Summary
Background
Hospital healthcare workers (HCW), in particular those involved in the clinical care of COVID-19 cases, are presumably exposed to a higher risk of acquiring the disease than the general population.
Methods
Between April 16 and 30, 2020 we conducted a prospective, SARS-CoV-2 seroprevalence study in HCWs in Southern Switzerland. Participants were hospital personnel with varying COVID-19 exposure risk depending on job function and working site. They provided personal information (including age, sex, occupation, and medical history) and self-reported COVID-19 symptoms. Odds ratio (OR) of seropositivity to IgG antibodies was estimated by univariate and multivariate logistic regressions.
Findings
Among 4726 participants, IgG antibodies to SARS-CoV-2 were detected in 9.6% of the HCWs. Seropositivity was higher among HCWs working on COVID-19 wards (14.1% (11.9–16.5)) compared to other hospital areas at medium (10.7% (7.6–14.6)) or low risk exposure (7.3% (6.4–8.3)). OR for high vs. medium wards risk exposure was 1.42 (0.91–2.22), P = 0.119, and 1.98 (1.55–2.53), P<0.001 for high vs. low wards risk exposure. The same was for true for doctors and nurses (10.1% (9.0–11.3)) compared to other employees at medium (7.1% (4.8–10.0)) or low risk exposure (6.6% (5.0–8.4)). OR for high vs. medium profession risk exposure was 1.37 (0.89–2.11), P = 0.149, and 1.75 (1.28–2.40), P = 0.001 for high vs. low profession risk exposure. Moreover, seropositivity was higher among HCWs who had household exposure to COVID-19 cases compared to those without (18.7% (15.3–22.5) vs. 7.7% (6.9–8.6), OR 2.80 (2.14–3.67), P<0.001).
Interpretation
SARS-CoV-2 antibodies are detectable in up to 10% of HCWs from acute care hospitals in a region with high incidence of COVID-19 in the weeks preceding the study. HCWs with exposure to COVID-19 patients have only a slightly higher absolute risk of seropositivity compared to those without, suggesting that the use of PPE and other measures aiming at reducing nosocomial viral transmission are effective. Household contact with known COVID-19 cases represents the highest risk of seropositivity.
Funding
Henry Krenter Foundation, Ente Ospedaliero Cantonale and Vir Biotechnology.
Keywords: COVID-19, Healthcare workers, Seroprevalence
Research in context.
Evidence before this study
Due to the rapidly emerging and evolving literature regarding the seroprevalence of SARS-CoV-2 antibodies among healthcare workers (HCWs), we performed a broad search without time limits in PubMed/Medline and among COVID-19 SARS-CoV-2 preprints from medRxiv and bioRxiv. Search terms used were “COVID-19″, “SARS-CoV-2 antibodies”, ”seroprevalence”, “HCW”, “hospital/health system reorganization”, and “hospital/health system redesign”. Due to the limited amount of studies available, particularly regarding the effect of hospital reorganization and redesign on the risk of serocoversion among HCWs, and the moderate quality of evidence, all retrieved studies were included and considered.
Added value of this study
The seroprevalence of SARS-CoV-2 antibodies among HCWs varies widely among studies. This study shows that even in a region with a high incidence of COVID-19, SARS-CoV-2 antibodies are detectable in nearly 10% of HCWs from acute care hospitals. Furthermore, this study adds to the existing evidence regarding the risk of infection among HCWs, showing that while direct patient contact seems to be associated with a slightly higher absolute risk of seroconversion compared to those without, the reorganization of the health system per se is not. It highlights that household contact with known COVID-19 cases represents the highest risk of seroconversion.
Implications of all the available evidence
The available evidence suggests that the use of PPE and other measures aiming at reducing nosocomial viral transmission are effective. Furthermore, hospital redesign, while effective in streamlining and managing the high burden of COVID-19 patients requiring hospital admission, seems not a major contributor in minimizing the risk of seroconversion among HCWs. This study provides information that can advance public health policies and outcomes, although future research is needed to assess whether these results are representative of other hospitals to help guide better infection control practices.
Alt-text: Unlabelled box
1. Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection continues to be a major public health concern [1,2]. There are significant differences across and within countries in the number of cases, hospitalizations, case fatality rates and in the health strategies that have been enforced to reduce the impact of the pandemic. Prior to the second wave, by 25 September 2020, Switzerland recorded 600 cases and 20 deaths per 100,000 population, respectively. In Southern Switzerland (Canton of Ticino), with over 1000 cases and 90 deaths per 100,000 population, the toll of COVID-19 was much heavier [3]. This is likely due to the fact that Ticino borders Lombardy, the first region outside Wuhan to be heavily affected by COVID-19 in February 2020 [4]. Thus, it was becoming apparent that a rapid surge of cases in Ticino could soon overwhelm hospitals and this called for unprecedented measures to mitigate the spreading of COVID-19 at the community level to avert exceeding healthcare service capacity [5].
In Ticino, with a population of approximately 350,000 inhabitants, the Ente Ospedaliero Cantonale (EOC) manages the public hospital network, which operates four acute care hospitals all equipped with an emergency department (ED) and intensive care units (ICU) for a total of 46 ICU beds. In addition, there is one private hospital, which is also equipped with 6 ICU beds. In February 2020, the regional government established a taskforce to devise an emergency plan aimed at adjusting the hospital capacity to cope with the projected inflow of COVID-19 patients. Two hospitals were assigned as COVID-19 dedicated facilities, whereas the other hospitals were kept COVID-19 free to cater for patients with other conditions. At the EOC, the COVID-19 hospital enhanced the ICU bed capacity to 83 ventilated beds. The privately operated COVID-19 hospital increased its ICU bed capacity to 32. The health services were reorganized so that the other acute care hospital facilities could not, and did not admit patients with SARS-CoV-2 infections, except for very short, temporary admissions into dedicated areas of their ED prior to transfer to one of the COVID-19 facilities.
Health services capacity and response are also greatly influenced by the availability and protection of the workforce. Healthcare workers (HCWs) are likely at higher risk of infection compared to the general population because of the occupational exposure to infectious droplets and other potentially infectious materials [6]. Controlling the impact of the epidemic on HCWs is crucial because they may not only infect patients they take care of, but also other HCWs they collaborate and interact with, which would cause detrimental reductions of the already highly strained capacity of health services.
