Abstract
Objectives
There is limited information on the performance of rapid antigen detection (RAD) tests to identify SARS-CoV-2-infected asymptomatic individuals. In this field study, we evaluated the Panbio™ COVID-19 Ag Rapid Test Device (Abbott Diagnostics, Jena, Germany) for this purpose.
Methods
A total of 634 individuals (355 female; median age, 37 years; range, 9–87) were enrolled. Two nasopharyngeal swabs were collected from household (n = 338) and non-household contacts (n = 296) of COVID-19 cases. RAD testing was carried out at the point of care. The RT-PCR test used was the TaqPath COVID-19 Combo Kit (Thermo Fisher Scientific, MA, USA).
Results
Household contacts were tested at a median of 2 days (range, 1–7) after diagnosis of the index case, whereas non-household contacts (n = 296) were tested at a median of 6 days (range, 1–7) after exposure. In total, 79 individuals (12.4%) tested positive by RT-PCR, of whom 38 (48.1%) yielded positive RAD results. The overall sensitivity and specificity of the RAD test was 48.1% (95% CI 37.4–58.9) and 100% (95% CI 99.3–100), respectively. Sensitivity was higher in household (50.8%; 95% CI 38.9–62.5) than in non-household (35.7%; 95% CI 16.3–61.2%) contacts. Individuals testing positive by RAD test were more likely (p < 0.001) to become symptomatic than their negative counterparts.
Discussion
The Panbio test displays low sensitivity in asymptomatic close contacts of COVID-19 patients, particularly in non-household contacts. Nonetheless, establishing the optimal timing for upper respiratory tract collection in this group seems imperative to pinpoint test sensitivity.
Keywords: Asymptomatic, Close contacts, COVID-19, Rapid antigen detection test, SARS-CoV-2
Introduction
Rapid antigen detection (RAD) immunoassays have emerged as a valuable alternative to RT-PCR for diagnosis of SARS-CoV-2 infection in patients presenting with clinically compatible COVID-19 [1]. RAD tests are simple to carry out and return results within a short time, thus being well-suited for point-of-care testing (POCT). Moreover, two recently evaluated RAD assays (BD Veritor System and the Panbio™ COVID-19 Ag Rapid Test Device) may be used as a proxy for SARS-CoV-2 cultured from respiratory tract specimens, thus allowing reasonably accurate prediction of contagiousness [2,3]; yet this assumption awaits further validation in larger cohorts. The possibility of using RAD tests to identify SARS-CoV-2-infected asymptomatic contacts of COVID-19 patients is appealing, as it could effectively contribute to minimize community SARS-CoV-2 spread through early detection of highly infectious individuals [1], yet little is known about how RAD tests perform in this population group [[4], [5], [6]]. Here, we report on the performance of the Panbio™ COVID-19 Ag Rapid Test Device (Abbott Diagnostic GmbH, Jena, Germany) conducted at POC in this setting.
Materials and methods
Patients
A total of 634 consecutive asymptomatic individuals (female, n = 355; median age, 37 years; range, 9–87 years) attended at the Clínico-Malvarrosa Health Department (Valencia, Spain) were enrolled between 16 October and 20 November 2020. Participants were either household (n = 338) or non-household (n = 296) close contacts of COVID-19 patients, as defined by the Spanish Ministry of Health [7]. COVID-19 diagnosis was made upon the presence of compatible signs or symptoms and a positive RT-PCR result in nasopharyngeal (NP) swabs. Timing of sample collection was prescribed at the discretion of either the physician in charge of the index case or local health authorities. The study was approved by the Hospital Clínico de Valencia (HCU) INCLIVA Research Ethics Committee.
SARS-CoV-2 testing
NPs for RAD and RT-PCR testing were collected by experienced nurses at the POC site located at Hospital Malvarrosa, as previously detailed [3]. For each patient, one swab, provided by the manufacturer, was taken from the left nostril for RAD testing, and another one, taken from the right nostril, was immediately placed in 3 mL of Universal Transport Medium (Becton Dickinson, Sparks, MD, USA) and used for RT-PCR testing. RAD testing was carried out at POC immediately after sampling. RT-PCRs were conducted within 24 hr of specimen collection at the Microbiology Service of Hospital Clínico Universitario (Valencia, Spain) with the TaqPath COVID-19 Combo Kit (Thermo Fisher Scientific, MA, USA). The AMPLIRUN® TOTAL SARS-COV-2 Control (Vircell S.A., Granada, Spain) was used as the reference material for SARS-CoV-2 RNA load quantitation (in copies/mL, considering RT-PCR CTs for the N gene). An Standard curve was constructed using ten-fold serial dilution (102 to 109 copies/mL) of the reference material; the linear regression equation was: Y = –0.31 × X + 13.77; R 2 = 9.89).
