable to metabolize the substrates. Genes encoding possible transporters were not found adjacent to the genes encoding C.eutactus-GH32-1 and C.eutactus-GH32-2, which are active against FOSs. These genes were linked with genes encoding conjugal transfer protein or integrase TN1549-like, suggesting that they are allochthonous and not actively used for FOS metabolism. A recent study found GH32 enzyme in Bacillus subtilis phages.52
Four strains in group N did not metabolize any of the oligosaccharides tested. These results are consistent with their GH profiles, as they lack proteins assigned to GH8, GH32, or GH36. These microbes were thus not directly activated by administration of the oligosaccharides tested. As some of the butyrate producers, including A. hallii, can obtain energy by a combination of acetate and lactate and produce butyrate,20 indirect stimulation by the acids produced through the metabolism of the oligosaccharides in other gut microbes may be possible. Butyricimonas spp. in the phylum Bacteroidetes are members of this poor GH holder group, although a previous study reported abundant GH family proteins (mean of 130 proteins) in 29 Bacteroidetes species (belonging to the genera Bacteroides, Parabacteroides, and Prevotella) originating from the human gut microbiota.53 Bacteroidetes organisms are able to produce biomass in the absence of fermentable carbohydrates,7,54 and similar results were obtained in the present study (Figure 1). Oligosaccharide metabolic properties in butyrate producers, as determined in the present study, are slightly inconsistent with a previous study,54 e.g. FOS metabolism in R. hominis and XOS metabolism in A. caccae, although the same strains were used. The discrepancy may be due to different oligosaccharides used in the studies. Roseburia hominis lacks GH32 protein and A. caccae lacks GH8 proteins (Table 2), consistent with their in vitro metabolic properties observed in the present study.
Phylogenetic analysis of GH32 enzymes produced four major clusters. Of the four clusters, enzymes classified in clusters 1, 2, and 3 had FOS degradation activity with different specific activities, except for A.butyraticus-GH32-2. Genes encoding GH32 enzymes in cluster 2 found in Anaerostipes spp. were located adjacent to genes encoding the PTS in each genome, suggesting that their innate substrates are phosphorylated-sucrose and -FOSs. Therefore, they are classified as S6PH.55 S6PH catalyzes the hydrolysis of both phosphorylated- and non-phosphorylated-products, but the enzyme has a markedly different Km for sucrose-6-phosphate and sucrose, 0.28 mM and 40 mM, respectively, in Fusobacterium mortiferum.56 Due to the commercial unavailability of phosphorylated-FOSs, specific activities of the phosphorylated-products were unable to be assessed in the present study. Substrate specificity of phospho-α-glucosidase in F. mortiferum was not influenced by the presence of phosphorylation.57,58 Two of the three GH32 enzymes in cluster 3 (A.butyraticus-GH32-3 and A.hadrus-GH32-4) contained components of signal sequences and CBM66, i.e., they are extracellular enzymes. On the other hand, A.butyraticus-GH32-3 is divided into two proteins (Locus_Tag ANBU17_10420 and ANBU17_10430) because of a stop codon. An intracellular enzyme only possessing the catalytic domain of A.butyraticus-GH32-3 (Locus_Tag ANBU17_10420) and fused extracellular whole A.butyraticus-GH32-3 (Locus_Tag ANBU17_10420 and ANBU17_10430), whose stop codon (TAG) was replaced with a glutamine codon (CAG) according to the sequences of Clostridium spiroforme (accession no. WP_087286452), exhibited similar FOS degradation activities, and degraded more kestose and nystose than sucrose. Similar degradation activities are also shared with A.hadrus-GH32-4. CBM66 binds the terminal fructosides of fructans in exo-acting β-fructosidase of Bacillus subtilis and removal of the CBM66 component resulted in an approximately 100-fold reduction in activity against levan.59 Extracellular GH enzymes equipped with CBMs are advantageous to obtain nutrients efficiently under the competitive gut microbiota49,60 and are also involved in symbiosis with other members in the microbiota by providing degradants.61,62 Similar functions may be observed for A.butyraticus-GH32-3 and A.hadrus-GH32-4 equipped with CBM66. Genes encoding four GH32 enzymes in cluster 1 (A.rectalis-GH32-1, F.prausnitzii-GH32, R.faecis-GH32, and R.inulinivorans-GH32) and one in cluster 3 (R.intestinalis-GH32) are adjacent to ABC transporter, suggesting that they are β-fructofuranosidase/levanase/inulinase. GH32 enzymes in these clusters had similar relative degradation activities against the three substrates or higher activities with the longer DP carbohydrates. These activities are consistent with the characteristics of adjacent transporters because ABC transporters generally import longer DP carbohydrates.63 PTSs are usually size-restricted transporters,63 and they were located adjacent to genes encoding cluster 2 enzymes hydrolyzing short DP carbohydrates. A.hadrus-GH32-4 and A.butyraticus-GH32-3 in cluster 3 are extracellular enzymes hydrolyzing longer DP carbohydrates, and adjacent PTS may be used to transport short DP carbohydrates after extracellular hydrolysis of long DP carbohydrates. GH32 enzymes in cluster 4 were not active against any of the tested FOSs, arabinan, inulin, or levan, suggesting that they have different unknown substrates. These enzymes were classified as GH32 by CAZy assignment but do not have the conserved NDPNG motif, suggesting that they are not GH32 enzymes. Previous studies found that the NDPNG motif is more associated with hydrolase/transferase activity than specificity,64 and amino acid replacement in this region (replacement of the initial N to S in the NDPNG motif) resulted in significant reduction of activity of the bacterial GH32 enzyme.65 The present study found that the NDPNG motif is involved in hydrogen bonding with substrates in GH32 enzymes. Based on BLASTP analysis, GH32 enzymes in cluster 4 are related to GH43 enzymes (data not shown). These enzymes were only found in one active-FOS metabolizer (i.e. A. hadrus) and two inactive-metabolizers (i.e. A. rectalis and C. eutactus), whereas A. hadrus possesses three alternative active GH32 enzymes to hydrolyze FOSs. Therefore, the presence of GH32 enzymes in cluster 4 does not impact the growth of butyrate producers.
