Abstract
Aim
It remains unclear whether cardiac arrest (CA) resuscitation generates aerosols that can transmit respiratory pathogens. We hypothesize that chest compression and defibrillation generate aerosols that could contain the SARS-CoV-2 virus in a swine CA model.
Methods
To simulate witnessed CA with bystander-initiated cardiopulmonary resuscitation, 3 female non-intubated swine underwent 4 min of ventricular fibrillation without chest compression or defibrillation (no-flow) followed by ten 2-min cycles of mechanical chest compression and defibrillation without ventilation. The diameter (0.3–10 μm) and quantity of aerosols generated during 45-s intervals of no-flow and chest compression before and after defibrillation were analyzed by a particle analyzer. Aerosols generated from the coughs of 4 healthy human subjects were also compared to aerosols generated by swine.
Results
There was no significant difference between the total aerosols generated during chest compression before defibrillation compared to no-flow. In contrast, chest compression after defibrillation generated significantly more aerosols than chest compression before defibrillation or no-flow (72.4 ± 41.6 × 104 vs 12.3 ± 8.3 × 104 vs 10.5 ± 11.2 × 104; p < 0.05), with a shift in particle size toward larger aerosols. Two consecutive human coughs generated 54.7 ± 33.9 × 104 aerosols with a size distribution smaller than post-defibrillation chest compression.
Conclusions
Chest compressions alone did not cause significant aerosol generation in this swine model. However, increased aerosol generation was detected during chest compression immediately following defibrillation. Additional research is needed to elucidate the clinical significance and mechanisms by which aerosol generation during chest compression is modified by defibrillation.
Keywords: Cardiac arrest, SARS-CoV-2, COVID-19, Swine model, Aerosol generation, Cardiopulmonary resuscitation, Chest compression, Defibrillation
Introduction
Respiratory infections can occur through the transmission of virus-containing aerosols from infected individuals. The SARS-CoV-2 pandemic has posed significant risk to healthcare workers during aerosol-generating procedures such as endotracheal intubation, bronchoscopy, and airway suctioning. However, it remains unclear whether cardiac arrest (CA) resuscitation generates aerosols that have the potential to transmit respiratory pathogens such as SARS-CoV-2. The World Health Organization categorized cardiopulmonary resuscitation (CPR) including chest compressions, defibrillation, and associated airway interventions as aerosol-generating procedure,1 while the European Resuscitation Council2 and American Heart Association3 suggested that defibrillation is unlikely to be an aerosol-generating procedure and can be undertaken with the healthcare provider wearing droplet-precaution personal productive equipment (PPE). A recent systematic review conducted by the International Liaison Committee on Resuscitation found inadequate evidence to definitely conclude whether defibrillation and chest compressions generate aerosols.4, 5 Given that bystander CPR and public access defibrillation improve the outcome of CA patients, it is important to determine whether these actions may generate aerosols that have the potential to transmit respiratory pathogens such as SARS-CoV-2 to lay rescuers who are unlikely to be wearing PPE. In addition, the appropriate PPE for first-responders performing chest compression and defibrillation should be guided by the risk of aerosol generation.
The diameter of the SARS-CoV-2 virus varies from 0.06 to 0.14 μm based on electron micrographs.6 The World Health Organization uses a cutoff limit of 5 μm to differentiate between aerosol (≤5 μm) and droplet (>5 μm) transmission routes.1 Small aerosols in the ≤5 μm range can penetrate airways down to the alveolar space, where the respiratory pathogens they carry can replicate and cause potentially more serious infections. Particles of <10 μm diameter can penetrate past the glottis, while the intermediate range of 10–20 μm particles may either settle or remain suspended. Large droplets with >20 μm diameter have a more ballistic trajectory and may be too large to follow inhalation airflow streamlines.7, 8, 9 Based on these information, we hypothesize that chest compression and defibrillation can generate aerosols of 0.3–10 μm diameter that could contain respiratory pathogens. In this study, we examined the effects of chest compression before and after defibrillation on aerosol generation in the absence of an advanced airway to simulate witnessed out-of-hospital CA with bystander-initiated CPR in a swine CA model.