Statistical analyses
Agreement between RAD and RT-PCR tests was assessed using Cohen's Kappa (κ) statistics. Differences between medians were compared using the Mann–Whitney U test. The chi-squared test was used for frequency comparisons. Two-sided p values < 0.05 were considered statistically significant. Statistical analyses were performed using SPSS version 25.0 (SPSS, Chicago, IL, USA).
Results
Overall performance of the RAD test in asymptomatic close contacts
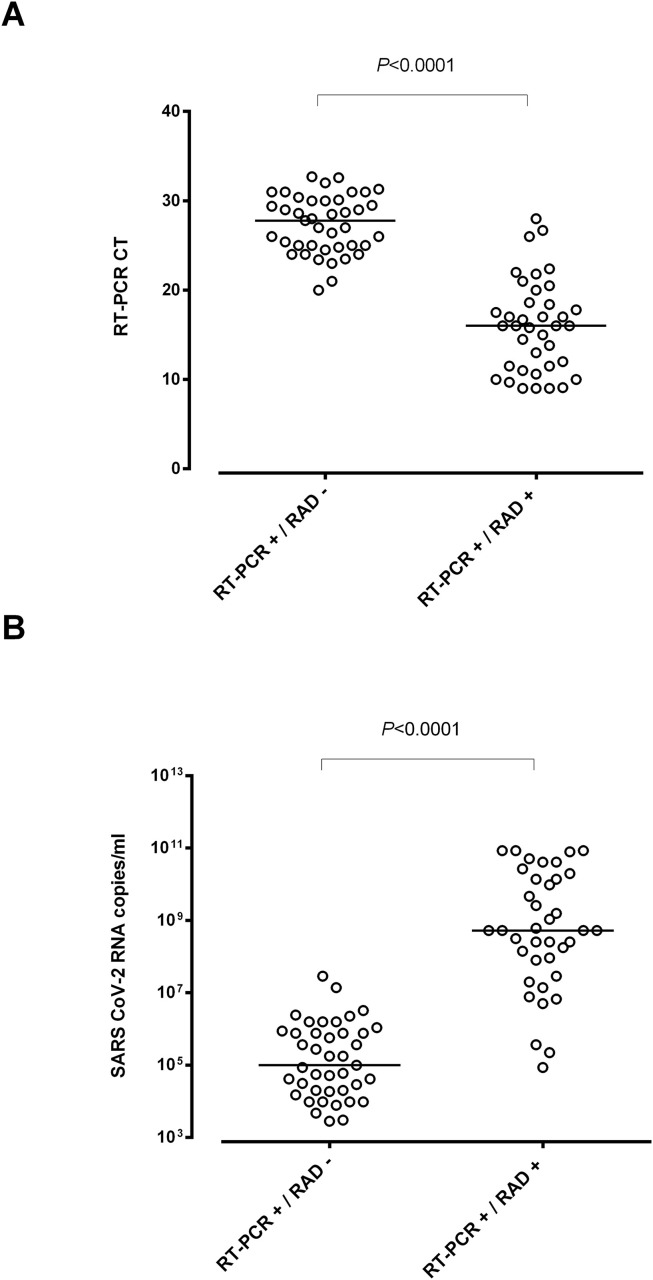
A total of 79 out of 634 individuals (12.4%) tested positive by RT-PCR, of whom 38 (48.1%) returned positive RAD test results. There were no RAD positive/RT-PCR negative cases. Accordingly, concordance between RT-PCR and RAD results was moderate (κ index, 0.61; 95% CI 0.5–0.73). As shown in Fig. 1 , SARS-CoV-2 RNA load in NPs was significantly higher (p < 0.001) in RAD-positive (median, 8.7 log10 copies/ml) than in RAD-negative individuals (4.9 log10 copies/mL).
Fig. 1.
RT-PCR cycle thresholds (Ct) (A) and SARS-CoV-2 RNA load (B) in asymptomatic close contacts of COVID-19 patients testing either positive or negative by Panbio™ COVID-19 Ag Rapid Test Device (RAD). The AMPLIRUN® TOTAL SARS-CoV-2 Control (Vircell S.A., Granada, Spain) was used as the reference material for SARS-CoV-2 RNA load quantitation (in copies/mL, considering RT-PCR Cts for the N gene). p Values for comparisons are shown.