Previous studies have found great variations in the rates of SARS-CoV-2 infection in HCWs [7]. In cohorts including at least 1000 participants, rates of infections varied between 0.4% in Japan [8] and 31.6% in the UK [9]. Direct comparisons may not be appropriate because of the differences in health systems structure and organization, the availability and enforcement of healthcare protocols and personal protection equipment (PPE), and variations in COVID-19 attack rates between and within countries. Whether and to what extent SARS-CoV-2 infections in HWCs vary as a function of health service reorganization intended to reduce the impact of nosocomial transmission is not known. The aim of the present study was to ascertain the seroprevalence of SARS-CoV-2 antibodies in HCWs in a region severely hit by the pandemic during the first wave in spring 2020, and to explore if the emergency reorganization of the hospital, inpatients healthcare services, and system in Ticino was associated with variations in SARS-CoV-2 infections amongst the HCWs.
2. Methods
2.1. Study design, setting and procedures
This is a prospective, whole population cohort study of HCWs. Here we focus primarily on the cross-sectional, baseline phase of the study. A collaborative team from the Institute for Research in Biomedicine (IRB), the EOC, Humabs BioMed SA (a subsidiary of Vir Biotechnology), and the Institute of Public Health (IPH) of the Università della Svizzera italiana (USI) carried out, with the coordination of the EOC Clinical Trial Unit (CTU-EOC), a COVID-19 serology surveillance program among HCWs in the Ticino region of Switzerland. The study was broadly advertised across the hospital facilities, providing standard information about procedures, potential risks and benefits associated with participation. We invited HCWs also via personal emails using the employer's official mailing lists. All participants signed an informed consent upon participation. The study was approved by the Cantonal Ethics Committee of Ticino, Switzerland (CE-TI-3428, 2018–02,166).
2.2. Participants and measurements
The only eligibility criterion was to be employed at one of the hospitals facilities in Ticino, including the administrative and other support staff who were considered HCWs for the purpose of the study, and were provided with PPE from March 15, 2020, onward. Surgical masks and hand-sanitizers were provided to all staff. All frontline HCWs had also access to standard long sleeved gowns, gloves, goggles or face shields. Additional PPE were available to staff at risk of airborne exposure and included hoods, FFP2/N95 masks, FFP3 masks when performing bronchoscopies, long sleeved fluid-resistant gowns. Staff with comorbidities or over the age of 60 years was generally not allowed to work at COVID-19 hospitals and was exempt from working in high risk COVID-19 areas. Moreover, for non-essential services home working was encouraged. SARS-CoV-2 testing by reverse transcriptase–polymerase chain reaction (rt-PCR) was promptly carried out in all HCWs who had COVID-19-related symptoms, including fever, dry cough, and respiratory distress.
From April 16 to April 30, 2020, we offered antibody testing, regardless of symptoms to 7293 HCWs. We took samples of 7/8 mL of peripheral venous blood using tubes containing clot activator, followed by centrifugation to separate sera, which were stored at +4 °C until the laboratory testing. At the time of blood sampling, we used a standardized questionnaire to collect data on the participants’ socio-demographic and health characteristics (including co-morbidities, smoking habit, body height and weight), work location, type of occupation, frequency and duration of contacts with COVID-19 patients (hospital, household, or community/social contacts), precautionary measures taken during contact with COVID-19 patients to reduce risk of transmission, and a travel history to other countries and regions. We used the participants’ year of birth to calculate age as of 2020, and computed three age groups (13 to 40, 41 to 50, and 51 years or more). Body mass index (BMI) was calculated from reported height and weight data. In addition, we explicitly asked about any contacts with individuals (i.e. non-patients) who tested positive for COVID-19 based on rt-PCR using the following question: “In your private life, have you had close contacts (less than two meters) with people who tested positive for COVID-19?”. Answer options were “No”, “Yes, at home”, and “Yes, outside home (i.e. community)”. We generated a dichotomous variable for “any contact with COVID-19 cases” combining the ‘at home’ and ‘community’ exposure. Next, all participants self-reported COVID-19 clinical symptoms, if any, that occurred in the previous weeks including: a, diarrhea; b, fatigue; c, muscle-bone pain; d, headache; e, fever; f, cough; g, sore throat; h, common cold; i, taste/smell loss; and j, shortness of breath or dyspnea. We defined 4 distinct symptom categories according to CDC criteria as follows: asymptomatic; mild symptoms (any symptoms of a-i); moderate symptoms (any of a-i plus j); atypical symptoms (all other non-COVID-19-related symptoms). We defined categories of professional risk exposure to COVID-19 infection in HCWs according to the hospital site (COVID-19 vs. non-COVID-19 hospital), the occupation within the hospital (doctors, nurses, administrative staff, and support staff), and the hospital unit (COVID-19 ward, standard ward, back office, store, kitchen, etc.) (Table 1). The CTU-EOC organized and managed all data collection, entry, cleaning, and management in collaboration with the IPH, including the linkage of questionnaire-based datasets with the serology results using coded participant IDs, which were used to label and uniquely identify tubes and questionnaires anonymously.
Table 1.
Healthcare worker (HCW) risk category coding.
| High risk | Intermediate risk | Low risk | |
|---|---|---|---|
| Hospital site | COVID-19 dedicated | – | All others |
| Hospital ward/unit type | COVID-19 ward | Emergency department | All others |
| HCW category | Doctors and nurses | Support staff working on COVID-19 ward | All others |
2.3. Laboratory analysis and assays
Qualitative/semi-quantitative measurement of SARS-CoV-2 IgG, IgM, and IgA antibodies was performed with enzyme-linked immunosorbent assays (ELISAs) developed by Humabs BioMed, based on a SARS-CoV-2 receptor-binding domain (RBD) antigen, as described elsewhere [10,11]. Briefly, 96-well ELISA plates were coated with SARS-CoV-2 RBD at 5 µg/ml in Phosphate-Buffered Saline (PBS), pH 7.2, and plates were subsequently blocked with Blocker Casein (1%) in PBS (Thermo Fisher Scientific) supplemented with 0.05% Tween 20 (Sigma Aldrich). Sera were diluted 1:100 and added, each in duplicate, to the plates together with positive control sera (seven 1:2 serial dilutions starting from 1:100 for IgG and 1:25 for IgM and IgA) and negative control sera. The positive control was a pool of 10 representative sera from COVID-19 convalescent patients with previously established clinical diagnosis based on symptoms, clinical history, risk exposure, and laboratory (i.e. molecular) and radiological testing. The negative control was a pool of 10 pre-pandemic sera from healthy individuals collected between 2017 and 2019. Plates were incubated for 1 h at room temperature and then washed with PBS containing 0.1% Tween-20 (PBS-T). Alkaline phosphatase-conjugated goat anti-human IgG, IgM, or IgA antibodies (SouthernBiotech) were added and incubated for 1 h. Plates were washed three times with PBS-T and 4-NitroPhenyl Phosphate (pNPP, Sigma-Aldrich) substrate was added and incubated for 1 h (IgG) or 2 h (IgA and IgM). The optical density (OD) was measured at 405 nm on a microplate reader (BioTek).