Methods
This study adhered to the principles stated in the Guide for the Care and Use of Laboratory Animals10 and was approved by the Institutional Animal Care and Use Committee as well as the institutional COVID-related Research Prioritization committee.
Animal model
Three female Yorkshire swine (weight range 50−66 kg) were fasted overnight with ad libitum access to water prior to experimentation. Anesthesia was induced with a combination of tiletamine/zolazepam (4−6 mg/kg) and xylazine (2 mg/kg) followed by 1–2% isoflurane delivered in 30–40% O2 balanced with room air. The animals were intubated using a 7.5 mm cuffed endotracheal tube and mechanically ventilated with a tidal volume of 7−8 ml/kg and PEEP of 5 mmH2O to achieve an end-tidal CO2 (PetCO2) between 35 and 45 mmHg and SpO2 above 95%. Core body temperature was maintained between 37.0° and 38.0 °C during instrumentation using a closed loop feedback temperature blanket (Blanketrol, Sub-Zero Medical, Cincinnati, OH). Standard 3-lead configuration was placed for ECG monitoring.
The right femoral artery was cannulated for measurement of blood pressure. The right external jugular vein was cannulated with a 9F introducer (Arrow, Teleflex, Morrisville, NC) via ultrasound guided percutaneous Seldinger technique. Following instrumentation, animals were gradually weaned from isoflurane and transitioned to up to 20 mg/kg/hr of intravenous propofol infusion after they started to show signs of spontaneous breathing. The animals did not receive neuromuscular blocking agents during this study.
Baseline measurements
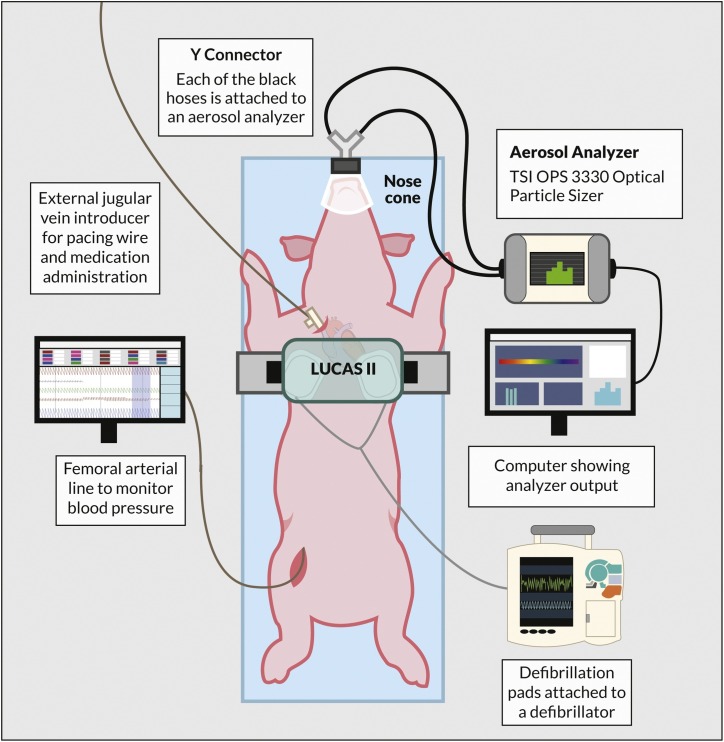
Arterial blood pressure, heart rate, ECG, SpO2, and PetCO2 were monitored and recorded continuously with Biopac MP160 data acquisition system (Biopac inc, Goleta, CA). Arterial and venous blood samples were collected for blood gas, electrolytes, glucose, and lactate measurements. The animals were extubated, and a nose cone was placed over the animal snout to cover its mouth and nostrils and connected through a Y-connector to the particle analyzer for aerosol measurements (Fig. 1 ). Baseline aerosol measurements were obtained for 2–5 min during spontaneous ventilation prior to each CA sequence.