Eight amino acid residues each are involved in hydrogen bonds with β-fructofuranose in the reference B.longum-GH32 β-fructofuranosidase33 and L.gasseri-GH32 S6PH (Table 3). The eight amino acids in B.longum-GH32 were all conserved in GH32 enzymes in cluster 1. B.longum-GH32 degrades sucrose, kestose, and nystose well,33 and this activity is consistent with that recorded for GH32 enzymes in cluster 1 (Figure 5). GH32 enzymes in cluster 3 also had similar FOS degradation activity to B.longum-GH32; however, Ser114 in B.longum-GH32 was substituted with threonine or alanine. GH32 enzymes in cluster 2 exhibited degradation activity for sucrose and kestose, but it was low or absent with nystose, and these enzymes substituted Trp78 with glycine or histidine. Trp105 in GH32 β-fructofuranosidase of Xanthophyllomyces dendrorhous, an equivalent locus to Trp78 in B.longum-GH32, mainly accommodates binding with 6-kestose at subsite +2.66 This suggests that Trp78, but not Ser114, is among the key factors for the degradation activity of nystose. The eight amino acids involved in hydrogen bonds with β-fructofuranose of L.gasseri-GH32 were conserved in GH32 enzymes in cluster 2. Although specific activities of L.gasseri-GH32 and nystose metabolic properties of the host strain 224–1 have not been characterized, other strains of L. gasseri are known to metabolize kestose, but not nystose.26,67 Two or three of the eight amino acid residues involved in hydrogen bonds with β-fructofuranose of B.longum-GH32 (Asn53, Gln70, and Trp78) were substituted with other amino acids in GH32 enzymes of cluster 4. These amino acid replacements and lack of the NDPNG motif, as described above, would result in no activity against FOSs.
A previous study reported that most genes encoding GH formed PULs with genes encoding transporters and regulators in the genomes of Roseburia spp. and A. rectalis.34 The study defined PUL as being a locus encoding, at minimum, one polysaccharide-degrading enzyme, a carbohydrate transport system, and a transcriptional regulator. Based on this definition, of the 17 GH32 proteins found in the present study, genes encoding 12 proteins formed 11 PULs, and A.hadrus-GH32-2 and A.hadrus-GH32-3 were located in a single PUL (Figure 6). Genes encoding carbohydrate kinase were found in seven of the 11 PULs. The number of genes encoding transporters and regulators, and arrangements of the genes in the 11 PULs are highly divergent among PULs, whereas relatively similar PUL structures were reported for GH32 proteins of butyrate producers in a previous study.34 Most PULs containing genes encoding GH32 enzymes found in the present study contain a single GH enzyme, whereas those for other GHs in butyrate producers contain multiple genes encoding several GHs, up to seven genes.34
In conclusion, 14 gut butyrate producers exhibited different FOS metabolic properties among organisms. Profiles of GH storage suggested diverse polysaccharide metabolic properties in the strains. Phylogenetic analysis separated GH32 enzymes found in butyrate producers into four clusters, and the enzymes generally had similar degradation activities among the clusters. GH32 enzymes exhibiting FOS degradation activities were conserved in all six strains metabolizing FOS and in three of the eight strains that did not metabolize FOS, suggesting that GH32 enzymes in the three strains are not actively used in metabolism. The present study highlighted that even if functional genes are present in microbes, they are sometimes unable to metabolize the substrates. This should be carefully considered in metagenomic studies to understand the metabolic potential of gut microbiota. The present study sheds light on the important characteristics of GH32 enzymes and their relationship with metabolic properties in important health-related microbes to discuss the potential of FOS as prebiotics.
Materials and methods
Bacterial strains and pre-culturing
Fourteen strains of gut butyrate-producing bacteria were used in the present study (Table 1). These strains were obtained from the Japan Collection of Microorganisms (JCM) and the American Type Culture Collection (ATCC). They included the predominant gut butyrate-producing bacteria in humans, i.e. F. prausnitzii in the Clostridium cluster IV and Roseburia spp., A. rectalis, A. hallii and Anaerostipes spp. in the Clostridium cluster XIVa.13,68 A few more human gut butyrate producers belonging to the Clostridium clusters IV and XIVa, and the phylum Bacteroidetes were also included to assess diverse metabolic characteristics in butyrate-producing bacteria. Basal YCFA broth supplemented with 0.5% (w/v) glucose was purged with N2 gas using an O2-removal unit (Model AG-2, Sanshin, Kanagawa, Japan) and used for pre-culturing. The composition of YCFA broth was described elsewhere.69 Bacterial cells were inoculated into the broth by injection and cultured at 37°C for 24 or 48 h.