The antibody titres were determined from the OD values using a software developed at Humabs BioMed. The software performs a non-linear regression dose-response analysis (4 parameters) of the standard positive control dilutions and determines the fifty percent effective dilution (ED50). For each sample, the software interpolates the OD value with the fitted curve and calculates the resulting dilution factor (DF). In case of multiple replicates, the software calculates the average of the sample DF. The DF is converted into Relative Units (RU) with the following formula: DF/ED50. The software produces an output Excel file with a list of sample IDs flanked by respective RU values and a qualitative evaluation of the result based on the following criteria: 1) "positive" if the sample RU is higher than the RU of the cut-off point of the fitted curve; 2) "negative" if the sample RU is lower than the cut-off point. The cut-off point is the RU corresponding to the lower bend point of the fitted curve, which is defined by one of the roots of the 3rd derivative of the fitted curve and delimits its linear range.
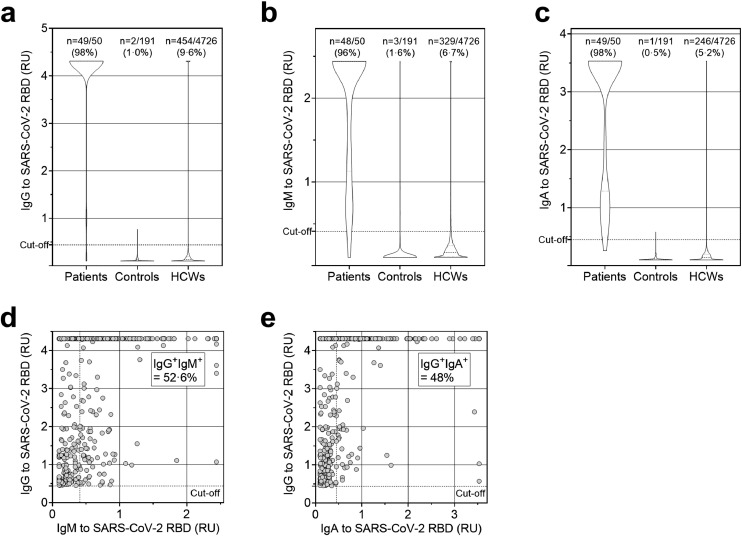
The in house-developed IgG, IgM, and IgA ELISAs were validated by testing serum samples from 50 convalescent COVID-19 patients and from 191 healthy controls collected between 2017 and 2019 (Fig. 1A-C). Based on the IgG results, we set sensitivity to 0.98 and specificity to 0.99 for the subsequent analyses plotting the sensitivity and the false positive rate (1-specificity) on a receiver operating characteristic (ROC) curve, and varying discrimination thresholds as standard (Supplementary Table 1). Discrimination thresholds (cut-offs) were chosen among those that maximized sensitivity and specificity with a lower limit of 0.95.
Fig. 1.
Analysis of serum antibody titres to SARS-CoV-2 RBD in HCWs. a-c. Shown are the distributions of titres expressed as Relative Units (RU) of serum IgG (a), IgM (b) and IgA (c) in 4726 HCWs, compared to the clinical group of 50 COVID-19 convalescent patients and 191 healthy donors (controls) whose serum was collected before the COVID-19 pandemic. d-e. Analysis of serum IgM (d) and IgA (e) in 454 SARS-CoV-2 RBD-IgG+ HCWs.
2.4. Statistical methods
Estimated prevalence of seropositivity to IgG antibodies against SARS-CoV-2 across categories of risk exposure was calculated accounting for specificity and sensitivity of the adopted assay. We calculated true prevalence with 95% confidence intervals using the function epi.prev in the R library epiR, version 1.0–15 [12,13]. We ran univariate and multivariate logistic regressions using the logitem command in STATA (StataCorp, College Station, TX, version 15) (see Supplementary Material) to correct for the assay's specificity and sensitivity [14,15], and estimated odds ratio (OR) of seropositivity to IgG antibodies associated with categories of risk exposure, age, gender, BMI, smoking, flu vaccination, and household or community/social contacts. Three separate models were tested: unadjusted (univariate model that included the main exposure of interest only), fully adjusted (adjusted for all covariates listed above, and for single categories of risk exposure alternatively), and mutually adjusted (adjusted for categories of risk exposure only). Testing both fully and mutually adjusted models is ideal when collider-stratification biases cannot be excluded [16], as in our case given categories of risk exposure among HCWs are likely not mutually exclusive, and a potential causal influence on the main exposures (i.e. categories of risk) of two or more covariates included in the adjusted models cannot be excluded. No statistically significant deviation from randomness using Little's MCAR test was detected (P = 0.067) for missing data on age (n = 2), gender (n = 1), and BMI (n = 112). Thus, complete-case analysis was adopted.
2.5. Role of the funding source
Henry Krenter Foundation, Ente Ospedaliero Cantonale and Vir Biotechnology supported study design, data collection, data analysis, interpretation, and writing of the report.
3. Results
In total, 4726 HCWs (65% of those invited) took part in the serology surveillance program. In the study sample, there were more women (68.3%) than men, and 46.4% were in the 16–40 age group, 27.3% in the 41–50 age group and 26.3% were over 51. Overall, 46.2% were vaccinated against seasonal influenza, 24.4% were smokers, 12.2% had comorbid conditions associated with poorer COVID-19 outcome, and 8% were obese (BMI ≥30 kg/m2) (Table 2). The distribution of gender, smoking status, flu vaccination, BMI category and co-morbidities was overall similar across categories of risk according to hospital site, hospital ward, or unit type, and HCW function. There was a higher proportion of HCWs aged 16 to 40 in high-risk hospital wards or units (57.4%) in comparison with high-risk hospital sites (48.2%) and HCW function (51.6%).
Table 2.