Fig. 1.
Swine cardiac arrest model setup for aerosol quantification during defibrillation and chest compression.
Particle analyzer
An optical particle sizer (TSI Optical Particle Sizer [OPS] Model 3330, TSI Incorporated, Shoreview, MN) was used to analyze aerosol particle concentration and size distribution during chest compression and defibrillations. It provided continuous 1 Hz particle size distributions, total particle counts for 0.3–10 μm particles in up to 16 channels, and wide concentration range from 0 to 3000 particles/cm3. Total aerosol count was calculated by multiplying the measured particle concentration (in particles/cm3) by the duration of time measured (in minute) and OPS flow rate of 1000 cm3/min. We performed several quality assurance measurements in between experiments to ensure device accuracy and reliability. First, the instrument was used to sample the ambient aerosol in the laboratory. As the negative control, it was checked for sensitivity by connection to a HEPA filter (99.97% capture for ≥0.3 μm particles). As the positive control, the instrument was used to sample nebulized saline at 15 L/min to verify its function for capturing a well characterized aerosol size distribution and instrument integrity in between experimental procedures. The ambient temperature in our large animal operating room ranged from 19 to 20 °C, and the ambient humidity ranged from 37 to 50%.
Experimental design
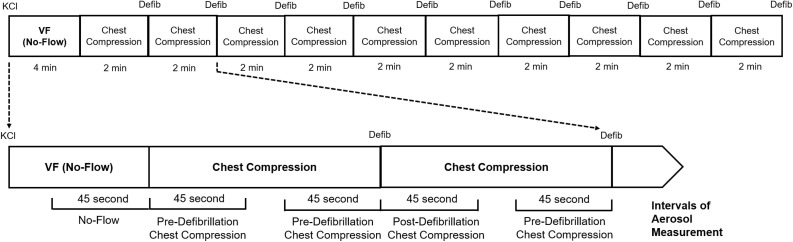
This is a pilot study aimed to generate preliminary data on aerosol generation during chest compression and defibrillation in a swine CA model. To simulate witnessed CA with bystander-initiated CPR, 2 mEq/kg of potassium chloride solution was administered to induce ventricular fibrillation. The animals underwent 4 min of ventricular fibrillation without chest compression or ventilation followed by ten 2-min cycles of mechanical chest compression performed with a 20% anterior-posterior displacement and compression rate of 100/min with LUCAS 2 (Physio-Control, Redmond, WA), and anterior-lateral defibrillation at biphasic 200J with HeartStart MRX (Philips, Amsterdam, Netherlands) without ventilation until euthanasia. In order to characterize aerosol generation during different phases of CA resuscitation, we categorized the experimental timeline into four distinct 45-s intervals: Baseline (before the induction of CA), no-flow (ventricular fibrillation without chest compression or ventilation), pre-defibrillation chest compression, and post-defibrillation chest compression. The diameter, concentration, and quantity of aerosols generated during 45-s intervals of baseline, no-flow, and chest compression before and after each defibrillation were collected via a nose cone that is connected to the particle sizer (Fig. 2 ). Aerosol generated from the coughs of 4 healthy human subjects were also compared to the swine data.
Fig. 2.
Experimental timeline. Defib = defibrillation. KCl = potassium chloride. VF = ventricular fibrillation.
Data analysis
Data were presented as mean and standard deviation (SD). One-way analysis of variance (ANOVA) with post-hoc Tukey’s correction for multiple comparisons was used to compare continuous variables. Bartlett’s and Brown–Forsythe tests were used to assess SD equality. The mean aerosol quantity generated by each of the 10 rounds of 45-s interval of chest compression before and after defibrillation were averaged from all 3 animals. The total number of aerosols were compared between no-flow, chest compression before defibrillation, and chest compression after defibrillation using one-way ANOVA with post-hoc Tukey’s. A p value < 0.05 was considered statistically significant. Prism 8 (GraphPad, LaJolla, CA) and MATLAB R2017b (The Mathworks, Inc., Natick, MA) were used for data analysis.
