Abstract
COVID-19 rapidly turned to a global pandemic posing lethal threats to overwhelming health care capabilities, despite its relatively low mortality rate. The clinical respiratory symptoms include dry cough, fever, anosmia, breathing difficulties, and subsequent respiratory failure. No known cure is available for COVID-19. Apart from the anti-viral strategy, the supports of immune effectors and modulation of immunosuppressive mechanisms is the rationale immunomodulation approach in COVID-19 management. Diet and nutrition are essential for healthy immunity. However, a group of micronutrients plays a dominant role in immunomodulation. The deficiency of most nutrients increases the individual susceptibility to virus infection with a tendency for severe clinical presentation. Despite a shred of evidence, the supplementation of a single nutrient is not promising in the general population. Individuals at high-risk for specific nutrient deficiencies likely benefit from supplementation. The individual dietary and nutritional status assessments are critical for determining the comprehensive actions in COVID-19.
Keywords: Immunomodulation, COVID-19, SARs-CoV-2, Nutrients, Vitamins, Trace elements
1. Introduction
The coronavirus disease 2019 (COVID-19) is a respiratory disorder that is the consequence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [1]. Since the first identified case in Wuhan, China, it took only three months for a global pandemic of the disease due to the highly contagious of this virus through droplets transmission [[2], [3], [4], [5]].
The host responses to SARS-CoV-2 infection diverse; 78% of newly infected persons may remain asymptomatic, while 84.3%, 9.6%, and 6.1 % of clinical patients present with mild, moderate, and severe symptoms, respectively [6,7]. The viral responses of innate and adaptive immune machinery differ upon the host metabolic determinants, including age, sex, nutritional status, smoking habits, and co-existing medical conditions [6,[8], [9], [10], [11], [12]]. The findings of asymptomatic and mild clinical symptoms in younger individuals signify the role of host status in SARS-CoV-2 infection [9,[13], [14], [15]].
In contrast to the current anti-viral approach targeting the specific pathogen, an emerging strategy aims the host immunity activation to fight the virus [16,17]. With the expanding knowledge of cellular immunity mechanisms, the development of drugs, substances, or measures that modulate immune responses contributes to the management of many life-threatening infections [[18], [19], [20], [21], [22], [23], [24]]. Small clinical trials in the severe infection conditions explored the compassionate options to activate the early-responding immune effector cells through various immunoadjuvant agents, such as interleukin-7, anti-programmed death 1, interferon-γ, and granulocyte-macrophage colony-stimulating factor [16,25]. The clinical management of severe infections commonly includes the modulation of immunosuppressive mechanisms, i.e., the alleviation of T-cell exhaustion, myeloid-derived suppressor cells, or regulatory T cells [16,21,24,26,27].
The balance between the immune activation and the counter-regulatory immunosuppression is crucial upon the virus-host encountering responses [28,29]. This balance determines the variation of subsequent clinical manifestations. Several micronutrients contribute to these immunomodulatory effects [16,[30], [31], [32], [33], [34]]. This article reviews some nutrients that can potentially modulate immunity to SARs-CoV-2 infection.
2. Nutrients and virus-host immunologic responses
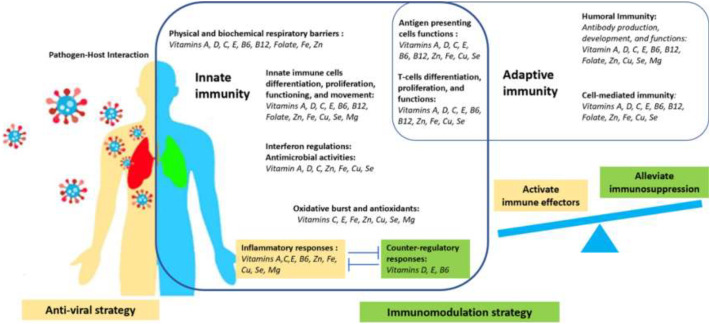
Micronutrients involve in the continuum of host immune responses to the virus from the initial virus-host interaction, innate immune activation, to adaptive immune responses, as summarized in Fig. 1 [30,35]. The healthy immunity requires the synergistic contribution from multiple micronutrients, and single nutrient barely drives the whole immune machinery. However, the viral-host resistance relies on the support from a dominant group of nutrients, including vitamins A, C, D, E, B6, B12, folate, iron (Fe), zinc (Zn), copper (Zn), selenium (Se), and magnesium (Mg) [30,[36], [37], [38]].
Fig. 1.
The immunomodulation strategy and roles of a group of nutrients in different processes of virus-host immune responses.
The first-line defenses against the virus are the physical and biochemical barriers of the respiratory tract, which their normal epithelial differentiation and growth require vitamin A and Fe [36,37]. Vitamins A, C, D, and Zn regulate membrane fluidity, membrane integrity, gap-junction communication, and membrane repair [37,[39], [40], [41], [42], [43], [44], [45], [46]]. Vitamin E mitigates the membrane lipid peroxidation from reactive oxygen species [37]. Vitamins A, D, C, and the trace elements Zn, Fe, Cu, and Se regulate the membrane-bounded antimicrobial peptide activities and mucosal-associated microbiota [30,47,48]. The mucosal migration and regulation of immune cell functions also synchronize with the integrated pathways of vitamins B6, B12, and folate [47,49].
Interferon (IFN) is a crucial anti-viral innate immune response than regulates and shapes the balance of Th1 and Th2 phenotypes in adaptive immunity [50]. IFN-λs are key antiviral cytokines at the epithelial barriers, and induce the inflammatory response and apoptotic cell death [50,51]. Apart from that, type I IFNs increase in responses to the viral activation of the toll-like receptor 7 and the mitochondrial antiviral-signaling [52,53]. Vitamins A, C, D, C, Zn, Fe, Cu, and Se regulate IFNs production [30,36,44,45,[54], [55], [56], [57], [58]].
Upon the intrusion of SARS-CoV-2 into the airway epithelial cells, innate immune cells respond through their movement, migration, differentiation, proliferation, and activation to counteract the viral replication. The cytokines and oxidative burst induce the pro-inflammatory milieu, while the virus delays and suppress type I IFNs responses [35]. Without the optimal counter-regulatory immune reactions, the activation of the Th1/Th17 phenotypes of adaptive immunity further exacerbates the hyperinflammatory conditions and the ‘cytokine storms’ [27,35,59]. However, healthy immunity eventually proceeds to the production of SARS-CoV-2 specific antibodies that neutralize the virus and resolve the infection [59,60].
Vitamins A, C, D, E, B6, B12, and folate, and the trace elements Zn, Fe, Cu, Se, as well as the mineral Mg comprise a group of nutrients that support the entire continuum of virus-host immune responses. Their contributions range from the regulation of number and function of innate immune cells such as neutrophils, natural killer cells, monocytes, and macrophages [36,37,45,54,[61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73]], the production of pro-, and anti-inflammatory cytokines, the responses to inflammation, the oxidative burst function, the reductive-oxidative hemodynamics [36,37,45,[61], [62], [63], [64],71,72,[74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85]], to the responses of adaptive immunity, including differentiation, proliferation, and functions of T-cells [32,36,37,45,54,71,77,84,[86], [87], [88], [89], [90], [91], [92], [93], [94], [95]], the interactions with the presenting viral antigens [37,54,71,73,96], and the production and development of virus-specific antibodies [36,37,45,71,73,97,98].
Despite their synergistic contributions to virus-host responses, the deficiency state of specific nutrients increases an individual susceptibility to the severe clinical manifestation of SARS-CoV-2 infection. The following sections explore the consequences of some micronutrient deficiencies and the potential effects of their supplementations to COVID-19.
3. Vitamins
3.1. Vitamin D
Vitamin D is involved in a wide range of immunomodulatory activities, including the maintenance of immune barrier integrity [[40], [41], [42], [43], [44],47,48], the production of antimicrobial peptides [[99], [100], [101], [102]], the support of monocytes, macrophages, and dendritic cells functions [36,37,[62], [63], [64]], the modulation of oxidative burst potential [37,[62], [63], [64]], the promotion of anti-inflammatory cytokine production [62,[74], [75], [76]], the inhibition of IFN γ [[54], [55], [56], [57], [58]], nuclear factor κB [103], other pro-inflammatory cytokines [104,105], and the subsequent responses of adaptive immune cells [32,54,71,[87], [88], [89], [90], [91]].