Population characteristics by categories of risk exposure.
| N | Hospital site |
Hospital ward/unit type |
HCW category |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Low risk n = 3729 | High risk n = 997 | Low risk n = 3426 | Medium risk n = 317 | High risk n = 983 | Low risk n = 1108 | Medium risk n = 445 | High risk n = 3173 | ||
| Age group | |||||||||
| 16–40 | 2191 (46.4) | 1710 (45.9) | 481 (48.2) | 1453 (42.4) | 174 (54.9) | 564 (57.4) | 434 (39.2) | 121 (27.2) | 1636 (51.6) |
| 41–50 | 1289 (27.3) | 1015 (27.2) | 274 (27.5) | 970 (28.3) | 77 (24.3) | 242 (24.6) | 299 (27.0) | 145 (32.6) | 845 (26.6) |
| >51 | 1244(26.3) | 1002 (26.9) | 242 (24.3) | 1001 (29.2) | 66 (20.8) | 177 (18.0) | 375 (33.8) | 179 (40.2) | 690 (21.8) |
| missing | 2 (<0.1) | ||||||||
| Gender | |||||||||
| Men | 1496 (31.7) | 1133 (30.4) | 363 (36.4) | 1048 (30.6) | 128 (40.4) | 320 (32.6) | 304 (27.4) | 111 (24.9) | 1081 (34.1) |
| Women | 3229 (68.3) | 2595 (69.6) | 634 (63.6) | 2377 (69.4) | 189 (59.6) | 663 (67.4) | 804 (72.6) | 334 (75.1) | 2091 (65.9) |
| missing | 1 (<0.1) | ||||||||
| BMI (kg/m2) | |||||||||
| <25 | 3116 (65.9) | 2454 (67.4) | 662 (68.1) | 2217 (66.5) | 219 (69.5) | 680 (70.5) | 745 (69.2) | 237 (55.2) | 2134 (68.7) |
| 25–29.9 | 1121 (23.7) | 889 (24.4) | 232 (23.9) | 824 (24.7) | 82 (26.0) | 215 (22.3) | 233 (21.6) | 125 (29.1) | 763 (24.5) |
| ≥30 | 377 (8.0) | 299 (8.2) | 78 (8.0) | 293 (8.8) | 14 (4.4) | 70 (7.3) | 99 (9.2) | 67 (15.6) | 211 (6.8) |
| missing | 112 (2.4) | ||||||||
| Chronic disease | |||||||||
| Diabetes | 57 (1.2) | 43 (1.2) | 14 (1.4) | 48 (1.4) | 6 (1.9) | 3 (0.3) | 8 (0.7) | 11 (2.5) | 38 (1.2) |
| Hypertension | 266 (5.7) | 210 (5.7) | 56 (5.6) | 218 (6.4) | 18 (5.7) | 30 (3.1) | 69 (6.3) | 33 (7.6) | 164 (5.2) |
| Heart disease | 57 (1.2) | 46 (1.2) | 11 (1.1) | 45 (1.3) | 3 (1.0) | 9 (0.9) | 14 (1.3) | 10 (2.3) | 33 (1.0) |
| Lung disease | 137 (2.9) | 116 (3.1) | 21 (2.1) | 103 (3.0) | 10 (3.2) | 24 (2.5) | 21 (1.9) | 17 (3.9) | 99 (3.1) |
| Renal disease | 17 (0.4) | 7 (0.2) | 10 (1.0) | 11 (0.3) | 1 (0.3) | 5 (0.5) | 5 (0.5) | 2 (0.5) | 10 (0.3) |
| Cancer | 38 (0.8) | 30 (0.8) | 8 (0.8) | 31 (0.9) | 4 (1.3) | 3 (0.3) | 14 (1.3) | 5 (1.1) | 19 (0.6) |
| Immunosuppressive therapies | |||||||||
| No | 4696 (99.4) | 3710 (99.5) | 986 (98.9) | 3401 (99.3) | 316 (99.7) | 979 (99.6) | 1103 (99.5) | 441 (99.1) | 3152 (99.3) |
| Yes | 30 (0.6) | 19 (0.5) | 11 (1.1) | 25 (0.7) | 1 (0.3) | 4 (0.4) | 5 (0.5) | 4 (0.9) | 21 (0.7) |
| Smoking | |||||||||
| Non-smoker/Ex-smoker | 3572 (75.6) | 2821 (75.7) | 751 (75.3) | 2630 (76.8) | 240 (75.7) | 702 (71.4) | 842 (76.0) | 309 (69.4) | 2421 (76.3) |
| Current smoker | 1154 (24.4) | 908 (24.3) | 246 (24.7) | 796 (23.2) | 77 (24.3) | 281 (28.6) | 266 (24.0) | 136 (30.6) | 752 (23.7) |
| Flu vaccination | |||||||||
| No | 2543 (53.8) | 2027 (54.4) | 516 (51.8) | 1936 (56.5) | 135 (42.6) | 472 (48) | 767 (69.2) | 281 (63.1) | 1495 (47.1) |
| Yes | 2183 (46.2) | 1702 (45.6) | 481 (48.2) | 1490 (43.5) | 182 (57.4) | 511 (52) | 341 (30.8) | 164 (36.9) | 1678 (52.9) |
| Contact COVID-19 | |||||||||
| No | 4325 (89.6) | 3348 (89.8) | 887 (89) | 3093 (90.3) | 283 (89.3) | 859 (87.4) | 994 (89.7) | 396 (89) | 2845 (89.7) |
| Yes | 491 (10.4) | 381 (10.2) | 110 (11) | 333 (9.7) | 34 (10.7) | 124 (12.6) | 114 (10.3) | 49 (11) | 328 (10.3) |
Values are frequencies (percentages).
BMI: Body Mass Index.
The majority of participants reported mild symptoms (68.7%) possibly related to COVID-19, while 23.1% reported no symptoms and only 6.6% moderate COVID-19-related symptoms (Supplementary Table 2). The distribution of COVID-19-related symptoms categories was overall similar in relation of HCW risk of exposure. Of the 4726 HCWs, 517 underwent SARS-CoV-2 molecular testing with rt-PCR because of COVID-19-like symptoms or suspected exposure, of these 123 tested positive (23.8%). Community acquired, rather than nosocomial transmission, was identified as the source of infection in 46% of cases and only 8 HCWs required hospital admission, none of whom was admitted to ICU and all of them recovered.