Results
Animal characteristics
The mean weight of animals was 59 ± 8 kg. The descriptive statistics of the baseline haemodynamic and arterial blood gas of the three animals are reported in Table 1 .
Table 1.
Baseline animal haemodyamics and arterial blood gas.
| Swine 1 | Swine 2 | Swine 3 | Mean | SD | |
|---|---|---|---|---|---|
| Weight (kg) | 66 | 62 | 50 | 59 | 8 |
| SBP (mmHg) | 154 | 171 | 170 | 165 | 9 |
| DBP (mmHg) | 92 | 117 | 117 | 109 | 14 |
| MAP (mmHg) | 118 | 138 | 138 | 131 | 12 |
| Heart rate (bpm) | 94 | 75 | 75 | 81 | 11 |
| PetCO2 (mmHg) | 39 | 37 | 37 | 37 | 1 |
| pH | 7.31 | 7.32 | 7.33 | 7.32 | 0.01 |
| pCO2 (mmHg) | 42 | 39 | 34 | 38 | 4 |
| pO2 (mmHg) | 272 | 479 | 299 | 350 | 113 |
| BE (mmol/L) | −4.5 | −5.5 | −7.6 | −5.9 | 1.6 |
| Lactate (mmol/L) | 1.5 | 2.5 | 0.8 | 1.6 | 0.9 |
| Glucose (mg/dL) | 119 | 113 | 122 | 118 | 5 |
SBP = systolic blood pressure; DBP = diastolic blood pressure; MAP = mean arterial pressure; bpm = beat per minute; PetCO2 = end tidal CO2; BE = base excess; SD = standard deviation.
Aerosol generation during CA resuscitation in a swine model
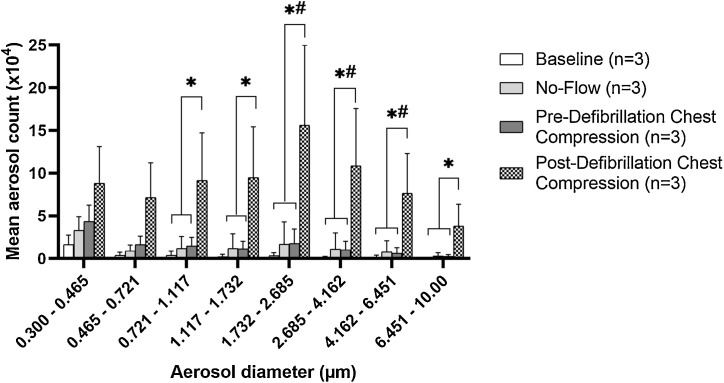
There was no significant difference between the mean total aerosols nor their size distribution generated during pre-defibrillation chest compression compared to baseline or no-flow. In contrast, post-defibrillation chest compression generated significantly more aerosols than pre-defibrillation chest compression or no-flow (72.4 ± 41.6 × 104 vs 12.3 ± 8.3 × 104 vs 10.5 ± 11.2 × 104; p < 0.05), particularly at the larger-size aerosols with >0.721 μm diameter (Fig. 3 ; p < 0.05 for all subgroup post-hoc Tukey’s comparisons). These changes were observed across all 3 animals and all 10 rounds of defibrillation and chest compressions for each animal. Individual animal data is provided to summarize aerosol generation during the different phases of CA resuscitation (Supplemental Fig. S1) and during the individual rounds of pre- and post-defibrillation chest compression for each animal (Supplemental Figs. S2–S4).
Fig. 3.