The low level of vitamin D increases the risks, severity, morbidity, and mortality of several respiratory conditions, such as rhinitis, asthma, tuberculosis, chronic pulmonary disorders, viral respiratory infections, and possibly also the COVID-19 [[106], [107], [108], [109], [110]]. The potential role of vitamin D in the modulation of immune response to viral respiratory tract infection (ALRI) has been evidenced in a study involving a young patient with individual genetic polymorphisms of vitamin D receptors [111]. Vitamin D influences lung structures, size, volume, and functions. Vitamin D deficiency thus worsens several pulmonary conditions [[112], [113], [114]].
A recent meta-analysis reported the associations of individuals with adequate vitamin D levels or daily oral supplementation with vitamin D and the reduced risk of respiratory infections [115,116]. The previous studies also suggested this risk reduction benefit of the supplementation, but only in the vitamin D deficient individuals [61,107]. With this information, vitamin D supplementation is a potential preventive strategy of COVID-19 in the individual with an established deficient state or has a high risk of vitamin D deficiency.
3.2. Vitamin C
Vitamin C supports the epithelial barrier integrity through its contributions to the collagen synthesis, keratinocyte differentiation, fibroblast migration, and proliferation [37,45]. Innate immune cells require vitamin C to maintain their activities, movement, functions, proliferation, and differentiation [36,37,45,54,61,65]. Vitamin C promotes the antimicrobial activities, increases serum complement proteins, and stimulated the production of IFN-γ [36,45]. Vitamin C is a powerful antioxidant, thus, maintains the intracellular reductive-oxidative homeostasis during the active immune responses [37,45,77]. It also plays roles in antibody production from plasma cells together with the supports of differentiation and proliferation of T-cells, particularly the cytotoxic T-cells [36,45].
Vitamin C deficiency increased the risk and severity of several respiratory infections, including pneumonia [37,45,107,110,117]. Despite many conflicting and inconclusive pieces of evidence, the oral supplementation of vitamin C potentially shortens the symptoms of the common cold in children. It also reduces the incidence of pneumonia in the elderly [107,[117], [118], [119]]. The combination of vitamin C and red ginseng reduced the influenza virus-induced lung inflammation and increased the survival rate in mice [120]. The treatment with high dose intravenous vitamin C shortens the recovery periods of severely ill patients with virus-induced acute respiratory distress syndrome [77,121,122]. Concerning its affordability, availability, and safety, vitamin C is still a functional option to consider in the management of COVID-19.
3.3. Vitamin A
Vitamin A is an essential micronutrient for maintaining the barrier integrity and normal differentiation of epithelial tissues [37,123]. It supports the mucosal immune responses and acts as an anti-inflammatory agent [39,47,124,125]. Vitamin A regulates the number and function of natural killer cells and supports the phagocytic and oxidative burst activities of macrophages [37,61]. The Th1/Th2 phenotypic differentiation and development of T-cells require vitamin A [37,86]. It downregulates IFN-γ, interleukin 2, and tumor-necrosis factor α productions by Th1 cells, thus, maintains the normal antibody-mediated Th2 responses [36,37,45]. Vitamin A also supports antibody production by B cells [37].
Vitamin A deficiency is a common risk factor for the increased susceptibility to virus-induced respiratory tract infections, measles, and diarrhea [37,107,126,127]. Young cows with vitamin A deficiency failed to mount the protective immunologic responses to the BRSV-NP vaccine (amphiphilic polyanhydride nanoparticle-based vaccine encapsulating the fusion and attachment proteins from bovine respiratory syncytial virus), with the subsequent lung infections after challenging by the virus [128]. Vitamin A supplementation improves antibody titer responses to vaccines [37]. Supplementation of vitamin A to deficient individuals reduces the incidence of Mycoplasma pneumoniae infection, which is a common post-viral secondary bacterial infection in COVID-19 [129,130]. The supplementation in vitamin A deficit children, 6-month to 5-year of age, decreased their risk of all-cause mortality and morbidity from infectious diseases. Nevertheless, vitamin A supplementation showed no benefits for pneumonia [107,131,132]. Concerning the potential adverse effects of vitamin A, the supplementation is sensible in the COVID-19 management of undernourished individuals or those with the evidence of vitamin A deficiency [132].
3.4. Vitamin E
Vitamin E is a potent lipid-soluble antioxidant that protects cell membranes against oxidative damage and supports the integrity of respiratory epithelial barriers [37,133,134]. It enhances the natural killer cell cytotoxic activity and decreases prostaglandin E2 production by macrophages [36,37,54,61,66,78]. Vitamin E modulates the production of IFN-γ and interleukin 2 [36,132,135]. It supports lymphocyte proliferation, T-cell-mediated functions, Th1 response optimization, and Th2 response suppression [36,37,61]. The active immune synapses between Th cells require vitamin E supports [54]. Vitamin E also increases the proportion of antigen-experienced memory T-cells [96].
Vitamin E deficiency is rare in humans. The deficit state impairs the functions of both humoral and cell-mediated adaptive immunity, thus facilitates the viral infection with high virulent strains, severe subsequent pathologies, and abnormal immune responses [71,132,133,136]. Vitamin E supplementation improves overall immune functions, reduces respiratory tract infection incidences, severity, lowers virus load in lung tissues, and increases the antibody titers, particularly in the elderly [37,107,135,137]. Malnourished individuals should benefit from the inclusion of vitamin E supplementation in COVID-19 management.
4. Essential trace elements and magnesium
4.1. Zinc
Zinc is an essential trace element that modulates the functions of approximately 2,000 enzymes and 750 transcription factors involved in various biological and physiological processes, including immunity, growth, and development [46,138,139]. Zinc also possesses a variety of direct and indirect antiviral properties. For instance, the pyrrolidine dithiocarbamate - a Zn ionophore - inhibits the RNA-dependent RNA polymerase enzyme that promotes SARS-CoV-2 replication [138,140,141]. Zinc maintains the integrity of immune barriers through its cofactor function in metalloenzymes [46,142]. It enhances the cytotoxic activity of natural killer cells and supports the cellular functions, growth, and differentiation of innate immune cells [37,54,61,67,68]. Zinc involves in complement protein activities and the IFN-γ production [36,45]. It has anti-inflammatory properties by the modulation of the cytokine release and the Th17 and Th9 development [36,54,77,[81], [82], [83],92,93]. Zinc also exerts anti-oxidant effects through its influences on antioxidant proteins [37,77]. It supports the proliferation of cytotoxic T cells, the differentiation, development, and activation of T-cells, the cytokine production of Th1 cells, and the development of regulatory T cells [36,54,77,[92], [93], [94]]. Zinc is involved in antibody production, mainly the immunoglobulin G antibody [37,97,98].
Zinc deficiency increases the risk and morbidity of inflammatory disorders, infections, and viral pneumonia, particularly in children and the elderly [37,93,107,110,127,143,144]. Supplementation of Zn in children reduced their susceptibilities, severity of symptoms, and the duration of common colds and viral pneumonia [77,107,138,145,146]. Zinc supplementation in nursing home elderly increased their serum Zn levels and their number of T-cells [147]. Despite the few shreds of confirming evidence, Zn supplementation can benefit in the management of COVID-19, particularly in high-risk individuals for Zn deficiency.
4.2. Selenium
Selenium is a trace component of selenoproteins that are essential for the functions of the immune system and the reductive-oxidative homeostasis [61,148]. It modulates the activities of virus-induced innate and adaptive immunity through the regulation of IFN-α, IFN-γ, and IFN-β production, the influences on the functions and differentiation of natural killer cells and T-cells, and the antibody production [36,45,71,84,95,149,150].
