To the Editor:
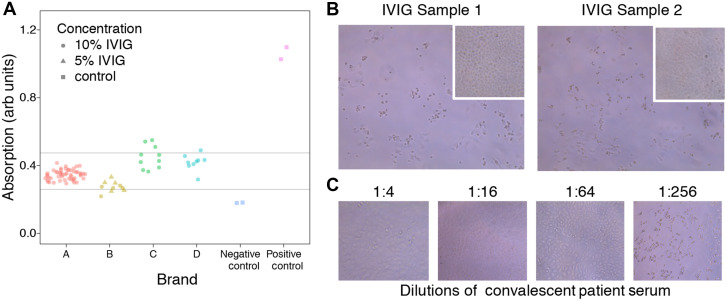
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is one of the greatest modern public health crises. COVID-19–specific treatments, while being studied, are not yet readily available. We examined whether commercial prepandemic intravenous immunoglobulin (IVIG) contains cross-reactive antibodies that could bind and neutralize SARS-CoV-2. The receptor-binding domain (RBD), contained within the S1 subunit of the coronavirus spike protein, mediates viral entry by binding to the angiotensin-converting enzyme 2 receptor on host cells. The spike protein, including the RBD, is a known target for neutralizing antibodies in natural infection, and shares some epitopes with S proteins from common, circulating strains of human coronaviruses. We took 82 samples from 4 different brands manufactured in the United States and Europe (OctaPharma, Hoboken, NJ; Grifols, Barcelona, Spain and Durham, NC; CSL, Bern, Switzerland) and tested them for SARS-CoV-2 RBD binding using a standard ELISA. We found that all samples demonstrated the presence of cross-reactive antibodies above the negative controls; however, binding activity varied between individual lots and among brands (Fig 1 , A).
Fig 1.
A, SARS-CoV-2 RBD antibody binding from commercially available IVIG at dilution 1:50 by ELISA. Each dot represents a different lot number of immunoglobulin product. The positive control is anti-spike antibody (CR3022, Creative Biolabs) at dilution 1:1000.1 The negative control is human whole serum obtained from Sigma. Concentration indicates the protein percentage (wt/vol) in the commercial lot, before dilution for the ELISA. The 5% and 10% IVIG samples were first diluted to 0.66% (wt/vol) to achieve a common concentration of immunoglobulin, then diluted 1:50 for the ELISA. The dilution solution was PBS (Fisher Scientific, item no. BP3994) with 0.05% (vol/vol) Tween-20 (Sigma Aldrich, item no. P1379) and 1% (wt/vol) BSA (Rockland Antibodies & Assays, item no. BSA-50). The final concentration of immunoglobulin is approximately 0.0133% (wt/vol) or roughly 133 μg/mL of immunoglobulin. B, SARS-CoV-2 (Isolate USA-WA1/2020) was obtained from the Centers for Disease Control and Prevention and expanded in Vero E6 cells (ATCC). The virus titer used was 1 × 105 plaque-forming units (PFU)/mL. Vero cells were seeded at 7500/well in 96-well plates and cultured overnight. IVIG diluted 1:4 (20 μL) was mixed with 100 PFU of SARS-CoV-2 in 100 μL DMEM, incubated at 37 °C for 1 hour, and added to monolayers of Vero E6 cells in duplicate. Cytopathic effect was assessed after 3 days. Images of Vero E6 cells by microscopy (10×) after 72 hours in culture. Cytopathic effect (CPE) is visible in SARS-CoV-2–infected Vero E6 cells mixed with IVIG. Images show results of 2 of the 4 highest RBD-binding samples; the 3 lowest RBD-binding samples showed similar results (not shown). The insets show co-culture of Vero E6 cells with immunoglobulin but without virus, demonstrating that IVIG does not kill Vero E6 cells. C, Reduction of CPE in SARS-CoV-2–infected Vero E6 cells mixed with convalescent patient serum at dilutions of 1:4, 1:16, and 1:64; however, CPE was seen when serum was diluted to 1:256. DMEM, Dulbecco modified Eagle medium.