Distribution of aerosol size and quantity during swine cardiac arrest resuscitation. *p < 0.05, one-way ANOVA with post-hoc Tukey’s comparing mean aerosol count of defibrillation + chest compression to baseline, no flow, and chest compression groups. #p < 0.005, one-way ANOVA with post-hoc Tukey’s comparing mean aerosol count of defibrillation + chest compression to baseline group.
Comparison between aerosol generation during CA resuscitation in a swine CA model with healthy human coughs
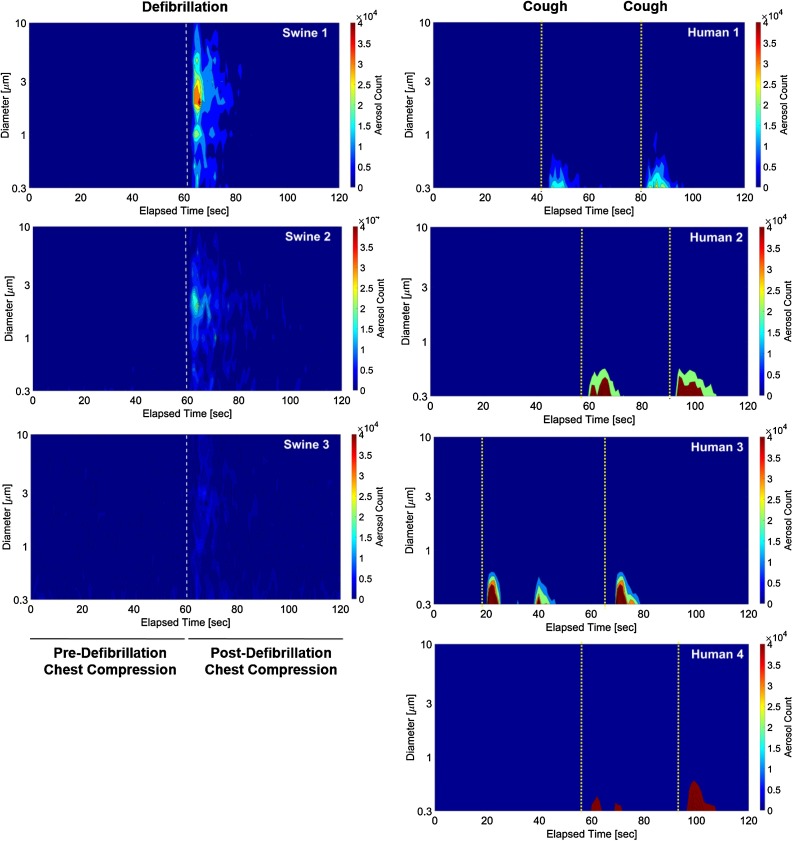
Two consecutive human coughs generated mean 54.7 ± 33.9 × 104 total aerosols with a size distribution smaller than post-defibrillation chest compression (Table 2 ). Human coughs generated significantly more particles in the 0.3–0.465 μm diameter range than pre-defibrillation chest compression or no-flow (39.9 ± 24.8 × 104 vs 4.3 ± 1.9 × 104 vs 3.3 ± 1.6 × 104; p < 0.05). In contrast, post-defibrillation chest compression generated significantly more aerosols of >1.117 μm diameter than human coughs (p < 0.005 for all subgroup post-hoc Tukey’s comparisons). The aerosol size distribution and quantity generated by the coughs of each human subjects were compared to that of each of the animals in contour diagrams (Fig. 4 ).
Table 2.