Selenium deficiency increases the risk and virulence of virus-induced pulmonary infections through the aberrant immune responses and excessive cytokines production, particularly in infants during their first six weeks of life [107,132,150,151]. At the same time, the maintaining of optimal Se status through an adequate diet protects against several viral infections [[148], [149], [150]]. Dietary selenium supplementation potentiates innate antiviral immune responses reducing, for instance, the pathogenicity of avian influenza virus infection [152]. Consequently, Se supplementation in deficit individuals distinctively enhances the immune responses to the virus [37,132]. Selenium supplementation is the rationale management of COVID-19 in susceptible hosts.
4.3. Magnesium
Magnesium is a crucial mineral for healthy physiologic functions, including bioenergetics, immune responses, and acid-base balance; it is involved in nucleic acid metabolism, DNA replication, leukocyte activation, antigen-binding to macrophages, and apoptotic regulation [38,72,73]. Magnesium influences both the cell-mediated and humoral adaptive immunity [73,153]. It can protect DNA from oxidative damages and reduce the superoxide anion production at high concentrations [72,85]. Magnesium deficiency increases the susceptibility to recurrent upper respiratory tract infections [73,153,154]. A deficiency of Mg promotes chronic low-grade inflammation through the production of pro-inflammatory cytokines, acute-phase proteins, and free radicals [155]. Normal Mg levels maintain healthy lung structure and functions, while its lower levels are often associated with increased respiratory complications [156,157]. To date, no available study explores the impact of Mg supplementation on the clinical virus-induced respiratory infection.
5. Other potential immunomodulators for the COVID-19 management
5.1. N-acetylcysteine
N-acetylcysteine (NAC) is a precursor of glutathione that is a thiol reducing agent with antioxidant and anti-inflammatory properties. NAC reduces the elasticity and viscosity of mucus and improves the clearance of pulmonary secretions. NAC reduces oxidative stress and inflammation in chronic obstructive pulmonary disease patients. With the exposure to the influenza virus, NAC inhibits the production of TNF-α in alveolar macrophages, the expression of intercellular adhesion molecule 1 in respiratory epithelial cells, and increases the heme oxygenase 1 level in cells [[158], [159], [160]]. The combination of NAC and glutathione reduced the antigen levels of human immunosuppressive virus 1 and their reverse transcriptase activities in a cell line study [161]. A murine model study reported the synergistic actions of NAC and Oseltamivir combination in survival rate improvement- up to 100%- from the lethal strain of influenza infection [162].
The clinical application of NAC in patients with community-acquired pneumonia reported the reduction of oxidative stress and inflammation, as shown by the improved levels of TNF-α and malondialdehyde [163]. The long-term administration of NAC in elderly persons reduced the severity and duration of influenza-like symptoms [164]. Concerning the safety profile of NAC, it can be a sensible option to include in COVID-19 management despite a few pieces of clinical evidence.
5.2. Polyphenolic compounds
Polyphenolic compounds are a major class of phytonutrients with several biological and pharmacological properties, including antioxidant, anti-inflammatory, antibacterial, and antiviral potentials [165]. The span of the antiviral property of polyphenols involves the viruses from the Coronaviriade family. Resveratrol inhibits the Middle East Respiratory Syndrome coronavirus in vitro [166]. Anti-viral face masks and the cleaning wipes have their fiber filter surface grafted with the polyphenol catechin [167,168].
A recent computerized virtual screening of molecular structures identified six polyphenol molecules, i.e., sanguiin, theaflavin gallate, theaflavin digallate, kaempferol, punicalagin, and protocatechuic acid, that potentially target the main protease of SARS-CoV- 2 [169]. Stilbene and flavonoid derivatives, such as herbacetin, isobavachalcone, quercetin 3-β-d-glucoside, and helichrysetin, inhibit 3C-like protease [170,171]. Resveratrol inhibits the virus nucleocapsid protein synthesis, thus suppress viral replication [166]. Quercetin inhibits the helicase, an enzyme for viral replication [172]. Other polyphenols such as delphinidin and epigallocatechin gallate inhibit the entry attachment of the virus [173,174]. These polyphenols and flavonoids have potential effects on COVID-19 management. However, these pieces of evidence are all from in vitro studies; to date, there is no available in vivo study. It is then premature to conclude their clinical applications.
6. Concluding remarks
Due to the great impact on medical services and the massive demand for health care, COVID-19 rapidly turned into a global pandemic, posing a lethal threat to the population despite its low mortality rate. The clinical respiratory symptoms include dry cough, fever, anosmia, breathing difficulties, and subsequent respiratory failure. No known cure is available for COVID-19. Apart from the anti-viral strategy, the supports of immune effectors and modulation of immunosuppression is the rationale immunomodulation approach in COVID-19 management. Diet and nutrition are essential for healthy immunity, but a group of micronutrients somehow plays a dominant role in immunomodulation [175]. This paper reviews the mechanisms, the effects of their deficiency states, and the potential impacts of their supplementations in COVID-19, as summarized in Table 1 . The deficiency states of most nutrients increase the individual susceptibility to virus infection with a tendency for severe clinical presentation. Despite a shred of evidence, the supplementation of a single nutrient is not promising in the general population. The high-risk individual of a specific micronutrient deficiency is likely to benefit from the supplementation. The individual dietary and nutritional status assessments are critical for determining the comprehensive actions in COVID-19.
Table 1.
The immunomodulating properties, the risk for the deficiency states, and the impacts of supplementation of a group of nutrients.
| Nutrients | Immunomodulating properties | Risks for deficiency states | Impacts of supplementation |
|---|---|---|---|
| Vitamin A |
|
|
|
| Vitamin C |
|
|
|
| Vitamin D |
|
|
|
| Vitamin E |
|
||
| Zinc |
|
||
| Selenium |
|
||
| Magnesium |
|
References
- 1.Valencia D.N. Brief review on COVID-19: the 2020 pandemic caused by SARS-CoV-2. Lancet. 2020;12 doi: 10.7759/cureus.7386. e7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg. Microb. Infect. 2020;9:727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabi F.A., Al Zoubi M.S., Kasasbeh G.A., Salameh D.M., Al-Nasser A.D. SARS-CoV-2 and coronavirus disease 2019: what we know so far. Pathogens. 2020;9:231. doi: 10.3390/pathogens9030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhama K., Sharun K., Tiwari R., Dadar M., Malik Y.S., Singh K.P., Chaicumpa W. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum. Vaccin. Immunother. 2020:1–7. doi: 10.1080/21645515.2020.1735227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan W.-j., Ni Z.-y., Hu Y., Liang W.-h., Ou C.-q., He J.-x., Liu L., Shan H., Lei C.-l., Hui D.S.C., Du B., Li L.-j., Zeng G., Yuen K.-Y., Chen R.-c., Tang C.-l., Wang T., Chen P.-y., Xiang J., Li S.-y., Wang J.-l., Liang Z.-j., Peng Y.-x., Wei L., Liu Y., Hu Y.-h., Peng P., Wang J.-m., Liu J.-y., Chen Z., Li G., Zheng Z.-j., Qiu S.-q., Luo J., Ye C.-j., Zhu S.-y., Zhong N.-s. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day M. Covid-19: four fifths of cases are asymptomatic, China figures indicate. BMJ. 2020;369 doi: 10.1136/bmj.m1375. [DOI] [PubMed] [Google Scholar]