Of the 4726 HCWs tested, 572 (12.1%) were seropositive for IgM and/or IgG and/or IgA against SARS-CoV-2 RBD (Fig. 1A-C). RBD-specific IgG antibodies were detected in 9.6% (454) of the serum samples, indicating current or previous COVID-19 infection. RBD-specific IgM and IgA antibodies were detected in 6.7% (329) and 5.2% (246) of the serum samples, respectively. Among IgG seropositive subjects, IgM antibodies were found in 52.6% (218 out of 454) and IgA antibodies were found in 48% (236 out of 454) of cases (Fig. 1D-E and Supplementary Table 3). Among IgG seronegative subjects, IgM antibodies were found in 2.1% (90 out of 4272) and IgA antibodies were found in 0.7% (28 out of 4272) of cases.
The 997 HCWs working in COVID-19 dedicated hospitals were exposed to a peak of 415 COVID-19 patients between March and June 2020, when strict PPE protocols were enforced since March 10th and progressively updated over time (Supplementary Figure 1). In the unadjusted model, the SARS-CoV-2 seroprevalence was higher among HCWs in COVID-19 dedicated hospitals compared to the other hospital facilities (11.17% vs. 8.25%; OR 1.40, 95% CI: 1.10–1.78, P = 0.007) (Table 3). Seropositivity was also significantly higher in high-risk wards where healthcare to COVID-19 patients was provided (14.07%) compared to low-risk wards not dedicated to patient care (7.27%; OR 2.09, 95% CI: 1.65–2.64, P<0.001). However, there was no significant difference in seroprevalence between high- and medium-risk wards (14.07% vs. 10.73%; OR 1.47, 95% CI: 0.95–2.26, P = 0.081). Similar trends were observed according to HCW risk categories: seropositivity was significantly higher in high-risk professions (i.e. doctors and nurses) compared to low-risk ones (10.11% vs. 6.56%; OR 1.75, 95% CI: 1.30–2.35, P<0.001). There was no significant difference in seroprevalence between high- and medium-risk professions (10.11% vs. 7.08%; OR 1.48, 95% CI: 0.98–2.23, P = 0.063). All significant associations between higher risk exposures and SARS-CoV-2 seroprevalence remained so also after full adjustment for relevant covariates (Table 3). Fully adjusted OR for high vs. medium wards risk exposure was 1.42 (0.91–2.22), P = 0.119, and 1.98 (1.55–2.53), P<0.001 for high vs. low wards risk exposure. Fully adjusted OR for high vs. medium profession risk exposure was 1.37 (0.89–2.11), P = 0.149, and 1.75 (1.28–2.40), P = 0.001 for high vs. low profession risk exposure. Next, we found that reported household or community/social contact with COVID-19 cases carried a higher risk of seropositivity (18.71%) compared to no contact 7.73% (OR 2.80, 95% CI: 2.14–3.67, P<0.001). We found a significant difference in the prevalence of seropositivity by age (P = 0.002), but not by sex (P = 0.167). Smokers were 40% less likely, while HCWs who had influenza vaccine were 30% more likely to be seropositive for SARS-CoV-2 IgG. HCWs in the low and middle risk categories were less likely to be vaccinated than those in the high risk category, but the interactions between risk-exposure and vaccination were not statistically significant in multivariate logistic models (Supplementary Table 4). Finally, only the association between hospital site and SARS-CoV-2 seroprevalence were no longer significant (P = 0.932) after mutual adjustment for ward/unit type and HCW high-risk categories (Table 3). Collinearity diagnostics for these multivariate analyses [17] indicated that multicollinearity was not an issue (Supplementary Table 5). As a sensitivity analysis, we imputed the missing values for BMI (n = 112; 2.4%) with a multivariate normal regression approach in Stata adopting an iterative Markov chain Monte Carlo method. As shown in Supplementary Table 6, results from the fully adjusted models using imputed BMI values did not diverge from the primary analysis. The prevalence SARS-CoV-2 IgG antibodies was higher among participants reporting moderate (21.4%) or mild symptoms (9.8%) in the previous two months compared to those reporting no symptoms (2.4%). Seropositivity was relatively low in HCWs who reported non COVID-19-related symptoms (7.6%), and lowest in those who reported no symptoms (2.4%) (Table 4). Finally, of the 123 HCWs with a positive rt-PCR, 118 (95.9%) were IgG seropositive and of the 394 HCWs with a negative rt-PCR, 358 (90.9%) were seronegative (Supplementary Table 7).
Table 3.
Adjusted prevalence and odds ratio of seropositivity to IgG antibodies against SARS-CoV-2 by categories of risk exposure and covariates.
| SARS-CoV-2 IgG seropositivity |
Odds Ratio (95%CI), p-value |
|||||
|---|---|---|---|---|---|---|
| N | n | % (95%CI) | Unadjusted | Fully adjusted | Mutually adjusted | |
| Hospital site | ||||||
| Low risk | 3729 | 336 | 8.25 (7.33–9.25) | 1 (ref) | 1 (ref) | 1 (ref) |
| High risk | 997 | 118 | 11.17 (9.21–13.37) | 1.40 (1.10–1.78), 0.007 | 1.38 (1.08–1.78), 0.011 | 1.01 (0.76–1.34), 0.932 |
| Hospital ward /unit type | ||||||
| Low risk | 3426 | 276 | 7.27 (6.36–8.27) | 1 (ref) | 1 (ref) | 1 (ref) |
| Medium risk | 317 | 34 | 10.73 (7.60–14.57) | 1.42 (0.94–2.15), 0.096 | 1.39 (0.91–2.13), 0.126 | 1.30 (0.86–1.98), 0.211 |
| High risk | 983 | 144 | 14.07 (11.88–16.51) | 2.09 (1.65–2.64), <0.001 | 1.98 (1.55–2.53), <0.001 | 1.91 (1.45–2.51), <0.001 |
| HCW category | ||||||
| Low risk | 1032 | 76 | 6.56 (5.02–8.37) | 1 (ref) | 1 (ref) | 1 (ref) |
| Medium risk | 445 | 35 | 7.08 (4.78–10.01) | 1.18 (0.74–1.91), 0.485 | 1.28 (0.78–2.09), 0.332 | 1.08 (0.67–1.75), 0.759 |