Characteristics and comparisons of aerosol generation during swine cardiac arrest resuscitation and health human coughs.
| Aerosol count (×104) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (n = 3) |
No-flow (n = 3) |
Pre-defibrillation chest compression (n = 3) |
Post-defibrillation chest compression (n = 3) |
Human cough (n = 4) |
|||||||
| Diameter (μm) | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | p Value |
| 0.300–0.465 | 1.7 | 1.1 | 3.3 | 1.6 | 4.3 | 1.9 | 8.8 | 4.3 | 39.9 | 24.8 | <0.05 |
| 0.465–0.721 | 0.4 | 0.4 | 0.9 | 0.7 | 1.6 | 1.0 | 7.1 | 4.1 | 10.0 | 6.6 | <0.05 |
| 0.721–1.117 | 0.4 | 0.5 | 1.2 | 1.4 | 1.5 | 1.0 | 9.1 | 5.6 | 3.1 | 2.1 | <0.05 |
| 1.117–1.732 | 0.2 | 0.3 | 1.2 | 1.7 | 1.2 | 0.9 | 9.5 | 6.0 | 1.1 | 0.7 | <0.01 |
| 1.732–2.685 | 0.3 | 0.4 | 1.7 | 2.6 | 1.8 | 1.7 | 15.6 | 9.4 | 0.4 | 0.3 | <0.005 |
| 2.685–4.162 | 0.1 | 0.2 | 1.1 | 1.9 | 1.0 | 1.0 | 10.9 | 6.7 | 0.1 | 0.1 | <0.005 |
| 4.162–6.451 | 0.2 | 0.2 | 0.8 | 1.3 | 0.6 | 0.6 | 7.6 | 4.7 | 0.0 | 0.0 | <0.005 |
| 6.451–10.00 | 0.0 | 0.0 | 0.3 | 0.4 | 0.3 | 0.2 | 3.8 | 2.6 | 0.0 | 0.0 | <0.01 |
| Total | 3.2 | 3.0 | 10.5 | 11.2 | 12.3 | 8.3 | 72.4 | 41.6 | 54.7 | 33.9 | <0.05 |
SD = Standard deviation; p values are from one-way ANOVA.
Fig. 4.
Contour diagram comparisons of aerosol size and quantity generated during swine cardiac arrest resuscitation and by healthy human coughs.
Discussion
To our knowledge, this is the first study to quantify aerosol quantity and particle size generated during chest compressions and defibrillation in a large animal CA model. We found that chest compression alone did not generate significantly more aerosols than without chest compression during CA. However, we found that chest compressions immediately after defibrillation generated significantly more aerosols with a shift toward larger-size particles. To the best of our knowledge, this is the first direct observation suggesting that defibrillation followed by chest compression is an aerosol generating procedure. The mechanism by which defibrillation led to a greater number and larger-size aerosol generation during subsequent chest compression remains unclear. It is possible that defibrillation generates aerosols within the lung that are then exhaled during chest compression.
Our results are not consistent with a recent manikin and human cadaver study by Ott et al., which reported that chest compression can generate aerosols.11 However, the aerosol spread in that study was simulated by nebulizing ultraviolet sensitive detergent into the artificial airway of the manikin and cadaver while chest compression was being performed, and neither the quantity nor the size of the aerosol were measured. As such, it is possible that the aerosols generated in the study by Ott et al. were smaller than 0.3 μm, larger than 10 μm, or from differences in the experimental model.