- 8.Bornstein S.R., Dalan R., Hopkins D., Mingrone G., Boehm B.O. Endocrine and metabolic link to coronavirus infection. Nat. Rev. Endocrinol. 2020 doi: 10.1038/s41574-020-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedrich C.M. COVID-19 - considerations for the paediatric rheumatologist. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciceri F., Castagna A., Rovere-Querini P., De Cobelli F., Ruggeri A., Galli L., Conte C., De Lorenzo R., Poli A., Ambrosio A., Signorelli C., Bossi E., Fazio M., Tresoldi C., Colombo S., Monti G., Fominskiy E., Franchini S., Spessot M., Martinenghi C., Carlucci M., Beretta L., Scandroglio A.M., Clementi M., Locatelli M., Tresoldi M., Scarpellini P., Martino G., Bosi E., Dagna L., Lazzarin A., Landoni G., Zangrillo A. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin. Immunol. 2020;217 doi: 10.1016/j.clim.2020.108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urra J.M., Cabrera C.M., Porras L., Rodenas I. Selective CD8 cell reduction by SARS-CoV-2 is associated with a worse prognosis and systemic inflammation in COVID-19 patients. Clin. Immunol. 2020;217 doi: 10.1016/j.clim.2020.108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y., Liu Y., Liu L., Wang X., Luo N., Ling L. Clinical outcome of 55 asymptomatic cases at the time of hospital admission infected with SARS-Coronavirus-2 in Shenzhen, China. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Z., Song C., Xu C., Jin G., Chen Y., Xu X., Ma H., Chen W., Lin Y., Zheng Y., Wang J., Hu Z., Yi Y., Shen H. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci. China Life Sci. 2020 doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kloc M., Ghobrial R.M., Kuchar E., Lewicki S., Kubiak J.Z. Development of child immunity in the context of COVID-19 pandemic. Clin. Immunol. 2020;217 doi: 10.1016/j.clim.2020.108510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotchkiss R.S., Opal S.M. Activating immunity to fight a foe — a new path. N. Engl. J. Med. 2020;382:1270–1272. doi: 10.1056/NEJMcibr1917242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felsenstein S., Herbert J.A., McNamara P.S., Hedrich C.M. COVID-19: Immunology and treatment options. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amezcua Vesely M.C., Pallis P., Bielecki P., Low J.S., Zhao J., Harman C.C.D., Kroehling L., Jackson R., Bailis W., Licona-Limón P., Xu H., Iijima N., Pillai P.S., Kaplan D.H., Weaver C.T., Kluger Y., Kowalczyk M.S., Iwasaki A., Pereira J.P., Esplugues E., Gagliani N., Flavell R.A. Effector T(H)17 Cells Give Rise to Long-Lived T(RM) Cells that Are Essential for an Immediate Response against Bacterial Infection. Cell. 2019;178:1176–1188. doi: 10.1016/j.cell.2019.07.032. e1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zander R., Schauder D., Xin G., Nguyen C., Wu X., Zajac A., Cui W. CD4(+) T cell help is required for the formation of a cytolytic CD8(+) T cell subset that protects against chronic infection and cancer. Immunity. 2019;51:1028–1042.e1024. doi: 10.1016/j.immuni.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Opal S.M. Non-antibiotic treatments for bacterial diseases in an era of progressive antibiotic resistance. Criti. Care (London, England) 2016;20 doi: 10.1186/s13054-016-1549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conti P., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Frydas I., Kritas S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34 doi: 10.23812/conti-e. [DOI] [PubMed] [Google Scholar]
- 22.Vellingiri B., Jayaramayya K., Iyer M., Narayanasamy A., Govindasamy V., Giridharan B., Ganesan S., Venugopal A., Venkatesan D., Ganesan H., Rajagopalan K., Rahman P., Cho S.G., Kumar N.S., Subramaniam M.D. COVID-19: A promising cure for the global panic. Sci. Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin L., Luo S., Qin R., Yang M., Wang X., Yang Q., Zhang Y., Wang Q., Zhu R., Fan H., Wang H., Hu Y., Wang L., Hu D. Long-term infection of SARS-CoV-2 changed the body's immune status. Clin. Immunol. 2020 doi: 10.1016/j.clim.2020.108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X., Zeng X., Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francois B., Jeannet R., Daix T., Walton A.H., Shotwell M.S., Unsinger J., Monneret G., Rimmelé T., Blood T., Morre M., Gregoire A., Mayo G.A., Blood J., Durum S.K., Sherwood E.R., Hotchkiss R.S. Interleukin-7 restores lymphocytes in septic shock: the IRIS-7 randomized clinical trial. JCI Insight. 2018;3 doi: 10.1172/jci.insight.98960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hotchkiss R.S., Moldawer L.L., Opal S.M., Reinhart K., Turnbull I.R., Vincent J.L. Sepsis and septic shock. Nat. Rev. Dis. Primers. 2016;2 doi: 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kane B.A., Bryant K.J., McNeil H.P., Tedla N.T. Termination of immune activation: an essential component of healthy host immune responses. J. Innate Immun. 2014;6:727–738. doi: 10.1159/000363449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malik Y.S., Sircar S., Bhat S., Sharun K., Dhama K., Dadar M., Tiwari R., Chaicumpa W. Emerging novel coronavirus (2019-nCoV)—current scenario, evolutionary perspective based on genome analysis and recent developments. Vet. Q. 2020;40:68–76. doi: 10.1080/01652176.2020.1727993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gombart A.F., Pierre A., Maggini S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients. 2020;12:236. doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gasmi A., Noor S., Tippairote T., Dadar M., Menzel A., Bjorklund G. Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sassi F., Tamone C., D'Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018;10 doi: 10.3390/nu10111656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun J., de Vos P. Editorial: immunomodulatory functions of nutritional ingredients in health and disease. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goswami T., Bhar R., Jadhav S.E., Joardar S., Ram G.C. Role of dietary zinc as a nutritional immunomodulator. Asian Australas. J. Anim. Sci. 2005;18 doi: 10.5713/ajas.2005.439. [DOI] [Google Scholar]
- 35.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38:1–9. doi: 10.12932/ap-200220-0772. [DOI] [PubMed] [Google Scholar]
- 36.Haryanto B., Suksmasari T., Wintergerst E., Maggini S., Bayer Multivitamin supplementation supports immune function and ameliorates conditions triggered by reduced air quality. Vitam. Min. 2015;4 doi: 10.4172/2376-1318.1000128. [DOI] [Google Scholar]
- 37.Maggini S., Beveridge S., Sorbara P.J.P., Senatore G. Feeding the immune system: the role of micronutrients in restoring resistance to infections, CAB reviews: perspectives in agriculture. Vet. Sci. Nutr. Nat. Resour. 2008;3:1–21. doi: 10.1079/PAVSNNR20083098. [DOI] [Google Scholar]
- 38.Zoroddu M.A., Aaseth J., Crisponi G., Medici S., Peana M., Nurchi V.M. The essential metals for humans: a brief overview. J. Inorg. Biochem. 2019;195:120–129. doi: 10.1016/j.jinorgbio.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Chew B.P., Park J.S. Carotenoid action on the immune response. J. Nutr. Biochem. 2004;134:257S–261S. doi: 10.1093/jn/134.1.257S. [DOI] [PubMed] [Google Scholar]
- 40.Clairmont A., Tessman D., Stock A., Nicolai S., Stahl W., Sies H. Induction of gap junctional intercellular communication by vitamin D in human skin fibroblasts is dependent on the nuclear Induction of gap junctional intercellular communication by vitamin D in human skin fibroblasts is dependent on the nuclear vitamin D receptor. Carcinogenesis. 1996;17:1389–1391. doi: 10.1093/carcin/17.6.1389. [DOI] [PubMed] [Google Scholar]