Overall sensitivity and specificity of RAD was 48.1% and 100% (Table 1 ). For the above-mentioned prevalence (12.4%), the negative predictive value (NPV) of the RAD test was 93.1.5%. As expected, overall RAD sensitivity was directly related to SARS-CoV-2 load in NP specimens (Table S1), reaching 96.8% when specimens with viral load ≥7.4 log10 copies/mL (Ct ≤ 20) were analysed separately.
Table 1.
Performance of the Panbio™ COVID-19 Ag Rapid Test Device for SARS-CoV-2 detection in asymptomatic household and non-household close contacts
| Parameter | Population group |
||
|---|---|---|---|
| All individuals | Household contacts | Non-household contacts | |
| Sensitivity % (95% CI) | 48.1 (37.4–58.9) | 50.8 (38.9–62.5) | 35.7 (16.3–61.2) |
| Specificity% (95% CI) | 100 (99.3–100) | 100 (98.6–100) | 100 (98.7–100) |
| Negative predictive valuea | 93.1 (90.8–94.9) | 89.5 (85.9–92.5) | 96.9 (94.2-98.4) |
| Positive predictive valuea | 100 (90.8–100) | 100 (89.6–100) | 100 (56.6–100) |
Adjusted to actual prevalence in the respective population group.
Performance of RAD test in household and non-household asymptomatic close contacts
Household contacts (n = 338; median age, 36.5; range, 10–86 years; 175 female) were tested at a median of 2 days (range, 1–7) after diagnosis of the presumed index case. Sixty-five (19.2%) tested positive by RT-PCR, of whom 33 (50.7%) were positive by RAD test. The likelihood of obtaining either a positive or a negative RAD result was unrelated to the time elapsed since diagnosis of the index case (p 0.33).
Non-household contacts (n = 296; median age, 38.5 years; range, 9–87 years; 180 female) were tested at a median of 6 days (range, 1–7) after self-reported exposure. Five individuals yielded RT-PCR-positive/RAD-positive results (1.6%) and 9 had RT-PCR-positive/RAD-negative results (3.0%). Overall, median time from exposure to testing was similar among individuals displaying either positive or negative RAD results (p 0.89).
The agreement level between RT-PCR and RAD results was significantly higher (p < 0.001) for household (κ, 0.61; 95% CI 0.50–0.75) than for non-household (κ, 0.51; 95% CI 0.20–0.83) contacts. RAD sensitivity was significantly higher (p < 0.001) in household contacts, while the opposite was true for NPV (Table 1).
SARS-CoV-2 RNA load was comparable (p 0.21) across household (median, 6.8 log10 copies/mL; range, 3.4–10.9) and non-household (median, 5.9 log10 copies/mL; range, 3.5–10.6) contacts, and was significantly higher (p < 0.001) in RAD-positive than in RAD-negative individuals, irrespective of the subcohort considered.
Clinical outcomes
Thirty-nine out of the 79 individuals testing positive by RT-PCR eventually became mildly symptomatic (49.3%), without requiring hospitalization. Individuals testing positive by RAD, either household or non-household contacts, were more likely (p < 0.001) to develop clinically compatible COVID-19 (30 out of 38) than those who did not (9 out of 41).
Discussion
In this field study, overall sensitivity of the Panbio™ COVID-19 Ag Rapid Test Device for identification of SARS-CoV-2-infected individuals among asymptomatic close contacts of confirmed COVID-19 cases was 48.1%, close to the figures reported by Linares et al. (54.5%) [4], Fenollar et al. (45.4%) [5] and Bulilete et al. (59.0%) [6], in apparently comparable cohorts. However, in two of these studies [4,5], the RAD test was carried out at a central laboratory, and timing of sample collection was not disclosed [4,5]. In the study by Bulilete et al. [5] most participants (70.6%) were tested within 5 days of exposure. Sensitivity of the Panbio™ test was lower than was previously found [[3], [4], [5], [6]] in symptomatic patients (around 80%), yet as reported for the latter patients, RAD sensitivity was directly related to the magnitude of SARS-CoV-2 RNA load in NP specimens. Such a striking difference might reflect dissimilarities across symptomatic and asymptomatic individuals in the kinetics of SARS-CoV-2 load in the upper respiratory tract [8,9]. While it is well known that SARS-CoV-2 load peaks around the time of symptoms onset in the former group [10,11], the timing is uncertain in asymptomatic cases.