In order to better understand the potential clinical relevance of our results, we compared aerosol generation during swine CA resuscitation to healthy human coughs. Our data showed that healthy human coughs generated significantly more particles in the 0.3–0.465 μm diameter range than pre-defibrillation chest compression or CA without chest compression. In contrast, post-defibrillation chest compression generated significantly more aerosols of >1.117 μm diameter than human coughs. The overall size distribution of aerosol generated from human coughs is smaller than post-defibrillation chest compression in swine. The aerosol characteristics generated by healthy subjects in our study was consistent with that from prior studies, which demonstrated that aerosol <5 μm is associated with normal breathing, talking, and occasional coughing of healthy individuals.12, 13, 14
There is significant variability in the definition and level of evidence regarding whether CA resuscitation is considered an aerosol-generating procedure in existing guidelines. The World Health Organization categorized CPR including chest compressions, defibrillation, and associated airway interventions as aerosol-generating procedure, but did not specify which components generate aerosol.1 The International Liaison Committee on Resuscitation suggests in its COVID-19 Consensus Statement5 that healthcare professionals should use PPE for aerosol-generating procedures during resuscitation (weak recommendation, very low certainty evidence), and that it may be reasonable for healthcare providers to consider defibrillation before donning PPE in situations where the provider assesses the benefits may exceed the risks (good practice statement). The European Resuscitation Council2 suggests that lay rescuers should consider placing a facemask or cloth/towel over the person’s mouth and nose before performing chest compressions and public-access defibrillation. They also suggest that delivering a shock from a defibrillator is unlikely to be an aerosol-generating procedure and thus could be performed with the healthcare provider wearing droplet precaution PPE (fluid-resistant surgical mask, eye protection, short-sleeved apron and gloves). However, healthcare personnel should always use airborne-precaution PPE for aerosol-generating procedures such as chest compressions, airway, and ventilation interventions during resuscitation. The American Heart Association COVID-19 Interim Guidance3 suggests that because defibrillation is not expected to be a highly aerosolizing procedure, lay rescuers should use an automated external defibrillator to assess and treat patients with out-of-hospital CA. Furthermore, they suggest that lay rescuers should perform at least hands-only CPR after recognition of a CA, if willing and able, especially if they are household members who have been exposed to the patient at home. Like the European Resuscitation Council,2 the American Heart Association also suggests that a facemask or cloth covering the mouth and nose of the rescuer and/or patient may reduce the risk of transmission to a non-household bystander.3
Our data demonstrated that more research is needed to determine the risk to unprotected rescuers who are performing chest compression following defibrillation attempts. The incremental risk for CA victims of delaying defibrillation and chest compression must also be balanced with the prevalence of SARS-CoV-2 in the community, comorbidities of the lay rescuers, and availability of PPE. If subsequent clinical studies confirm aerosol generation during post-defibrillation chest compression, then potential strategies to reduce lay rescuer risk may include placing a facemask or cloth/towel over the CA victim’s nose and mouth prior to defibrillation attempts in order to minimize aerosol spread.2, 3, 11, 15
This study has several limitations. Due to the lack of historical or other preliminary data, our study was designed as a pilot study. As such, the sample size and power analysis to detect a discrete difference in our primary outcome using well-defined variability was not feasible. Despite the limited sample size, our results were consistent between animals and across repeated measurements. Our data will enable future hypothesis testing on aerosol quantification during CPR in swine model with power analysis to detect predetermined differences in outcomes. The airway anatomy of swine differs from that of humans in that swine’s longer snout may affect aerosol generation during CA resuscitation. Furthermore, we did not measure the half-life and distance traveled by the aerosols, nor did we examine the effect of temperature, relative humidity, air flow, and ventilation on aerosol spread. Because we focused on the measurement of ≤10 μm diameter aerosols to assess particles that are most likely to transmit SARS-CoV-2, we do not know whether chest compression or defibrillation could generate particles larger than 10 μm. Emerging evidence suggest that aerosol and droplet size should be viewed as a continuum rather than dichotomy due to the dynamic impact of turbulent gas cloud16 and droplet evaporation17 on the half-life and distance traveled by respiratory pathogens. As such, our data may provide a simplistic view of aerosol dynamic during CA resuscitation. Finally, it remains unclear how the size and quantity of aerosols generated during CA resuscitation correlate with the infectivity of viral pathogens and risk to rescuers.
Conclusions
In this swine CA model, chest compressions alone did not cause significant aerosol generation. However, an increase in aerosol generation was detected during chest compression immediately following defibrillation. The mechanism and clinical significance of this observation require further investigation. If similar results are observed during human CA resuscitation, strategies to mitigate risk of disease transmission will need to be considered.
Credit author statement
This study was presented as a Lighting Round Oral Abstract in the 2020 Resuscitation Science Symposium on November 14, 2020.
Declaration of conflicts of interest
None.