- 41.Gniadecki R., Gajkowska B., Hansen M. 1,25-dihydroxyvitamin D3 stimulates the assembly of adherens junctions in keratinocytes: involvement of protein kinase C. Endocrinology. 1997;138:2241–2248. doi: 10.1210/endo.138.6.5156. [DOI] [PubMed] [Google Scholar]
- 42.Pálmer H.G., González-Sancho J.M., Espada J., Berciano M.T., Puig I., Baulida J., Quintanilla M., Cano A., de Herreros A.G., Lafarga M., Muñoz A. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J. Cell Biol. 2001;154:369–387. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mihajlovic M., Fedecostante M., Oost M.J., Steenhuis S.K.P., Lentjes E.G.W.M., Maitimu-Smeele I., Janssen M.J., Hilbrands L.B., Masereeuw R. Role of vitamin D in maintaining renal epithelial barrier function in uremic conditions. Int. J. Mol. Sci. 2017;18:2531. doi: 10.3390/ijms18122531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin Z., Pintea V., Lin Y., Hammock B.D., Watsky M.A. Vitamin D enhances corneal epithelial barrier function. Invest. Ophthalmol. Vis. Sci. 2011;52:7359–7364. doi: 10.1167/iovs.11-7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carr A.C., Maggini S. Vitamin C and immune function. Nutrients. 2017;9 doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin P.-H., Sermersheim M., Li H., Lee P.H.U., Steinberg S.M., Ma J. Zinc in wound healing modulation. Nutrients. 2017;10:16. doi: 10.3390/nu10010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biesalski H.K. Nutrition meets the microbiome: micronutrients and the microbiota. Ann. N. Y. Acad. Sci. 2016;1372:53–64. doi: 10.1111/nyas.13145. [DOI] [PubMed] [Google Scholar]
- 48.Clark A., Mach N. Role of vitamin D in the hygiene hypothesis: the interplay between vitamin D, vitamin D receptors, gut microbiota, and immune response. Front. Immunol. 2016;7:627. doi: 10.3389/fimmu.2016.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshii K., Hosomi K., Sawane K., Kunisawa J. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front. Nutr. 2019;6:48. doi: 10.3389/fnut.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou J., Wang Y., Chang Q., Ma P., Hu Y., Cao X. Type III interferons in viral infection and antiviral immunity. Cell. Physiol. Biochem. 2018;51:173–185. doi: 10.1159/000495172. [DOI] [PubMed] [Google Scholar]
- 51.Lee S.H., Kwon J.Y., Kim S.-Y., Jung K., Cho M.-L. Interferon-gamma regulates inflammatory cell death by targeting necroptosis in experimental autoimmune arthritis. Sci. Rep. 2017;7:10133. doi: 10.1038/s41598-017-09767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCarty M.F., DiNicolantonio J.J. Nutraceuticals have potential for boosting the type 1 interferon response to RNA viruses including influenza and coronavirus. Prog. Cardiovasc. Dis. 2020 doi: 10.1016/j.pcad.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang B., Li J., Zhang X., Zhao Q., Lu M., Lv Y. RIG-1 and MDA-5 signaling pathways contribute to IFN-β production and viral replication in porcine circovirus virus type 2-infected PK-15 cells in vitro. Vet. Microbiol. 2017;211:36–42. doi: 10.1016/j.vetmic.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 54.Wu D., Lewis E.D., Pae M., Meydani S.N. Nutritional modulation of immune function: analysis of evidence, mechanisms, and clinical relevance. Front. Immunol. 2018;9:3160. doi: 10.3389/fimmu.2018.03160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsui T., Takahashi R., Nakao Y., Koizumi T., Katakami Y., Mihara K., Sugiyama T., Fujita T. 1,25-Dihydroxyvitamin D3-regulated expression of genes involved in human T-lymphocyte proliferation and differentiation. Cancer Res. 1986;46:5827–5831. [PubMed] [Google Scholar]
- 56.Reichel H., Koeffler H.P., Tobler A., Norman A.W. Proceedings of the National Academy of Sciences of the United States of America. Vol. 84. 1987. 1 alpha,25-Dihydroxyvitamin D3 inhibits gamma-interferon synthesis by normal human peripheral blood lymphocytes; pp. 3385–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rigby W.F., Denome S., Fanger M.W. Regulation of lymphokine production and human T lymphocyte activation by 1,25-dihydroxyvitamin D3. Specific inhibition at the level of messenger RNA. J. Clin. Invest. 1987;79:1659–1664. doi: 10.1172/jci113004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inoue M., Matsui T., Nishibu A., Nihei Y., Iwatsuki K., Kaneko F. Regulatory effects of 1alpha,25-dihydroxyvitamin D3 on inflammatory responses in psoriasis. Eur. J. Dermatol. 1998;8:16–20. [PubMed] [Google Scholar]
- 59.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., Bucci E., Piacentini M., Ippolito G., Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020 doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shanmugaraj B., Siriwattananon K., Wangkanont K., Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19) Asian Pac. J. Allergy Immunol. 2020;38:10–18. doi: 10.12932/ap-200220-0773. [DOI] [PubMed] [Google Scholar]
- 61.Maggini S., Pierre A., Calder P.C. Immune function and micronutrient requirements change over the life course. Nutrients. 2018;10:1531. doi: 10.3390/nu10101531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wishart K. 2017. Increased Micronutrient Requirements during Physiologically Demanding Situations: Review of the Current Evidence. [DOI] [Google Scholar]
- 63.Sly L.M., Lopez M., Nauseef W.M., Reiner N.E. 1alpha,25-Dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J. Biol. Chem. 2001;276:35482–35493. doi: 10.1074/jbc.M102876200. [DOI] [PubMed] [Google Scholar]
- 64.Tanaka H., Hruska K.A., Seino Y., Malone J.D., Nishii Y., Teitelbaum S.L. Disassociation of the macrophage-maturational effects of vitamin D from respiratory burst priming. J. Biol. Chem. 1991;266:10888–10892. [PubMed] [Google Scholar]
- 65.Bozonet S.M., Carr A.C. The role of physiological vitamin C concentrations on key functions of neutrophils isolated from healthy individuals. Nutrients. 2019;11:1363. doi: 10.3390/nu11061363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu D., Meydani S.N. Age-associated changes in immune function: impact of vitamin E intervention and the underlying mechanisms. Endocr Metab Immune Disord Drug Targets. 2014;14:283–289. doi: 10.2174/1871530314666140922143950. [DOI] [PubMed] [Google Scholar]
- 67.Gao H., Dai W., Zhao L., Min J., Wang F. The role of zinc and zinc homeostasis in macrophage function. J Immunol Res. 2018;2018:6872621. doi: 10.1155/2018/6872621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sheikh A., Shamsuzzaman S., Ahmad S.M., Nasrin D., Nahar S., Alam M.M., Al Tarique A., Begum Y.A., Qadri S.S., Chowdhury M.I., Saha A., Larson C.P., Qadri F. Zinc influences innate immune responses in children with enterotoxigenic Escherichia coli-induced diarrhea. J. Nutr. Biochem. 2010;140:1049–1056. doi: 10.3945/jn.109.111492. [DOI] [PubMed] [Google Scholar]
- 69.Agoro R., Taleb M., Quesniaux V.F.J., Mura C. Cell iron status influences macrophage polarization. PLoS One. 2018;13:e0196921. doi: 10.1371/journal.pone.0196921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Besold A.N., Culbertson E.M., Culotta V.C. The Yin and Yang of copper during infection. J. Biol. Inorg. Chem. 2016;21:137–144. doi: 10.1007/s00775-016-1335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saeed F., Nadeem M., Ahmed R.S., Tahir Nadeem M., Arshad M.S., Ullah A. Studying the impact of nutritional immunology underlying the modulation of immune responses by nutritional compounds – a review. Food Agric. Immunol. 2016;27:205–229. doi: 10.1080/09540105.2015.1079600. [DOI] [Google Scholar]
- 72.Petrović J., Stanić D., Dmitrašinović G., Plećaš-Solarović B., Ignjatović S., Batinić B., Popović D., Pešić V. Magnesium supplementation diminishes peripheral blood lymphocyte DNA oxidative damage in athletes and sedentary young man. Oxidative Med. Cell. Longev. 2016;(2016) doi: 10.1155/2016/2019643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laires M.J., Monteiro C. Exercise, magnesium and immune function. Magnes. Res. 2008;21:92–96. [PubMed] [Google Scholar]