To assess biological relevance, we took 7 samples (4 highest and 3 lowest RBD-binding) and examined neutralizing activity against a clinical isolate of SARS-CoV-2 in culture. Wells inoculated with virus alone or IVIG alone served as positive and negative controls, respectively. None of the 7 samples demonstrated SARS-CoV-2–neutralizing capacity at dilution 1:4 (Fig 1, B); meanwhile, convalescent patient serum neutralized virus at dilutions of 1:4, 1:16, and 1:64, but not at 1:256 (Fig 1, C). These results show that although IVIG contains cross-reactive antibodies against novel SARS-CoV-2, this does not confer viral neutralization.
Studies of immunoglobulin utility in other pandemics, such as the 2003 severe acute respiratory syndrome coronavirus and the 2012 Middle East respiratory syndrome coronavirus outbreaks, were largely inconclusive.2 However, anti-infectious mechanisms by which immunoglobulin acts are complex and not limited to viral neutralization. For example, non-neutralizing influenza-specific antibodies can mediate complement fixation, phagocytosis, and antibody-dependent cellular cytotoxicity (ADCC). IVIG produced before the 2009 H1N1 pandemic had moderate titers of cross-reactive ADCC antibodies that eliminated H1N1-infected respiratory cells in vitro.3 IVIG can also have anti-inflammatory effects that target immune-mediated pathology frequently seen during and after infection. Thus, evidence supports therapeutic antiviral and anti-inflammatory activity of IVIG beyond neutralization.
Data on the clinical utility of IVIG in COVID-19 are limited. IVIG from 1 manufacturer contained antibodies with reactivity to components of various coronaviruses but neutralization studies were not performed.4 Over time, of course, all commercial immunoglobulin will contain SARS-CoV-2 antibodies. A case report described prompt recovery in a patient with severe COVID-19 after receiving plasma exchange and IVIG, suggesting that plasma exchange may clear pathogenic or inflammatory mediators while IVIG provides immunomodulatory and antiviral effects.5 Although limited by study size and confounding variables, other case series reported that IVIG improved clinical outcomes in severe COVID-19, supporting its potential as adjuvant therapy.6 , 7
In summary, even prepandemic IVIG contains cross-reactive SARS-COV-2 RBD, but does not neutralize viral spread. Nonetheless, activities beyond neutralization such as ADCC, complement activation, and anti-inflammation may warrant its use in COVID-19.
Footnotes
This work was supported by the Jeffrey Modell Foundation, the UCLA AIDS Institute, and the Charity Treks fund.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest. Immunoglobulin products were provided without financial support. The companies that provided immunoglobulin did not preview this manuscript and had no say in its content.
References
- 1.Mustafa S., Balkhy H., Gabere M.N. Current treatment options and the role of peptides as potential therapeutic components for Middle East respiratory syndrome (MERS): a review. J Infect Public Health. 2018;11:9–17. doi: 10.1016/j.jiph.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jegaskanda S., Vandenberg K., Laurie K.L., Loh L., Kramski M., Winnall W.R., et al. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity in intravenous immunoglobulin as a potential therapeutic against emerging influenza viruses. J Infect Dis. 2014;210:1811–1822. doi: 10.1093/infdis/jiu334. [DOI] [PubMed] [Google Scholar]
- 3.Díez J.M., Romero C., Gajardo R. Currently available intravenous immunoglobulin contains antibodies reacting against severe acute respiratory syndrome coronavirus 2 antigens. Immunotherapy. 2020;12:571–576. doi: 10.2217/imt-2020-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi H., Zhou C., He P., Huang S., Duan Y., Wang X., et al. Successful treatment with plasma exchange followed by intravenous immunogloblin in a critically ill patient with 2019 novel coronavirus infection. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao W., Liu X., Bai T., Fan H., Hong K., Song H., et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie Y., Cao S., Li Q., Chen E., Dong H., Zhang W., et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan M., Wu N.C., Zhu X., Lee C.C., So R.T., Lv H., et al. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020;368:630–633. doi: 10.1126/science.abb7269. [DOI] [PMC free article] [PubMed] [Google Scholar]