Growth on oligosaccharides
To study FOS metabolic properties, kestose (DP3; B Food Science, Japan), nystose (DP4; Wako Chemical, Japan), and a FOS mixture (FOSs, containing DP3-5; Wako Chemical) were used. XOSs (mainly DP2-5; B Food Science) and raffinose (Wako Chemical), which were reported as bifidogenic prebiotics, were also included in the present study. The composition of carbohydrates was described previously,7 and the purity of kestose, nystose, and raffinose was higher than 98% (w/w). Basal YCFA broth supplemented with 0.5% (w/v) oligosaccharide was used to study oligosaccharide metabolic properties of butyrate-producing bacteria. Sugar-free YCFA broth and YCFA broth supplemented with 0.5% glucose were included as controls. These tested broths were also purged with N2 gas to be oxygen-free. Twenty microliters of the pre-cultured cells were inoculated into 2 ml of the tested broth and incubated anaerobically at 37°C for 72 h. Growth was monitored at 660 nm using a spectrophotometer (model U-2800A, Hitachi, Japan) every 24 h. This experiment was performed in triplicate.
Butyrate production and metabolic profiles of oligosaccharides
Butyrate produced by the metabolism of oligosaccharides was measured by high-performance liquid chromatography (HPLC) combined with an Aminex HPX- 87 H column (Bio-Rad, Japan), and the oligosaccharide composition of FOSs and XOSs before/after culturing was determined using high-performance anion exchange chromatography coupled with a pulsed amperometric detection (HPAEC-PAD) system (model ICS-3000, Dionex, United Kingdom) and a Dionex CarboPac PA1 column (Thermo Scientific, Japan), as described previously.26 These were determined in culture supernatants after incubation of 72 h. These experiments were performed in triplicate.
Genome analysis and identification of GH32 enzymes
Of the 14 strains used in the in vitro oligosaccharide metabolic test, draft or complete genomes of ten strains were obtained from the GenBank, RefSeq, or the JGI Genome Portal70 database and used in the analysis. Genomes of the remaining four strains, which were A. butyraticus JCM 17466 T, Butyricicoccus faecihominis JCM 31056 T, A. hallii JCM 31263, and C. eutactus JCM 31265, were sequenced by the Illumina platform. Genomic DNA was isolated from cells as described previously.71 Assembly and annotation of the sequences, and quality check of the resulting genomic data were conducted by methods described previously.72 The genomic data were used to search for GH family enzymes using dbCAN2 in the CAZy database with HMMER, DIAMOND, and Hotpep tools.73 GH proteins were identified when detected by two of the three tools, as recommended by the database.73 Numbers of estimated proteins in each GH family of the strains were used to prepare a dendrogram using the hclust function with the Ward.D2 algorithm in the R package (version 3.6.2). The signal peptide in GH proteins was identified by CAZy and SignalP-5.0 programs.74 The phylogenetic tree was constructed using the program ClustalW, version 2.1.75 The number of bootstrapping replicates was 1,000.
To select amino acid residues that are essential for substrate specificity, the structures of two GH32 enzymes, B.longum-GH32 β-fructofuranosidase (PDB ID, 3PIJ) and L.gasseri-GH32 S6PH (PDB ID, 6NU8), were obtained. B.longum-GH32 was reported to hydrolyze sucrose, kestose, and nystose,33 whereas L.gasseri-GH32 is the only bacterial S6PH whose structure is currently available. Eight amino acid residues forming hydrogen bonds with β-fructofuranose at subsite −1 in B.longum-GH32 were obtained from elsewhere,33 and those in L.gasseri-GH32 were determined using the crystal structure of the enzyme complexed with β-fructofuranose (PDB ID 6NU7) and PyMOL software (version 2.4; https://pymol.org/2/) with the default setting (within 3.3 Å). GH32 enzymes found in 14 butyrate producers were aligned with the two reference GH32 enzymes described above, and amino acid residues at positions equivalent to the hydrogen bonds with β-fructofuranose in the two reference enzymes were compared among clusters.