- 74.Lin Z., Li W. The roles of vitamin D and its analogs in inflammatory diseases. Curr. Top. Med. Chem. 2016;16:1242–1261. doi: 10.2174/1568026615666150915111557. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y., Leung D.Y.M., Richers B.N., Liu Y., Remigio L.K., Riches D.W., Goleva E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 2012;188:2127–2135. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Topilski I., Flaishon L., Naveh Y., Harmelin A., Levo Y., Shachar I. The anti-inflammatory effects of 1,25-dihydroxyvitamin D3 on Th2 cells in vivo are due in part to the control of integrin-mediated T lymphocyte homing. Eur. J. Immunol. 2004;34:1068–1076. doi: 10.1002/eji.200324532. [DOI] [PubMed] [Google Scholar]
- 77.Wintergerst E.S., Maggini S., Hornig D.H. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann. Nutr. Metab. 2006;50:85–94. doi: 10.1159/000090495. [DOI] [PubMed] [Google Scholar]
- 78.Lee G.Y., Han S.N. The role of vitamin E in immunity. Nutrients. 2018;10:1614. doi: 10.3390/nu10111614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sakakeeny L., Roubenoff R., Obin M., Fontes J.D., Benjamin E.J., Bujanover Y., Jacques P.F., Selhub J. Plasma pyridoxal-5-phosphate is inversely associated with systemic markers of inflammation in a population of U.S. adults. J. Nutr. Biochem. 2012;142:1280–1285. doi: 10.3945/jn.111.153056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ueland P.M., McCann A., Midttun Ø., Ulvik A. Inflammation, vitamin B6 and related pathways. Mol. Asp. Med. 2017;53:10–27. doi: 10.1016/j.mam.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 81.Jarosz M., Olbert M., Wyszogrodzka G., Młyniec K., Librowski T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. 2017;25:11–24. doi: 10.1007/s10787-017-0309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Foster M., Samman S. Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients. 2012;4:676–694. doi: 10.3390/nu4070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wessels I., Rink L. Micronutrients in autoimmune diseases: possible therapeutic benefits of zinc and vitamin D. J. Nutr. Biochem. 2020;77 doi: 10.1016/j.jnutbio.2019.108240. [DOI] [PubMed] [Google Scholar]
- 84.Alpert P.T. The role of vitamins and minerals on the immune system. Home Health Care Manag. Pract. 2017;29:199–202. doi: 10.1177/1084822317713300. [DOI] [Google Scholar]
- 85.Bussière F.I., Mazur A., Fauquert J.L., Labbe A., Rayssiguier Y., Tridon A. High magnesium concentration in vitro decreases human leukocyte activation. Magnes. Res. 2002;15:43–48. [PubMed] [Google Scholar]
- 86.Ross A.C. Vitamin A and retinoic acid in T cell-related immunity. Am. J. Clin. Nutr. 2012;96:1166s–1172s. doi: 10.3945/ajcn.112.034637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sigmundsdottir H., Pan J., Debes G.F., Alt C., Habtezion A., Soler D., Butcher E.C. DCs metabolize sunlight-induced vitamin D3 to 'program' T cell attraction to the epidermal chemokine CCL27. Nat. Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 88.Cantorna M.T., Snyder L., Lin Y.-D., Yang L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients. 2015;7:3011–3021. doi: 10.3390/nu7043011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Penna G., Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 90.Piemonti L., Monti P., Sironi M., Fraticelli P., Leone B.E., Dal Cin E., Allavena P., Di Carlo V. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J. Immunol. 2000;164:4443–4451. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 91.Bscheider M., Butcher E.C. Vitamin D immunoregulation through dendritic cells. Immunology. 2016;148:227–236. doi: 10.1111/imm.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kitabayashi C., Fukada T., Kanamoto M., Ohashi W., Hojyo S., Atsumi T., Ueda N., Azuma I., Hirota H., Murakami M., Hirano T. Zinc suppresses Th17 development via inhibition of STAT3 activation. Int. Immunol. 2010;22:375–386. doi: 10.1093/intimm/dxq017. [DOI] [PubMed] [Google Scholar]
- 93.Maywald M., Wang F., Rink L. Zinc supplementation plays a crucial role in T helper 9 differentiation in allogeneic immune reactions and non-activated T cells. J. Trace Elem. Med. Biol. 2018;50:482–488. doi: 10.1016/j.jtemb.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 94.Wintergerst E.S., Maggini S., Hornig D.H. Contribution of selected vitamins and trace elements to immune function. Ann. Nutr. Metab. 2007;51:301–323. doi: 10.1159/000107673. [DOI] [PubMed] [Google Scholar]
- 95.Hurwitz B.E., Klaus J.R., Llabre M.M., Gonzalez A., Lawrence P.J., Maher K.J., Greeson J.M., Baum M.K., Shor-Posner G., Skyler J.S., Schneiderman N. Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: a randomized controlled trial. Arch. Intern. Med. 2007;167:148–154. doi: 10.1001/archinte.167.2.148. [DOI] [PubMed] [Google Scholar]
- 96.Han S.N., Adolfsson O., Lee C.K., Prolla T.A., Ordovas J., Meydani S.N. Vitamin E and gene expression in immune cells. Ann. N. Y. Acad. Sci. 2004;1031:96–101. doi: 10.1196/annals.1331.010. [DOI] [PubMed] [Google Scholar]
- 97.Shankar A.H., Prasad A.S. Zinc and immune function: the biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998;68:447s–463s. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 98.Rink L., Kirchner H. Zinc-altered immune function and cytokine production. J. Nutr. 2000;130:1407s–1411s. doi: 10.1093/jn/130.5.1407S. [DOI] [PubMed] [Google Scholar]
- 99.Wang T.T., Nestel F.P., Bourdeau V., Nagai Y., Wang Q., Liao J., Tavera-Mendoza L., Lin R., Hanrahan J.W., Mader S., White J.H. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 100.Gombart A.F., Borregaard N., Koeffler H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 101.Weber G., Heilborn J.D., Chamorro Jimenez C.I., Hammarsjo A., Törmä H., Stahle M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J. Invest. Dermatol. 2005;124:1080–1082. doi: 10.1111/j.0022-202X.2005.23687.x. [DOI] [PubMed] [Google Scholar]
- 102.Gombart A.F. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4:1151–1165. doi: 10.2217/fmb.09.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hansdottir S., Monick M.M., Lovan N., Powers L., Gerke A., Hunninghake G.W. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J. Immunol. 2010;184:965–974. doi: 10.4049/jimmunol.0902840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hughes D.A., Norton R. Vitamin D and respiratory health. Clin. Exp. Immunol. 2009;158:20–25. doi: 10.1111/j.1365-2249.2009.04001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hansdottir S., Monick M.M., Hinde S.L., Lovan N., Look D.C., Hunninghake G.W. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J. Immunol. 2008;181:7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Laird E., Kenny R.A. Vitamin D deficiency in Ireland –implications for COVID-19. Results from the Irish Longitudinal Study on Ageing (TILDA) Irish Longitud. Study Age. 2020 doi: 10.38018/TildaRe.2020-05. [DOI] [Google Scholar]
- 107.Prentice S. They are what you eat: can nutritional factors during gestation and early infancy modulate the neonatal immune response? Front. Immunol. 2017;8:1641. doi: 10.3389/fimmu.2017.01641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cannell J.J., Vieth R., Umhau J.C., Holick M.F., Grant W.B., Madronich S., Garland C.F., Giovannucci E. Epidemic influenza and vitamin D. Epidemiol. Infect. 2006;134:1129–1140. doi: 10.1017/s0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jat K.R. Vitamin D deficiency and lower respiratory tract infections in children: a systematic review and meta-analysis of observational studies. Trop. Dr. 2017;47:77–84. doi: 10.1177/0049475516644141. [DOI] [PubMed] [Google Scholar]
- 110.Caplan M. In: Scientific Review: The Role of Nutrients in Immune Function of Infants and Young Children Emerging Evidence for Long-chain Polyunsaturated Fatty Acids. Calder P., Prescott S., editors. 2007. [Google Scholar]