| High risk | 3173 | 343 | 10.11 (9.02–11.28) | 1.75 (1.30–2.35), <0.001 | 1.75 (1.28–2.40), 0.001 | 1.49 (1.10–2.03), 0.011 |
| Age group | ||||||
| 16–40 | 2191 | 237 | 10.12 (8.83–11.54) | 1 (ref) | 1 (ref) | – |
| 41–50 | 1289 | 123 | 8.81 (7.21–10.59) | 0.86 (0.67–1.10), 0.234 | 0.83 (0.64–1.08), 0.175 | – |
| >51 | 1244 | 94 | 6.76 (5.31–8.39) | 0.64 (0.49–0.85), 0.002 | 0.63 (0.47–0.85), 0.002 | – |
| Gender | ||||||
| Women | 3229 | 319 | 9.15 (8.11–10.27) | 1 (ref) | 1 (ref) | – |
| Men | 1496 | 135 | 8.27 (6.82–9.87) | 0.90 (0.71–1.13), 0.355 | 0.84 (0.65–1.08), 0.167 | – |
| BMI (kg/m2) | ||||||
| <25 | 3116 | 293 | 8.66 (6.64–9.78) | 1 (ref) | 1 (ref) | – |
| 25–29.9 | 1121 | 101 | 8.26 (6.61–10.11) | 0.95 (0.73–1.24), 0.698 | 1.04 (0.79–1.37), 0.776 | – |
| ≥30 | 377 | 47 | 11.82 (8.56–15.70) | 1.41 (0.99–2.02), 0.057 | 1.51 (1.04–2.17), 0.028 | – |
| Smoking | ||||||
| No | 3572 | 370 | 9.65 (8.64–10.73) | 1 (ref) | 1 (ref) | – |
| Yes | 1154 | 84 | 6.47 (4.99–8.15) | 0.65 (0.49–0.86), 0.003 | 0.63 (0.47–0.84), 0.002 | – |
| Flu vaccination | ||||||
| No | 2543 | 220 | 7.93 (6.83–9.12) | 1 (ref) | 1 (ref) | – |
| Yes | 2183 | 233 | 9.97 (8.68–11.38) | 1.29 (1.04–1.59), 0.021 | 1.27 (1.01–1.58), 0.038 | – |
| Contact COVID-19 | ||||||
| No | 4325 | 360 | 7.73 (6.89–8.64) | 1 (ref) | 1 (ref) | – |
| Yes | 491 | 94 | 18.71 (15.28–22.54) | 2.75 (2.11–3.58), <0.001 | 2.80 (2.14–3.67), <0.001 | |
95%CI: 95% Confidence Intervals.
Ref = Referent category.
Unadjusted estimates are from univariate models. Fully adjusted estimates are from models adjusted for all covariates (age group, gender, BMI, smoking, flu vaccination, and contact COVID-19) and one single category of risk exposure at a time (fully adjusted estimates pertaining to the covariates are from a model including all covariates and no categories of risk exposure). Mutually adjusted estimates are from a model including only categories of risk exposure.
Table 4.
Adjusted prevalence of seropositivity to IgG antibodies against SARS-CoV-2 by categories of self-reported symptoms.
| SARS-CoV-2 IgG seropositivity |
||||
|---|---|---|---|---|
| N | n | % | 95%CI | |
| Symptoms category | ||||
| No symptoms | 1089 | 36 | 2•4 | 1•4–3•6 |
| Atypical symptoms | 77 | 5 | 5•7 | 1•6–13•9 |
| Mild symptoms | 3247 | 341 | 9•8 | 8•7–10•9 |
| Moderate symptoms | 331 | 72 | 21•4 | 16•9–26•3 |
95%CI: 95% Confidence Intervals.
4. Discussion
In spring 2020, we conducted a study to assess seroprevalence and risk of SARS-CoV-2 infection associated to hospital reorganization in 4726 HCWs employed in acute care hospitals in Southern Switzerland, a region which was severely affected by COVID-19 during the first wave of the pandemic. We found that nearly 10% of HCWs developed SARS-CoV-2 IgG antibodies, and that while the direct contact with patients was associated with a higher risk of infection, the reorganization of the health system per se was not.
The relatively low seroprevalence observed in our HCW cohort is consistent with the one reported in similar studies in 3056 HCWs at a single hospital in Belgium (6.4%) and in 40,329 HCWs in the greater New York City area (13.7%) [18,19], while other studies in 2004 HCWs at an integrated care organization in London, UK [9], and in 2590 HCWs at a teaching hospital in Madrid, Spain [16] reported much higher seroprevalences (up to 31.6%) [9,20]. This high prevalence in the UK may be ascribed, at least in part, to selection bias, because 51.5% of those tested reported COVID-19-like illness, with typical symptoms listed on the request form [9] Nonetheless, another, yet smaller UK prospective study of unselected frontline HCWs also demonstrated a seroprevalence of 45%, of whom 20% seroconverted during the study period [21]. Similarly, among the Spanish HCWs, 25.2% reported no symptoms and 23.3% only mild symptoms of COVID-19-like illness.
In this study, HCW-reported symptoms suggestive of virus exposure and prior positive rt-PCR testing results were most strongly associated with seropositivity, which is consistent with the New York study [19]. Seroprevalence was low in personnel who did not report any symptoms. However, because rt-PCR screening was offered only to staff with symptoms potentially related to COVID-19, we cannot determine the precise rate of seroconversion among asymptomatic cases who were not tested with the molecular test. A point prevalence screening by rt-PCR conducted among HCWs in Oxford showed that only 3% of asymptomatic subjects had a positive nasopharyngeal swab [22], which is in line with the seroprevalence we found in the asymptomatic staff of our study. Despite only those with suggestive symptoms were tested, out of 517 of these, only 123 were positive to SARS-CoV-2 by rt-PCR, and only eight required hospital admission. At the time of the serosurvey the rate of nosocomial COVID-19 cases among HCWs was 5.4% at the COVID-19 hospitals and 1.5% at non-COVID-19 hospitals. The COVID-19 hospitalization rate among the HCWs who took part in this study was extremely low in participants exposed to contagion and slightly higher, but still below 2% in those with SARS-CoV-2 IgG antibodies, which is not surprising because HCWs are likely healthier and younger than the general population. Next, we found that half of undiagnosed infections occurred in HCWs who reported symptoms considered being atypical for COVID-19. Further investigations are warranted to investigate whether enforcement of rt-PCR screening programs for all HCWs, irrespective of COVID-19 symptoms, would reduce the risk of hospital-acquired SARS-CoV-2 infections.