Interestingly, individuals testing positive by RAD were more likely to become (mildly) symptomatic than their negative counterparts, pointing to a pathogenetic link between SARS-CoV-2 RNA load and development of overt COVID-19.
The strength of the current study is that it reflects the real-life performance of the RAD test at POC. Among its limitations are the relative low number of cases, and the possibility that samples were collected too early after exposure, particularly in non-household contacts, in whom RAD sensitivity was strikingly low. In this sense, Linares et al. [4] reported the sensitivity of the Panbio™ test as very low in close contacts at less than 7 days from exposure.
In summary, we found the Panbio™ test to display low overall sensitivity in asymptomatic contacts of COVID-19 patients, although it may identify those displaying higher SARS-CoV-2 RNA loads, who are presumed to be more contagious. Nevertheless, establishing the optimal timeframe for NP collection in household and non-household contacts seems crucial to accurately determine the sensitivity of the test.
Transparency declaration
This work received no public or private funds. The authors declare no conflicts of interest.
Author contributions
I.T., S.P. and E.A.: Methodology and data validation. E.A., I.T. and J.C.: Formal analysis. D.N.: Conceptualization, supervision, writing the original draft. All authors reviewed the original draft.
Acknowledgements
We are grateful to Abbott Diagnostics for providing the Panbio™ COVID-19 Ag Rapid Test Device kits. We thank all personnel working at Clinic University Hospital and primary healthcare centres belonging to the Clínico Malvarrosa Health Department for their unwavering commitment in the fight against COVID-19. We would also like to thank María José Beltrán, Pilar Botija and Ana Sanmartín for assistance in organizing RAD testing in primary healthcare centres and Salvador Peiró for critical revision of the manuscript.
Editor: F. Allerberger
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.12.022.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Options for the use of rapid antigen tests for COVID-19 in the EU/EEA and the UK. https://www.ecdc.europa.eu/en/publications-data/options-use-rapid-antigen-tests-covid-19-eueea-and-uk
- 2.Pekosz A., Cooper C., Parvu V., Li M., Andrews J., Manabe Y.C. Antigen-based testing but not real-time PCR correlates with SARS-CoV-2 virus culture. medRxiv. 2020 doi: 10.1101/2020.10.02.20205708. [DOI] [Google Scholar]
- 3.Albert E., Torres I., Bueno F., Huntley D., Molla E., Fernández-Fuentes M.Á. Field evaluation of a rapid antigen test (Panbio™ COVID-19 Ag Rapid Test Device) for COVID-19 diagnosis in primary healthcare centers. Clin Microbiol Infect. 2021;27:472.e7–472.e10. doi: 10.1016/j.cmi.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linares M., Pérez-Tanoira R., Romanyk J., Pérez-García F., Gómez-Herruz P., Arroyo T. Panbio antigen rapid test is reliable to diagnose SARS-CoV-2 infection in the first 7 days after the onset of symptoms. J Clin Virol. 2020;133:104659. doi: 10.1016/j.jcv.2020.104659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenollar F., Bouam A., Ballouche M., Fuster L., Prudent E., Colson P. Evaluation of the Panbio Covid-19 rapid antigen detection test device for the screening of patients with Covid-19. J Clin Microbiol. 2020 doi: 10.1128/JCM.02589-20. JCM.02589-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bulilete O., Lorente P., Leiva A., Carandell E., Oliver A., Rojo E. Evaluation of the Panbio™ rapid antigen test for SARS-CoV-2 in primary health care centers and test sites. medRxiv. 2020 doi: 10.1101/2020.11.13.20231316. [DOI] [Google Scholar]
- 7.Estrategia de detección precoz, vigilancia y control de COVID-19. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/COVID19_Estrategia_vigilancia_y_control_e_indicadores.pdf
- 8.Zhang Z., Xiao T., Wang Y., Yuan J., Ye H., Wei L. Early viral clearance and antibody kinetics of COVID-19 among asymptomatic carriers. medRxiv. 2020:20083139. doi: 10.3389/fmed.2021.595773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chau N.V.V., Thanh Lam V., Thanh Dung N., Yen L.M., Minh N.N.Q., Hung L.M. The natural history and transmission potential of asymptomatic SARS-CoV-2 infection. Clin Infect Dis. 2020:ciaa711. doi: 10.1093/cid/ciaa711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baggio S., L'Huillier A.G., Yerly S., Bellon M., Wagner N., Rohr M. SARS-CoV-2 viral load in the upper respiratory tract of children and adults with early acute COVID-19. Clin Infect Dis. 2020:ciaa1157. doi: 10.1093/cid/ciaa1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.