- 111.Roth D.E., Jones A.B., Prosser C., Robinson J.L., Vohra S. Vitamin D receptor polymorphisms and the risk of acute lower respiratory tract infection in early childhood. J. Infect. Dis. 2008;197:676–680. doi: 10.1086/527488. [DOI] [PubMed] [Google Scholar]
- 112.Zosky G.R., Berry L.J., Elliot J.G., James A.L., Gorman S., Hart P.H. Vitamin D deficiency causes deficits in lung function and alters lung structure. Am. J. Respir. Crit. Care Med. 2011;183:1336–1343. doi: 10.1164/rccm.201010-1596OC. [DOI] [PubMed] [Google Scholar]
- 113.Beyhan-Sagmen S., Baykan O., Balcan B., Ceyhan B. Association between severe vitamin D deficiency, lung function and asthma control. Arch. Bronconeumol. 2017;53:186–191. doi: 10.1016/j.arbres.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 114.Bener A., Ehlayel M.S., Bener H.Z., Hamid Q. The impact of Vitamin D deficiency on asthma, allergic rhinitis and wheezing in children: an emerging public health problem. J. Fam. Community Med. 2014;21:154–161. doi: 10.4103/2230-8229.142967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hejazi M.E., Modarresi-Ghazani F., Entezari-Maleki T. A review of Vitamin D effects on common respiratory diseases: asthma, chronic obstructive pulmonary disease, and tuberculosis. J. Res. Pharm. Pract. 2016;5:7–15. doi: 10.4103/2279-042X.176542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P., Dubnov-Raz G., Esposito S., Ganmaa D., Ginde A.A., Goodall E.C., Grant C.C., Griffiths C.J., Janssens W., Laaksi I., Manaseki-Holland S., Mauger D., Murdoch D.R., Neale R., Rees J.R., Simpson S., Jr., Stelmach I., Kumar G.T., Urashima M., Camargo C.A., Jr. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356 doi: 10.1136/bmj.i6583. i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hemilä H. Vitamin C and infections. Nutrients. 2017;9:339. doi: 10.3390/nu9040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hemila H., Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst. Rev. 2013 doi: 10.1002/14651858.CD005532.pub3. [DOI] [PubMed] [Google Scholar]
- 119.Cai Y., Li Y.F., Tang L.P., Tsoi B., Chen M., Chen H., Chen X.M., Tan R.R., Kurihara H., He R.R. A new mechanism of vitamin C effects on A/FM/1/47(H1N1) virus-induced pneumonia in restraint-stressed mice. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/675149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim H., Jang M., Kim Y., Choi J., Jeon J., Kim J., Hwang Y.I., Kang J.S., Lee W.J. Red ginseng and vitamin C increase immune cell activity and decrease lung inflammation induced by influenza A virus/H1N1 infection. J. Pharm. Pharmacol. 2016;68:406–420. doi: 10.1111/jphp.12529. [DOI] [PubMed] [Google Scholar]
- 121.Hemilä H., Chalker E. Vitamin C can shorten the length of stay in the ICU: a meta-analysis. Nutrients. 2019;11 doi: 10.3390/nu11040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fowler III A.A., Kim C., Lepler L., Malhotra R., Debesa O., Natarajan R., Fisher B.J., Syed A., DeWilde C., Priday A., Kasirajan V. Intravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome. World J. Crit. Care Med. 2017;6:85–90. doi: 10.5492/wjccm.v6.i1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang Z., Liu Y., Qi G., Brand D., Zheng S.G. Role of vitamin A in the immune system. J. Clin. Med. 2018;7 doi: 10.3390/jcm7090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Levy M., Thaiss C.A., Elinav E. Metabolites: messengers between the microbiota and the immune system. Genes Dev. 2016;30:1589–1597. doi: 10.1101/gad.284091.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sirisinha S. The pleiotropic role of vitamin A in regulating mucosal immunity. Asian Pac. J. Allergy Immunol. 2015;33:71–89. [PubMed] [Google Scholar]
- 126.Calder P.C. Feeding the immune system. Proc. Nutr. Soc. 2013;72:299–309. doi: 10.1017/s0029665113001286. [DOI] [PubMed] [Google Scholar]
- 127.Ross A.C. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2014. Modern Nutrition in Health and Disease. [Google Scholar]
- 128.McGill J.L., Kelly S.M., Guerra-Maupome M., Winkley E., Henningson J., Narasimhan B., Sacco R.E. Vitamin A deficiency impairs the immune response to intranasal vaccination and RSV infection in neonatal calves. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-51684-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xing Y., Sheng K., Xiao X., Li J., Wei H., Liu L., Zhou W., Tong X. Vitamin A deficiency is associated with severe Mycoplasma pneumoniae pneumonia in children. Ann. Transl. Med. 2020;8:120. doi: 10.21037/atm.2020.02.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fan B.E., Lim K.G.E., Chong C.L., Chan S.S.W., Ong K.H., Kuperan P. COVID-19 and mycoplasma pneumoniae coinfection. Am. J. Hematol. 2020 doi: 10.1002/ajh.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.D S.R. In: Nestlé Nutrition Workshop Series Pediatric Program. Pettifor J.M., Zlotkin S., editors. Nestec Ltd.; Basel: 2004. Micronutrient deficiencies during the weaning period and the first years of life; pp. 137–152. [DOI] [Google Scholar]
- 132.Center M.I. In: Immunity In Brief. L.P. Institute, editor. 2017. [Google Scholar]
- 133.Traber M.G., Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mileva M., Galabov A.S. 2018. Vitamin E and Influenza Virus Infection. [Google Scholar]
- 135.Han S.N., Wu D., Ha W.K., Beharka A., Smith D.E., Bender B.S., Meydani S.N. Vitamin E supplementation increases T helper 1 cytokine production in old mice infected with influenza virus. Immunology. 2000;100:487–493. doi: 10.1046/j.1365-2567.2000.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Beck M.A. Selenium and vitamin E status: impact on viral pathogenicity. J. Nutr. 2007;137:1338–1340. doi: 10.1093/jn/137.5.1338. [DOI] [PubMed] [Google Scholar]
- 137.De la Fuente M., Hernanz A., Guayerbas N., Victor M., Arnalich F. Vitamin E ingestion improves several immune functions in elderly men and women. Free Radic. Res. 2008;42:272–280. doi: 10.1080/10715760801898838. [DOI] [PubMed] [Google Scholar]
- 138.Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Adv. Nutr. 2019;10:696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chasapis C.T., Ntoupa P.A., Spiliopoulou C.A., Stefanidou M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020;94:1443–1460. doi: 10.1007/s00204-020-02702-9. [DOI] [PubMed] [Google Scholar]
- 140.Suara R.O., Crowe J.E., Jr. Effect of zinc salts on respiratory syncytial virus replication. Antimicrob. Agents Chemother. 2004;48:783–790. doi: 10.1128/aac.48.3.783-790.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ghaffari H., Tavakoli A., Moradi A., Tabarraei A., Bokharaei-Salim F., Zahmatkeshan M., Farahmand M., Javanmard D., Kiani S.J., Esghaei M., Pirhajati-Mahabadi V., Monavari S.H., Ataei-Pirkooh A. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. J. Biomed. Sci. 2019;26 doi: 10.1186/s12929-019-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chasapis C.T., Ntoupa P.A., Spiliopoulou C.A., Stefanidou M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020;94:1443–1460. doi: 10.1007/s00204-020-02702-9. [DOI] [PubMed] [Google Scholar]
- 143.Savino W., Dardenne M. Nutritional imbalances and infections affect the thymus: consequences on T-cell-mediated immune responses. Proc. Nutr. Soc. 2010;69:636–643. doi: 10.1017/s0029665110002545. [DOI] [PubMed] [Google Scholar]
- 144.Sandström B., Cederblad A., Lindblad B.S., Lönnerdal B. Acrodermatitis enteropathica, zinc metabolism, copper status, and immune function. Arch. Pediatr. Adolesc. Med. 1994;148:980–985. doi: 10.1001/archpedi.1994.02170090094017. [DOI] [PubMed] [Google Scholar]