Our study also assessed the role of sex, weight and age in determining the risk of SARS-CoV-2 infection in the HCW cohort. While seropositivity to anti-SARS-CoV-2 antibodies did not differ by sex, overweight and obese HCWs were more likely to be seropositive compared to those with a normal body weight. Evidence suggests that obesity in COVID-19 patients is associated to a significantly less favourable course of the disease, and to invasive mechanical ventilation [23], but it is unclear whether obesity imparts a higher susceptibility to SARS-CoV-2 infection in people exposed to the virus. Being older than 50 years was associated with a lower seroprevalence in our population. This could be explained by the older staff's better awareness of the risks of severe COVID-19, and their consequent attitude to follow personal protective measures more stringently than the younger counterpart. Moreover, compared to young, older HCWs were less represented in high risk sites and wards, and more likely to be redirected to tasks with reduced exposure to infectious patients. However, age-related declines in humoral immunity cannot be excluded because vaccine responses are reduced in older adults [24,25].
We studied the impact of the reorganization of acute care hospitals on risk of infection in HCWs. We found that SARS-CoV-2 IgG antibodies were up to 75% more likely in HCWs in direct contact with COVID-19 patients, compared to those not exposed to COVID-19 patients. This is not consistent with evidence from a study conducted in a Belgian hospital, in which neither being directly involved in clinical care nor working in a COVID-19 unit increased the odds of being seropositive [18]. However, the overall seroprevalence and the proportion of doctors and nurses in our sample (67.1%) were higher compared to the Belgian sample (51.4%). Moreover, the seroprevalence among frontline workers was less than double the one observed among administrative, service, and maintenance staff, which is consistent with evidence from a recent study conducted in HCWs in Denmark [26].
Preliminary results from the Corona Immunitas study in a representative sample of the Ticino population suggest that 11% of adults between 20 and 64 years of age developed detectable antibodies against SARS-CoV-2 (https://www.usi.ch/it/feeds/14492). Other previous serological studies in the Geneva Canton, as well as in France, Italy and Spain showed similar results [27], [28], [29], [30]. Infection rates and community transmission in the general population might be slightly higher compared to the overall seroprevalence in HCWs, and the seropositivity among HCWs at high risk of exposure was only 3% higher than in the local general population. Thus, our data do not support widespread nosocomial transmission as the source of infection in HCWs. In fact, the availability and widespread use of PPE, and the stringent adherence to measures aiming at reducing viral transmission compared to the general population may have contributed to keep contagions relatively at bay in HCWs despite their high risk. The importance of appropriate PPE when dealing with COVID-19 patients, including full PEE when in the isolated chamber or performing airways procedures generating aerosol, has been highlighted early [31,32]. In a multinational study, the incidence of laboratory-confirmed COVID-19 in HCWs after a tracheal intubation episode performed with PPE conforming to standards was reported to be 10.7% [33], which is comparable to the incidence of seropositivity in our cohort. Finally, while transmission of COVID-19 infection to family members may be a key concern for HCWs, our results may suggest that household proximity and contact with COVID-19 cases in our cohort was strongly associated with the development of SARS-CoV-2 IgG antibodies. The direction of travel of the exposure from HCW to household member and vice versa are equally possible and likely. However, personnel reporting of a COVID-19 positive family member in their household was low, and asymptomatic infections can be assumed to not vary as a function of the direction of infections in the household from or to HCWs.
Some limitations of this study are worth noting. The reorganization of health services implies that the likelihood of exposure to COVID-19 patients, and the enforcement of PPE and other preventive measures varied across the study sites. Next, despite the very large sample, response rate was overall around 65% of the eligible HCWs. However, response rates were not differential across hospital site, ward/ unit type, and HCWs category (i.e. the three main exposures to risk of infection). We did not explore the reasons for not participating, but it is unlikely that the study cohort is an overrepresentation of staff who were not exposed to or did not develop detectable antibodies against SARS-CoV-2, because while through data collection and blood sampling 12.6% of the staff was on sick leave, only 2.2% was due to COVID-19 related illness or quarantine. Moreover, participation rate was highest (82%) in frontline HCWs, which might contribute to a slight over rather than under estimation of the true seroprevalence. Conversely, because we conducted the study during the first wave of the pandemic, latency in seroconversion may have contributed to an underestimation of the true rates of infection in HCWs, because testing might have been performed too early. Indeed, 90 HCWs had detectable SARS-CoV-2 IgM but not IgG antibodies. The former develop (and decay) earlier than the latter [34,35], and including IgM positive subjects, the overall seroprevalence would reach 11.5%. A follow-up sampling is under way to ascertain whether seroprevalence increased with time in our cohort. Finally, recall and social desirability biases in the reporting of symptoms by participants cannot be excluded. However, this seems unlikely, and information bias is likely low also because of the high health literacy and level of awareness of COVID-19 in a sample of HCWs who were well aware of being at the forefront of the societal response to the epidemic during the lockdown months.
In conclusion, our results suggest that in a region struck extremely hard during the first wave of the COVID-19 pandemic, SARS-CoV-2 IgG seroprevalence in HCWs may be lower than feared and expected. Higher availability of PPE, stringent adherence to hand hygiene and physical distancing rules could explain the contained viral transmission within hospitals. Health services reorganization, while effective in streamlining and managing the high burden of COVID-19 patients requiring hospital admission, was not a major contributor in minimizing the risk of infection based on serological data among HCWs. Further studies are needed to assess whether these results are representative of other hospitals to help guide better infection control practices.
Contributors
FS, LP, AC, PF, LE, EB, AL, OG and CG contributed to study concept and design.
AC, PF, LE, EB, CG and TT contributed to acquisition of data.
EC, SJa and NS contributed to production of reagents for the ELISA tests.
FM, SJ, BF and IG-S contributed to preparation and management of sera for the ELISA tests.
FM, SJ, BFR and CS-F contributed the ELISA screenings.
LP, IB and DC contributed to informatic elaboration of the ELISA data.
FS, LP, AC, PF, EA and GP contributed to data analysis.
PF, AC, FS, LP and EA contributed to initial drafting of the manuscript.
EC and MU contributed to Ethics protocol.
AC, PF and FS contributed to study supervision.
All authors contributed to interpretation of data and critical revision of the manuscript.
Declaration of Competing Interests
LP, C.S-F and NS report that the work was supported in part by Vir Biotechnology. DC, AL, IB, EC and SJ report that they owns shares of Vir Biotechnology and that the work was supported in part by Vir Biotechnology. FS owns shares of Vir Biotechnology. EB reports other from Gilead Sciences, other from Merck Sharp & Dohme, other from ViiV Healthcare, other from Pfizer, other from Abbvie, other from Sandoz, outside the submitted work. CG reports to be an external scientific consultant of Humabs BioMed SA, outside the submitted work. AC, PF, LE, OG, MU, EA, BFR, IG-S, SJ, FM, GP and TT have nothing to disclose.