Activity of recombinant GH32 enzymes expressed in E. coli
Genomic DNA was isolated from cells of the butyrate producers by a previously described method71 and used as templates to amplify genes encoding GH32 enzymes. Plasmids were constructed using the In-Fusion Cloning kit (Takara-bio, Japan). Plasmids and the genes encoding GH32 enzymes were amplified using the KOD Plus DNA polymerase kit (Toyobo, Japan) combined with primers listed in Supplemental Table S2, and fused using the In-Fusion Cloning kit according to the manufacturer’s instructions. pET28a (Merck, Germany) was used for cloning of intracellular GH32 enzymes and pCDF-PgsA76 was used for extracellular GH32 enzymes. The latter plasmid was developed to study the activities of extracellular GH32 enzymes using the surface display system on E. coli cells.76 Escherichia coli JM109 (Takara-bio, Japan) were transformed with the fused plasmids, which were extracted from the transformed strains using the FastGene Plasmid Mini Kit (Nippon Genetics, Japan) and used to transform E. coli BL21 (DE3, Takara-bio). Transformed E. coli strains were cultured in Terrific broth (Sigma-Aldrich, Japan) supplemented with 25 μg/ml of kanamycin sulfate (for pET28a-derivative plasmids) or 12.5 μg/ml of streptomycin sulfate (for pCDF-PgsA -derivative plasmids) at 20°C or 24°C for 24 h. The transformed E. coli BL21 (DE3) cells were cultured in Overnight Express™ Instant TB Medium (Merck, Germany) supplemented with antibiotics at 20°C for 24 h. For intracellular enzymes, preparation of cell-free extracts, enzyme assays, and carbohydrate profiles after enzyme reactions were performed as described previously.32 For extracellular enzymes, overnight-cultured recombinant E.coli cells were collected by centrifugation, washed, re-suspended in solution containing 30% substrates, and used to measure substrate degradation activities, as described elsewhere.76 Sucrose, kestose, and nystose were used as substrates for this assay, and arabinan, inulin, and levan were also included for GH32 enzymes of cluster 4.
For cloning of A.butyraticus-GH32-3, two E. coli recombinants were generated. One recombinant produced intracellular partial A.butyraticus-GH32-3 (Locus_Tag ANBU_10420) and the other produced extracellular whole A.butyraticus-GH32-3 (Locus_Tag ANBU_10420 and 10430). To prepare the extracellular A.butyraticus-GH32-3, genes encoding whole A.butyraticus-GH32-3 (Locus_Tag ANBU_10420 and 10430) were amplified by the method described above. The amplified product was fused with pCDF-PgsA (pCDF_PgsA_AB_LamG_Pre), as described above. pCDF_PgsA_AB_LamG_Pre was amplified with the primer pair of AB_LamG_fusion(Q) and AB_LamG_fusion(Q)_R for linearization and replacement of the stop codon (TAG) with a glutamine codon (CAG) according to the sequence of GH32 β-fructofuranosidase of Clostridium spiroforme (accession no. WP_087286452), and self-ligated using T4 Polynucleotide Kinase (Toyobo, Japan) and DNA Ligation Kit Ver.2.1 (Takara-bio, Japan). The GH32 β-fructofuranosidase of Clostridium spiroforme (accession no. WP_087286452) had the highest similarity (63%) with A.butyraticus-GH32-3 by BLAST analysis (data not shown). Activities of the extracellular A.butyraticus-GH32-3 (Locus_Tag ANBU_10420 and 10430) enzyme were studied using the surface display system on E. coli cells, and those of the intracellular partial A.butyraticus-GH32-3 (Locus_Tag ANBU_10420) were determined by preparing cell-free extracts, as described above.
Supplementary Material
Acknowledgments
We are grateful to Ms. Naomi Sakurai (Microbe Division/Japan Collection of Microorganisms, RIKEN BioResource Research Center) for her technical assistance. Computational analysis was performed in part on the National Institute of Genetics (NIG) supercomputer at ROIS. The genome sequence data of Butyricimonas faecihominis DSM 105721T was produced by the US Department of Energy Joint Genome Institute (http://www.jgi.doe.gov/).
Funding Statement
This study was supported by Tokyo University of Agriculture and by a grant from B Food Science Co. Ltd.
Declaration of interests
Tadashi Fujii, Katsuaki Hirano, and Takumi Tochio are employees of B Food Science Co. Ltd, which is the producer of 1-kestose and XOSs used in the present study. The remaining authors declare no conflicts of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Meguid MM, Yang ZJ, Gleason JR.. The gut-brain brain-gut axis in anorexia: toward an understanding of food intake regulation. Nutrition (Burbank, Los Angeles County, Calif). 1996;12:S57–20. doi: 10.1016/0899-9007(95)00083-6. [DOI] [PubMed] [Google Scholar]
- 2.Cooke KR, Hill GR, Gerbitz A, Kobzik L, Martin TR, Crawford JM, Brewer JP, Ferrara JLM. Hyporesponsiveness of donor cells to lipopolysaccharide stimulation reduces the severity of experimental idiopathic pneumonia syndrome: potential role for a gut-lung axis of inflammation. J Immunol. 2000;165:6612–6619. doi: 10.4049/jimmunol.165.11.6612. [DOI] [PubMed] [Google Scholar]