- 145.Hulisz D. Efficacy of zinc against common cold viruses: an overview. J. Am. Pharm. Assoc. 2003;44(2004):594–603. doi: 10.1331/1544-3191.44.5.594.hulisz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hemilä H. Zinc lozenges may shorten the duration of colds: a systematic review. Open Respir. Med. J. 2011;5:51–58. doi: 10.2174/1874306401105010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Barnett J.B., Dao M.C., Hamer D.H., Kandel R., Brandeis G., Wu D., Dallal G.E., Jacques P.F., Schreiber R., Kong E., Meydani S.N. Effect of zinc supplementation on serum zinc concentration and T cell proliferation in nursing home elderly: a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2016;103:942–951. doi: 10.3945/ajcn.115.115188. [DOI] [PubMed] [Google Scholar]
- 148.Rayman M.P. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 149.Steinbrenner H., Al-Quraishy S., Dkhil M.A., Wunderlich F., Sies H. Dietary selenium in adjuvant therapy of viral and bacterial infections. Adv. Nutr. 2015;6:73–82. doi: 10.3945/an.114.007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nelson H.K., Shi Q., Van Dael P., Schiffrin E.J., Blum S., Barclay D., Levander O.A., Beck M.A. Host nutritional selenium status as a driving force for influenza virus mutations. FASEB J. 2001;15:1846–1848. doi: 10.1096/fj.01-0115fje. [DOI] [PubMed] [Google Scholar]
- 151.Guillin O.M., Vindry C., Ohlmann T., Chavatte L. Selenium, selenoproteins and viral infection. Nutrients. 2019;11 doi: 10.3390/nu11092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shojadoost B., Kulkarni R.R., Yitbarek A., Laursen A., Taha-Abdelaziz K., Negash Alkie T., Barjesteh N., Quinteiro-Filho W.M., Smith T.K., Sharif S. Dietary selenium supplementation enhances antiviral immunity in chickens challenged with low pathogenic avian influenza virus subtype H9N2. Vet. Immunol. Immunopathol. 2019;207:62–68. doi: 10.1016/j.vetimm.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 153.Chaigne-Delalande B., Li F.Y., O'Connor G.M., Lukacs M.J., Jiang P., Zheng L., Shatzer A., Biancalana M., Pittaluga S., Matthews H.F., Jancel T.J., Bleesing J.J., Marsh R.A., Kuijpers T.W., Nichols K.E., Lucas C.L., Nagpal S., Mehmet H., Su H.C., Cohen J.I., Uzel G., Lenardo M.J. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science. 2013;341:186–191. doi: 10.1126/science.1240094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Johnson S. The multifaceted and widespread pathology of magnesium deficiency. Med. Hypotheses. 2001;56:163–170. doi: 10.1054/mehy.2000.1133. [DOI] [PubMed] [Google Scholar]
- 155.Nielsen F.H. Magnesium deficiency and increased inflammation: current perspectives. J. Inflamm. Res. 2018;11:25–34. doi: 10.2147/JIR.S136742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Landon R.A., Young E.A. Role of magnesium in regulation of lung function. J. Am. Diet. Assoc. 1993;93:674–677. doi: 10.1016/0002-8223(93)91675-g. [DOI] [PubMed] [Google Scholar]
- 157.Janssen R. Magnesium to counteract elastin degradation and vascular calcification in chronic obstructive pulmonary disease. Med. Hypotheses. 2017;107:74–77. doi: 10.1016/j.mehy.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 158.Mahmoud Abd A., El Hafiz L., Mohammed El Wakeel H., Mohammed El Hady A.E.R. Mourad. High dose N-acetyl cysteine improves inflammatory response and outcome in patients with COPD exacerbations. Egypt. J. Chest Dis. Tuberc. 2013;62:51–57. doi: 10.1016/j.ejcdt.2013.02.012. [DOI] [Google Scholar]
- 159.Dick C.A., Brown D.M., Donaldson K., Stone V. The role of free radicals in the toxic and inflammatory effects of four different ultrafine particle types. Inhal. Toxicol. 2003;15:39–52. doi: 10.1080/08958370304454. [DOI] [PubMed] [Google Scholar]
- 160.Mata M., Sarrion I., Armengot M., Carda C., Martinez I., Melero J.A., Cortijo J. Respiratory syncytial virus inhibits ciliagenesis in differentiated normal human bronchial epithelial cells: effectiveness of N-acetylcysteine. PLoS One. 2012;7 doi: 10.1371/journal.pone.0048037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ho W.Z., Douglas S.D. Glutathione and N-acetylcysteine suppression of human immunodeficiency virus replication in human monocyte/macrophages in vitro. AIDS Res. Hum. Retrovir. 1992;8:1249–1253. doi: 10.1089/aid.1992.8.1249. [DOI] [PubMed] [Google Scholar]
- 162.Garozzo A., Tempera G., Ungheri D., Timpanaro R., Castro A. N-acetylcysteine synergizes with oseltamivir in protecting mice from lethal influenza infection. Int. J. Immunopathol. Pharmacol. 2007;20:349–354. doi: 10.1177/039463200702000215. [DOI] [PubMed] [Google Scholar]
- 163.Zhang Q., Ju Y., Ma Y., Wang T. N-acetylcysteine improves oxidative stress and inflammatory response in patients with community acquired pneumonia: A randomized controlled trial. Medicine (Baltimore) 2018;97 doi: 10.1097/MD.0000000000013087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.De Flora S., Grassi C., Carati L. Attenuation of influenza-like symptomatology and improvement of cell-mediated immunity with long-term N-acetylcysteine treatment. Eur. Respir. J. 1997;10:1535–1541. doi: 10.1183/09031936.97.10071535. [DOI] [PubMed] [Google Scholar]
- 165.Cory H., Passarelli S., Szeto J., Tamez M., Mattei J. The role of polyphenols in human health and food systems: a mini-review. Front. Nutr. 2018;5:87. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lin S.C., Ho C.T., Chuo W.H., Li S., Wang T.T., Lin C.C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017;17 doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Catel-Ferreira M., Tnani H., Hellio C., Cosette P., Lebrun L. Antiviral effects of polyphenols: Development of bio-based cleaning wipes and filters. J. Virol. Methods. 2015;212:1–7. doi: 10.1016/j.jviromet.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 168.Balachandar V., Mahalaxmi I., Kaavya J., Vivekanandhan G., Ajithkumar S., Arul N., Singaravelu G., Senthil Kumar N., Dev S. Mohana. COVID-19: emerging protective measures. Eur. Rev. Med. Pharmacol. Sci. 2020;24:3422–3425. doi: 10.26355/eurrev_202003_20713. [DOI] [PubMed] [Google Scholar]
- 169.Sonam B., Sabeena G., Arnica L.F., Shaminder S. 2020. Battle Against Coronavirus: Repurposing Old Friends (Food Borne Polyphenols) for New Enemy (COVID-19) [Google Scholar]
- 170.Li Y.Q., Li Z.L., Zhao W.J., Wen R.X., Meng Q.W., Zeng Y. Synthesis of stilbene derivatives with inhibition of SARS coronavirus replication. Eur. J. Med. Chem. 2006;41:1084–1089. doi: 10.1016/j.ejmech.2006.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jo S., Kim H., Kim S., Shin D.H., Kim M.-S. Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors. Chem. Biol. Drug Des. 2019;94:2023–2030. doi: 10.1111/cbdd.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Park H.R., Yoon H., Kim M.K., Lee S.D., Chong Y. Synthesis and antiviral evaluation of 7-O-arylmethylquercetin derivatives against SARS-associated coronavirus (SCV) and hepatitis C virus (HCV) Arch. Pharm. Res. 2012;35:77–85. doi: 10.1007/s12272-012-0108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vazquez-Calvo A., Jimenez de Oya N., Martin-Acebes M.A., Garcia-Moruno E., Saiz J.C. antiviral properties of the natural polyphenols delphinidin and epigallocatechin gallate against the flaviviruses West Nile virus, zika virus, and dengue virus. Front. Microbiol. 2017;8:1314. doi: 10.3389/fmicb.2017.01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sokmen M., Angelova M., Krumova E., Pashova S., Ivancheva S., Sokmen A., Serkedjieva J. In vitro antioxidant activity of polyphenol extracts with antiviral properties from Geranium sanguineum L. Life Sci. 2005;76:2981–2993. doi: 10.1016/j.lfs.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 175.Iyer M., Jayaramayya K., Subramaniam M.D., Lee S.B., Dayem A.A., Cho S.G., Vellingiri B. COVID-19: an update on diagnostic and therapeutic approaches. BMB Rep. 2020;53:191–205. doi: 10.5483/BMBRep.2020.53.4.080. [DOI] [PMC free article] [PubMed] [Google Scholar]