Acknowledgements
Dr. Hsu reports research grant K12HL133304-01 from the National Institutes of Health. Dr. Neumar reports research grants NIH-R01HL133129, NIH-R44HL091606, and NIH-R34HL130738 from the National Institutes of Health and equipment support for clinical and laboratory research from Physio-Control. The authors acknowledge financial support for the OPS instrument from the University of Michigan College of Engineering. The authors also acknowledge Katelyn Murphy for her assistance in graphic design for this manuscript and instrumentation assistance from Dr. Taehoon Han (University of Michigan) and Dr. Matthew Brusstar (U.S. EPA National Vehicle and Fuels Emissions Laboratory).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.resuscitation.2020.12.004.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Organization WH . Organization WH; 2014. Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in health care. p. 133. [PubMed] [Google Scholar]
- 2.Nolan J.P., Monsieurs K.G., Bossaert L., et al. European Resuscitation Council COVID-19 guidelines executive summary. Resuscitation. 2020;153:45–55. doi: 10.1016/j.resuscitation.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edelson D.P., Sasson C., Chan P.S., et al. Interim guidance for basic and advanced life support in adults, children, and neonates with suspected or confirmed COVID-19: from the Emergency Cardiovascular Care Committee and Get With the Guidelines((R))-Resuscitation Adult and Pediatric Task Forces of the American Heart Association in Collaboration with the American Academy of Pediatrics, American Association for Respiratory Care, American College of Emergency Physicians, The Society of Critical Care Anesthesiologists, and American Society of Anesthesiologists: Supporting Organizations: American Association of Critical Care Nurses and National EMS Physicians. Circulation. 2020;141(25):e933–e943. doi: 10.1161/CIRCULATIONAHA.120.047463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couper K., Taylor-Phillips S., Grove A., et al. COVID-19 in cardiac arrest and infection risk to rescuers: a systematic review. Resuscitation. 2020;151:59–66. doi: 10.1016/j.resuscitation.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perkins G.D., Morley P.T., Nolan J.P., et al. International Liaison Committee on Resuscitation: COVID-19 consensus on science, treatment recommendations and task force insights. Resuscitation. 2020;151:145–147. doi: 10.1016/j.resuscitation.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tellier R., Li Y., Cowling B.J., Tang J.W. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis. 2019;19:101. doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicas M., Nazaroff W.W., Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J Occup Environ Hyg. 2005;2:143–154. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gralton J., Tovey E., McLaws M.L., Rawlinson W.D. The role of particle size in aerosolised pathogen transmission: a review. J Infect. 2011;62:1–13. doi: 10.1016/j.jinf.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Council NR . 8th ed. The National Academies Press; Washington, DC: 2011. Guide for the care and use of laboratory animals. [Google Scholar]
- 11.Ott M., Milazzo A., Liebau S., et al. Exploration of strategies to reduce aerosol-spread during chest compressions: a simulation and cadaver model. Resuscitation. 2020;152:192–198. doi: 10.1016/j.resuscitation.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabian P., Brain J., Houseman E.A., Gern J., Milton D.K. Origin of exhaled breath particles from healthy and human rhinovirus-infected subjects. J Aerosol Med Pulm Drug Deliv. 2011;24:137–147. doi: 10.1089/jamp.2010.0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson G.R., Morawska L., Ristovski Z.D., et al. Modality of human expired aerosol size distributions. J Aerosol Sci. 2011;42:839–851. [Google Scholar]
- 14.Morawska L., Johnson G.R., Ristovski Z.D., et al. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J Aerosol Sci. 2009;40:256–269. [Google Scholar]
- 15.Asadi S., Cappa C.D., Barreda S., Wexler A.S., Bouvier N.M., Ristenpart W.D. Efficacy of masks and face coverings in controlling outward aerosol particle emission from expiratory activities. Sci Rep. 2020;10 doi: 10.1038/s41598-020-72798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- 17.Jayaweera M., Perera H., Gunawardana B., Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ Res. 2020;188 doi: 10.1016/j.envres.2020.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.