- 3.Berndt T, Thomas LF, Craig TA, Sommer S, Li X, Bergstralh EJ, Kumar R. Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption. Proc Natl Acad Sci U S A. 2007;104:11085–11090. doi: 10.1073/pnas.0704446104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ticinesi A, Milani C, Guerra A, Allegri F, Lauretani F, Nouvenne A, Mancabelli L, Lugli GA, Turroni F, Duranti S, et al. Understanding the gut-kidney axis in nephrolithiasis: an analysis of the gut microbiota composition and functionality of stone formers. Gut. 2018;67:2097–2106. doi: 10.1136/gutjnl-2017-315734. [DOI] [PubMed] [Google Scholar]
- 5.Hvatum M, Kanerud L, Hallgren R, Brandtzaeg P. The gut-joint axis: cross reactive food antibodies in rheumatoid arthritis. Gut. 2006;55:1240–1247. doi: 10.1136/gut.2005.076901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, Seino S, Simpson JA, Drucker DJ. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. 2013;19:567–575. doi: 10.1038/nm.3128. [DOI] [PubMed] [Google Scholar]
- 7.Ose R, Hirano K, Maeno S, Nakagawa J, Salminen S, Tochio T, Endo A. The ability of human intestinal anaerobes to metabolize different oligosaccharides: novel means for microbiota modulation? Anaerobe. 2018;51:110–119. doi: 10.1016/j.anaerobe.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Roediger WE. The colonic epithelium in ulcerative colitis: an energy-deficiency disease? Lancet (London, England). 1980;2:712–715. doi: 10.1016/S0140-6736(80)91934-0. [DOI] [PubMed] [Google Scholar]
- 9.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida M, Kijima M, Akita M, Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem. 1990;265:17174–17179. [PubMed] [Google Scholar]
- 11.Kinoshita M, Suzuki Y, Saito Y. Butyrate reduces colonic paracellular permeability by enhancing PPARgamma activation. Biochem Biophys Res Commun. 2002;293:827–831. doi: 10.1016/S0006-291X(02)00294-2. [DOI] [PubMed] [Google Scholar]
- 12.Donohoe DR, Holley D, Collins LB, Montgomery SA, Whitmore AC, Hillhouse A, Curry KP, Renner SW, Greenwalt A, Ryan EP, et al. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov. 2014;4:1387–1397. doi: 10.1158/2159-8290.CD-14-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louis P, Young P, Holtrop G, Flint HJ. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA: acetateCoA-transferase gene. Environ Microbiol. 2010;12:304–314. doi: 10.1111/j.1462-2920.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- 14.Scanlan PD, Shanahan F, O’Mahony C, Marchesi JR. Culture-independent analyses of temporal variation of the dominant fecal microbiota and targeted bacterial subgroups in Crohn’s disease. J Clin Microbiol. 2006;44:3980–3988. doi: 10.1128/JCM.00312-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63:1275–1283. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 16.de Goffau MC, Luopajarvi K, Knip M, Ilonen J, Ruohtula T, Harkonen T, Orivuori L, Hakala S, Welling GW, Harmsen HJ, et al. Fecal microbiota composition differs between children with beta-cell autoimmunity and those without. Diabetes. 2013;62:1238–1244. doi: 10.2337/db12-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang X, Jia W, Cai S, Zhao L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. Isme J. 2012;6:320–329. doi: 10.1038/ismej.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feehley T, Plunkett CH, Bao R, Choi Hong SM, Culleen E, Belda-Ferre P, Campbell E, Aitoro R, Nocerino R, Paparo L, et al. Healthy infants harbor intestinal bacteria that protect against food allergy. Nat Med. 2019;25:448–453. doi: 10.1038/s41591-018-0324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louis P, Duncan SH, McCrae SI, Millar J, Jackson MS, Flint HJ. Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. J Bacteriol. 2004;186:2099–2106. doi: 10.1128/JB.186.7.2099-2106.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duncan SH, Louis P, Flint HJ. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol. 2004;70:5810–5817. doi: 10.1128/AEM.70.10.5810-5817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vital M, Gao J, Rizzo M, Harrison T, Tiedje JM. Diet is a major factor governing the fecal butyrate-producing community structure across Mammalia, Aves and Reptilia. Isme J. 2015;9:832–843. doi: 10.1038/ismej.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Bourgot C, Ferret-Bernard S, Le Normand L, Savary G, Menendez-Aparicio E, Blat S, Appert-Bossard E, Respondek F, Le Huërou-Luron I. Maternal short-chain fructooligosaccharide supplementation influences intestinal immune system maturation in piglets. PloS One. 2014;9:e107508. doi: 10.1371/journal.pone.0107508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato T, Fukuda S, Fujiwara A, Suda W, Hattori M, Kikuchi J, Ohno H. Multiple omics uncovers host-gut microbial mutualism during prebiotic fructooligosaccharide supplementation. DNA Res: Int J Rapid Publ Rep Genes Genomes. 