Acknowledgments
Acknowledgments
We thank all participants to the study and the personnel at the hospitals for blood collection. The study was in part financed by the Henry Krenter Foundation, the EOC research funds and Vir Biotechnology. Federica Sallusto and the Institute for Research in Biomedicine are supported by the Helmut Horten Stiftung.
Data sharing statement
There are no restrictions to the availability of any materials or data.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2020.100013.
Contributor Information
Federica Sallusto, Email: federica.sallusto@irb.usi.ch.
Alessandro Ceschi, Email: alessandro.ceschi@eoc.ch.
Appendix. Supplementary materials
References
- 1.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVID-19 information for Switzerland, corondata dashboard. 2020. http://www.corona-data.ch/. Accessed 25 Septmeber 2020.
- 4.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 5.Palladino R., Bollon J., Ragazzoni L., Barone-Adesi F. Excess deaths and hospital admissions for COVID-19 due to a late implementation of the lockdown in Italy. Int J Environ Res Public Health. 2020;17(16) doi: 10.3390/ijerph17165644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verbeek J.H., Rajamaki B., Ijaz S. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in health care staff. Cochrane Database Syst Rev. 2020;5 doi: 10.1002/14651858.CD011621.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gómez-Ochoa S.A., Franco O.H., Rojas L.Z. COVID-19 in health care workers: a living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol. 2020 doi: 10.1093/aje/kwaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura A., Sato R., Ando S. Seroprevalence of antibodies to SARS-CoV-2 in health care workers in non-epidemic region: a hospital report in Iwate Prefecture, Japan. medRxiv. 2020 doi: 10.1101/2020.06.15.20132316. 2020.06.15.20132316. [DOI] [Google Scholar]
- 9.Grant J., Wilmore S., McCann N. Seroprevalence of SARS-CoV-2 antibodies in health care workers at a London NHS Trust. Infect Control Hosp Epidemiol. 2020 doi: 10.1017/ice.2020.402. 1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinto D., Park Y.J., Beltramello M. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583(7815):290–295. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- 11.Piccoli L., Park Y.-.J., Tortorici M.A. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell. 2020 doi: 10.1016/j.cell.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiczigel J., Foldi J., Ozsvari L. Exact confidence limits for prevalence of a disease with an imperfect diagnostic test. Epidemiol Infect. 2010;138(11):1674–1678. doi: 10.1017/S0950268810000385. [DOI] [PubMed] [Google Scholar]
- 13.Blaker H. Confidence curves and improved exact confidence intervals for discrete distributions. Can J Stat. 2000;28(4):783–798. [Google Scholar]
- 14.Cleves M., Tossetto A. Logistic regression when binary outcome is measured with uncertainty. Stat Tech Bull. 2001;10(55) [Google Scholar]
- 15.Magder L.S., Hughes J.P. Logistic regression when the outcome is measured with uncertainty. Am J Epidemiol. 1997;146(2):195–203. doi: 10.1093/oxfordjournals.aje.a009251. [DOI] [PubMed] [Google Scholar]
- 16.Westreich D., Greenland S. The Table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. 2013;177(4):292–298. doi: 10.1093/aje/kws412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabachnick B.G., Fidell L.S. 6th ed. Pearson; Boston, MA: 2013. Using multivariate statistics. ed. [Google Scholar]
- 18.Steensels D., Oris E., Coninx L. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020 doi: 10.1001/jama.2020.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moscola J., Sembajwe G., Jarrett M. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City Area. JAMA. 2020 doi: 10.1001/jama.2020.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galan I., Velasco M., Casas M.L. SARS-CoV-2 Seroprevalence among all workers in a teaching hospital in Spain: unmasking the risk. medRxiv. 2020 doi: 10.1101/2020.05.29.20116731. 2020.05.29.20116731. [DOI] [Google Scholar]
- 21.Houlihan C.F., Vora N., Byrne T. Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health care workers. Lancet. 2020;396(10246):e6–e7. doi: 10.1016/S0140-6736(20)31484-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivett L., Sridhar S., Sparkes D. Screening of health care workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife. 2020;9:e58728. doi: 10.7554/eLife.58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caussy C., Pattou F., Wallet F. Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diabetes Endocrinol. 2020;8(7):562–564. doi: 10.1016/S2213-8587(20)30160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frasca D., Blomberg B.B. Aging affects human B cell responses. J Clin Immunol. 2011;31(3):430–435. doi: 10.1007/s10875-010-9501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner A., Garner-Spitzer E., Jasinska J. Age-related differences in humoral and cellular immune responses after primary immunisation: indications for stratified vaccination schedules. Sci Rep. 2018;8(1):9825. doi: 10.1038/s41598-018-28111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iversen K., Bundgaard H., Hasselbalch R.B. Risk of COVID-19 in health care workers in Denmark: an observational cohort study. The Lancet Infectious Diseases. 2020 doi: 10.1016/S1473-3099(20)30589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stringhini S., Wisniak A., Piumatti G. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396(10247):313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollan M., Perez-Gomez B., Pastor-Barriuso R. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vena A., Berruti M., Adessi A. Prevalence of antibodies to SARS-CoV-2 in Italian adults and associated risk factors. J Clin Med. 2020;9(9) doi: 10.3390/jcm9092780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrat F., de Lamballerie X., Rahib D. Seroprevalence of SARS-CoV-2 among adults in three regions of France following the lockdown and associated risk factors: a multicohort study. medRxiv. 2020 doi: 10.1101/2020.09.16.20195693. 2020.09.16.20195693. [DOI] [Google Scholar]
- 31.Sorbello M., El-Boghdadly K., Di Giacinto I. The Italian coronavirus disease 2019 outbreak: recommendations from clinical practice. Anaesthesia. 2020;75(6):724–732. doi: 10.1111/anae.15049. [DOI] [PubMed] [Google Scholar]
- 32.Kursumovic E., Lennane S., Cook T.M. Deaths in health care workers due to COVID-19: the need for robust data and analysis. Anaesthesia. 2020;75(8):989–992. doi: 10.1111/anae.15116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Boghdadly K., Wong D.J.N., Owen R. Risks to health care workers following tracheal intubation of patients with COVID-19: a prospective international multicentre cohort study. Anaesthesia. 2020;75(11):1437–1447. doi: 10.1111/anae.15170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun B., Feng Y., Mo X. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microbes Infect. 2020;9(1):940–948. doi: 10.1080/22221751.2020.1762515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang A.T., Garcia-Carreras B., Hitchings M.D.T. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11(1):4704. doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.