2014;21:469–480. doi: 10.1093/dnares/dsu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ide K, Shinohara M, Yamagishi S, Endo A, Nishifuji K, Tochio T. Kestose supplementation exerts bifidogenic effect within fecal microbiota and increases fecal butyrate concentration in dogs. Journal Vet Med Sci. 2020;82:1–8. doi: 10.1292/jvms.19-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koga Y, Tokunaga S, Nagano J, Sato F, Konishi K, Tochio T, Murakami Y, Masumoto N, Tezuka J-I, Sudo N, et al. Age-associated effect of kestose on Faecalibacterium prausnitzii and symptoms in the atopic dermatitis infants. Pediatr Res. 2016;80:844–851. doi: 10.1038/pr.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Endo A, Nakamura S, Konishi K, Nakagawa J, Tochio T. Variations in prebiotic oligosaccharide fermentation by intestinal lactic acid bacteria. Int J Food Sci Nutr. 2016;67:1–8. doi: 10.3109/09637486.2016.1147019. [DOI] [PubMed] [Google Scholar]
- 27.Scott KP, Martin JC, Chassard C, Clerget M, Potrykus J, Campbell G, Mayer C-D, Young P, Rucklidge G, Ramsay AG, et al. Substrate-driven gene expression in Roseburia inulinivorans: importance of inducible enzymes in the utilization of inulin and starch. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4672–4679. doi: 10.1073/pnas.1000091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saulnier DM, Molenaar D, de Vos WM, Gibson GR, Kolida S. Identification of prebiotic fructooligosaccharide metabolism in Lactobacillus plantarum WCFS1 through microarrays. Appl Environ Microbiol. 2007;73:1753–1765. doi: 10.1128/AEM.01151-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan SM, Fitzgerald GF, van Sinderen D. Transcriptional regulation and characterization of a novel beta-fructofuranosidase-encoding gene from Bifidobacterium breve UCC2003. Appl Environ Microbiol. 2005;71:3475–3482. doi: 10.1128/AEM.71.7.3475-3482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finegold SM, Li Z, Summanen PH, Downes J, Thames G, Corbett K, Dowd S, Krak M, Heber D. Xylooligosaccharide increases bifidobacteria but not lactobacilli in human gut microbiota. Food Funct. 2014;5:436–445. doi: 10.1039/c3fo60348b. [DOI] [PubMed] [Google Scholar]
- 31.Fernando WM, Hill JE, Zello GA, Tyler RT, Dahl WJ, Van Kessel AG. Diets supplemented with chickpea or its main oligosaccharide component raffinose modify faecal microbial composition in healthy adults. Benef Microbes. 2010;1:197–207. doi: 10.3920/BM2009.0027. [DOI] [PubMed] [Google Scholar]
- 32.Tanno H, Fujii T, Ose R, Hirano K, Tochio T, Endo A. Characterization of fructooligosaccharide-degrading enzymes in human commensal Bifidobacterium longum and Anaerostipes caccae. Biochem Biophys Res Commun. 2019;518:294–298. doi: 10.1016/j.bbrc.2019.08.049. [DOI] [PubMed] [Google Scholar]
- 33.Bujacz A, Jedrzejczak-Krzepkowska M, Bielecki S, Redzynia I, Bujacz G. Crystal structures of the apo form of β-fructofuranosidase from Bifidobacterium longum and its complex with fructose. Febs J. 2011;278:1728–1744. doi: 10.1111/j.1742-4658.2011.08098.x. [DOI] [PubMed] [Google Scholar]
- 34.Sheridan PO, Martin JC, Lawley TD, Browne HP, Harris HMB, Bernalier-Donadille A, Duncan SH, O’Toole PW, P. Scott K, J. Flint H, et al. Polysaccharide utilization loci and nutritional specialization in a dominant group of butyrate-producing human colonic Firmicutes. Microb Genomics. 2016;2:e000043. doi: 10.1099/mgen.0.000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martens EC, Lowe EC, Chiang H, Pudlo NA, Wu M, McNulty NP, Abbott DW, Henrissat B, Gilbert HJ, Bolam DN, et al. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 2011;9:e1001221. doi: 10.1371/journal.pbio.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen TH, Huang YC, Yang CS, Yang CC, Wang AY, Sung HY. Insights into the catalytic properties of bamboo vacuolar invertase through mutational analysis of active site residues. Phytochemistry. 2009;70:25–31. doi: 10.1016/j.phytochem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. 2012;22:1147–1162. doi: 10.1093/glycob/cws074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quevrain E, Maubert MA, Michon C, Chain F, Marquant R, Tailhades J, Miquel S, Carlier L, Bermúdez-Humarán LG, Pigneur B, et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut. 2016;65:415–425. doi: 10.1136/gutjnl-2014-307649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eggertsson G, Söll D. Transfer ribonucleic acid-mediated suppression of termination codons in Escherichia coli. Microbiol Rev. 1988;52:354–374. doi: 10.1128/MR.52.3.354-374.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herring CD, Blattner FR. Global transcriptional effects of a suppressor tRNA and the inactivation of the regulator frmR. J Bacteriol. 2004;186:6714–6720. doi: 10.1128/JB.186.20.6714-6720.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valenzuela SV, Lopez S, Biely P, Sanz-Aparicio J, Pastor FI. The Glycoside Hydrolase Family 8 Reducing-End Xylose-Releasing Exo-oligoxylanase Rex8A from Paenibacillus barcinonensis BP-23 Is Active on Branched Xylooligosaccharides. Appl Environ Microbiol. 2016;82:5116–5124. doi: 10.1128/AEM.01329-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cervera-Tison M, Tailford LE, Fuell C, Bruel L, Sulzenbacher G, Henrissat B, Berrin JG, Fons M, Giardina T, Juge N, et al. Functional analysis of family GH36 alpha-galactosidases from Ruminococcus gnavus E1: insights into the metabolism of a plant oligosaccharide by a human gut symbiont. Appl Environ Microbiol. 2012;78:7720–7732. doi: 10.1128/AEM.01350-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang Y, Zhang H, Ben P, Duan Y, Lu M, Li Z, Cui Z. Characterization of a novel GH36 alpha-galactosidase from Bacillus megaterium and its application in degradation of raffinose family oligosaccharides. Int J Biol Macromol. 2018;108:98–104. doi: 10.1016/j.ijbiomac.2017.11.154. [DOI] [PubMed] [Google Scholar]
- 44.Mao B, Tang H, Gu J, Li D, Cui S, Zhao J, Zhang H, Chen W. In vitro fermentation of raffinose by the human gut bacteria. Food Funct. 2018;9:5824–5831. doi: 10.1039/C8FO01687A. [DOI] [PubMed] [Google Scholar]
- 45.Nakamichi Y, Fouquet T, Ito S, Matsushika A, Inoue H. Mode of Action of GH30-7 Reducing-End Xylose-Releasing Exoxylanase A (Xyn30A) from the Filamentous Fungus Talaromyces cellulolyticus. Appl Environ Microbiol. 2019;85: e00552–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou J, Bao L, Chang L, Zhou Y, Lu H. Biochemical and kinetic characterization of GH43 beta-D-xylosidase/alpha-L-arabinofuranosidase and GH30 alpha-L-arabinofuranosidase/beta-D -xylosidase from rumen metagenome. J Ind Microbiol Biotechnol. 2012;39:143–152. doi: 10.1007/s10295-011-1009-5. [DOI] [PubMed] [Google Scholar]
- 47.La Rosa SL, Leth ML, Michalak L, Hansen ME, Pudlo NA, Glowacki R, Pereira G, Workman CT, Arntzen MØ, Pope PB, et al. The human gut Firmicute Roseburia intestinalis is a primary degrader of dietary beta-mannans. Nat Commun. 2019;10:905. doi: 10.1038/s41467-019-08812-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kasahara K, Krautkramer KA, Org E, Romano KA, Kerby RL, Vivas EI, Mehrabian M, Denu JM, Bhed F, Lusis AJ, et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. 2018;3:1461–1471. doi: 10.1038/s41564-018-0272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leth ML, Ejby M, Madland E, Kitaoku Y, Slotboom DJ, Guskov A, Aachmann FL, Abou Hachem M. Molecular insight into a new low-affinity xylan binding module from the xylanolytic gut symbiont Roseburia intestinalis. Febs J. 2020;287:2105–2117. doi: 10.1111/febs.15117. [DOI] [PubMed] [Google Scholar]
- 50.Michalak L, La Rosa SL, Leivers S, Lindstad LJ, Rohr AK, Lillelund Aachmann F, Westereng B. A pair of esterases from a commensal gut bacterium remove acetylations from all positions on complex beta-mannans. Proc Natl Acad Sci U S A. 2020;117:7122–7130. doi: 10.1073/pnas.1915376117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang TY, Zhang XQ, Chen AL, Zhang J, Lv BH, Ma MH, Lian J, Wu YX, Zhou YT, Ma CC, et al. A comparative study of microbial community and functions of type 2 diabetes mellitus patients with obesity and healthy people. Appl Microbiol Biotechnol. 2020;104:7143–7153. [DOI] [PubMed] [Google Scholar]
- 52.Maaroufi H, Levesque RC. Glycoside hydrolase family 32 is present in Bacillus subtilis phages. Virol J. 2015;12:157. doi: 10.1186/s12985-015-0373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El Kaoutari A, Armougom F, Gordon JI, Raoult D, Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol. 2013;11:497–504. doi: 10.1038/nrmicro3050. [DOI] [PubMed] [Google Scholar]
- 54.Scott KP, Martin JC, Duncan SH, Flint HJ. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol Ecol. 2014;87:30–40. doi: 10.1111/1574-6941.12186. [DOI] [PubMed] [Google Scholar]
- 55.St Martin EJ, Wittenberger CL. Regulation and function of sucrose 6-phosphate hydrolase in Streptococcus mutans. Infect Immun. 1979;26:487–491. doi: 10.1128/IAI.26.2.487-491.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thompson J, Nguyen NY, Robrish SA. Sucrose fermentation by Fusobacterium mortiferum ATCC 25557: transport, catabolism, and products. J Bacteriol. 1992;174:3227–3235. doi: 10.1128/JB.174.10.3227-3235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pikis A, Immel S, Robrish SA, Thompson J. Metabolism of sucrose and its five isomers by Fusobacterium mortiferum. Microbiology. 2002;148:843–852. doi: 10.1099/00221287-148-3-843. [DOI] [PubMed] [Google Scholar]
- 58.Alberto F, Bignon C, Sulzenbacher G, Henrissat B, Czjzek M. The three-dimensional structure of invertase (beta-fructosidase) from Thermotoga maritima reveals a bimodular arrangement and an evolutionary relationship between retaining and inverting glycosidases. J Biol Chem. 2004;279:18903–18910. doi: 10.1074/jbc.M313911200. [DOI] [PubMed] [Google Scholar]
- 59.Cuskin F, Flint JE, Gloster TM, Morland C, Basle A, Henrissat B, Coutinho PM, Strazzulli A, Solovyova AS, Davies GJ, et al. How nature can exploit nonspecific catalytic and carbohydrate binding modules to create enzymatic specificity. Proc Natl Acad Sci U S A. 2012;109:20889–20894. doi: 10.1073/pnas.1212034109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cameron EA, Kwiatkowski KJ, Lee BH, Hamaker BR, Koropatkin NM, Martens EC. Multifunctional nutrient-binding proteins adapt human symbiotic bacteria for glycan competition in the gut by separately promoting enhanced sensing and catalysis. mBio. 2014;5:e01441–14. doi: 10.1128/mBio.01441-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gotoh A, Katoh T, Sakanaka M, Ling Y, Yamada C, Asakuma S, Urashima T, Tomabechi Y, Katayama-Ikegami A, Kurihara S, et al. Sharing of human milk oligosaccharides degradants within bifidobacterial communities in faecal cultures supplemented with Bifidobacterium bifidum. Sci Rep. 2018;8:13958. doi: 10.1038/s41598-018-32080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rakoff-Nahoum S, Foster KR, Comstock LE. The evolution of cooperation within the gut microbiota. Nature. 2016;533:255–259. doi: 10.1038/nature17626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buckwalter CM, King SJ. Pneumococcal carbohydrate transport: food for thought. Trends Microbiol. 2012;20:517–522. doi: 10.1016/j.tim.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trollope KM, van Wyk N, Kotjomela MA, Volschenk H. Sequence and structure-based prediction of fructosyltransferase activity for functional subclassification of fungal GH32 enzymes. Febs J. 2015;282:4782–4796. doi: 10.1111/febs.13536. [DOI] [PubMed] [Google Scholar]
- 65.Menéndez C, Martínez D, Pérez ER, Musacchio A, Ramírez R, López-Munguía A, Hernández L. Engineered thermostable β-fructosidase from Thermotoga maritima with enhanced fructooligosaccharides synthesis. Enzyme Microb Technol. 2019;125:53–62. doi: 10.1016/j.enzmictec.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 66.Ramírez-Escudero M, Gimeno-Pérez M, González B, Linde D, Merdzo Z, Fernández-Lobato M, Sanz-Aparicio J. Structural analysis of β-fructofuranosidase from xanthophyllomyces dendrorhous reveals unique features and the crucial role of N-glycosylation in oligomerization and activity. J Biol Chem. 2016;291:6843–6857. doi: 10.1074/jbc.M115.708495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Endo H, Tamura K, Fukasawa T, Kanegae M, Koga J. Comparison of fructooligosaccharide utilization by Lactobacillus and Bacteroides species. Biosci Biotechnol Biochem. 2012;76:176–179. doi: 10.1271/bbb.110496. [DOI] [PubMed] [Google Scholar]
- 68.Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294:1–8. doi: 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- 69.Lopez-Siles M, Khan TM, Duncan SH, Harmsen HJ, Garcia-Gil LJ, Flint HJ. Cultured representatives of two major phylogroups of human colonic Faecalibacterium prausnitzii can utilize pectin, uronic acids, and host-derived substrates for growth. Appl Environ Microbiol. 2012;78:420–428. doi: 10.1128/AEM.06858-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nordberg H, Cantor M, Dusheyko S, Hua S, Poliakov A, Shabalov I, Smirnova T, Grigoriev IV, Dubchak I. The genome portal of the department of energy joint genome institute: 2014 updates. Nucleic Acids Res. 2014;42:D26–31. doi: 10.1093/nar/gkt1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Endo A, Okada S. Lactobacillus satsumensis sp. nov., isolated from mashes of shochu, a traditional Japanese distilled spirit made from fermented rice and other starchy materials. Int J Syst Evol Microbiol. 2005;55:83–85. doi: 10.1099/ijs.0.63248-0. [DOI] [PubMed] [Google Scholar]
- 72.Maeno S, Tanizawa Y, Kajikawa A, Kanesaki Y, Kubota E, Arita M, Dicks L, Endo A. Pseudofructophilic leuconostoc citreum strain F192-5, isolated from satsuma mandarin peel. Appl Environ Microbiol. 2019;85:e01077–19. doi: 10.1128/AEM.01077-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang H, Yohe T, Huang L, Entwistle S, Wu P, Yang Z, Busk PK, Xu Y, Yin Y. dbCAN2: a meta server for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2018;46:W95–w101. doi: 10.1093/nar/gky418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Almagro Armenteros JJ, Tsirigos KD, Sonderby CK, Petersen TN, Winther O, Brunak S, von Heijne G, Nielsen H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat Biotechnol. 2019;37:420–423. doi: 10.1038/s41587-019-0036-z. [DOI] [PubMed] [Google Scholar]
- 75.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. Clustal W and Clustal X version 2.0. Bioinformatics (Oxford, England). 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 76.Fujii T, Tochio T, Hirano K, Tamura K, Tonozuka T. Rapid evaluation of 1-kestose producing β-fructofuranosidases from Aspergillus species and enhancement of 1-kestose production using a PgsA surface-display system. Biosci Biotechnol Biochem. 2018;82:1599–1605. doi: 10.1080/09168451.2018.1480347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


