Abstract
Introduction
Children account for a relatively small proportion of laboratory-confirmed SARS-CoV-2 infections. In children, COVID-19 usually has a relatively mild course. However, in rare cases, severe disorders can be observed, and clinical manifestations may differ from adults.
Purpose
The aim of this study is to analyse the frequency, clinical picture and outcome of COVID-19 in children based on the experience from the tertiary care centre and regional sanitary-epidemiological office.
Methods
We report a study regarding 106 cases of confirmed SARS-CoV-2 infection cases in PCR from a nasopharyngeal swab (age range 1-month – 17-years). In all cases, history was taken. In children who required hospital admission, physical examination and laboratory test were performed according to clinical indications.
Results
Twelve of the patients required admission to the hospital. The most common symptoms were anosmia and dysgeusia (75%) and headaches (49%) in outpatients and fever in hospitalised children (75%). Three children from the hospitalised group developed a severe course with increased inflammatory indexes. The clinical picture was more severe in younger children from the hospitalised group. Treatment options were regarded individually in all cases.
Conclusion
Our study is the first tour knowledge regarding the clinical course of COVID-19 in Polish children. In general, the clinical course of COVID-19 was mild with anosmia and dysgeusia as the most common symptoms. However, in hospitalised children, a severe progression of the disease and less typical signs as aplastic anaemia may be developed.
Keywords: COVID-19, Children, Clinical course, Anosmia, Dysgeusia, Aplastic anaemia
Introduction
At the end of 2019, a cluster of pneumonia cases was identified in Wuhan (Hubei Province, China), and a novel coronavirus was discovered as a causing factor [1]. The number of cases quickly increased with subsequent epidemic throughout China, followed by an increasing number of cases in other countries throughout the world. The World Health Organization defined the disease COVID-19 (coronavirus disease 2019) in February 2020. The virus causing COVID-19 is called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); initially referred to as 2019-nCoV (novel coronavirus) [2,3]. The pandemic was declared on 11 March 2020.
Between 31 December 2019 and 23 May 2020, there were 5 175 476 cases of COVID-19 (according to the definition from an affected country), including 338 039 deaths. Most cases so far have been reported in the United States (1 601 434), Brazil (330 890) and Russia (326 448). The highest number of deaths were in patients from the United States (96 007), the United Kingdom (36 393) and Italy (32 616) [4]. In Poland first case was reported on 4 March 2020 and until 31 May 2020, 23 686 cases with 1064 deaths were confirmed. Until 31 May 2020 in the region of Wielkopolska 2150 cases with 152 deaths were reported.
SARS-CoV-2 may spread from person to person by droplets from respiratory secretions. The infection may also occur through contact with an infected surface when the person touches the eyes, the nose or mouth subsequently. Although long-range airborne transmission of SARS-CoV-2 has not been verified, recommendations on airborne protections are generally recommended when aerosol-generating procedures are performed [5]. The incubation period of SARS-CoV-2 infection ranges from 1 to 14 days, mostly ranging from 3 to 7 days.
Children account for a relatively small proportion of laboratory-confirmed SARS-CoV-2 infections. In children, COVID-19 usually has a relatively mild course, which may be responsible for a lower number of diagnostic tests, since they may be performed less frequently. However, in rare cases, severe disorders can be observed, and clinical manifestations may differ from adults [[6], [7], [8], [9]].
The vast majority of cases were associated with household or community exposure; however, infections related to travel were also noticed. Healthcare-associated outbreaks have also been reported in children [[5], [6], [7], [8]].
The aim of this study is to analyse the frequency, clinical picture and outcome of COVID-19 in children based on the experience from the tertiary care centre and regional sanitary-epidemiological office.
Methods
The study was performed between 15 February and 31 May 2020. Due to the decision of local authorities starting from 10 March all day-care facilities including kindergartens, primary and secondary schools were closed in the region (and the whole country).
Outpatients were tested by Regional Sanitary-Epidemiological Station. The indications for the testing were: contact with the individual infected with SARS-CoV-2 (family members, health care professionals). Patients were visited at homes. In all cases history was taken, complaints were noted. If clinical indications were present, patients were referred to infectious diseases specialist for physical examination.
In all children, the nasopharyngeal swabs were taken, and test for SARS-CoV-2 by real-time polymerase chain reaction (RT-PCR) was performed at least seven days after the exposure. The test was performed in the Virological Laboratory of Sanitary-Epidemiological Station – CE IVD Bosphore Novel Coronavirus Detection Kit v2. (cut-off value 61.5 copies/mL for gen E; 193.5 copies/mL for gen ORF1ab).
Children with clinical suspicion of COVID-19 based on clinical symptoms including fever > 38 C, cough, dyspnea or respiratory infections were referred to a tertiary care centre. The department was designated for children with clinical suspicion or confirmed infections with SARS-CoV-2 (Department of Infectious Diseases and Child Neurology, Poznan University of Medical Sciences).
In all cases, a detailed history was taken, including potential exposure to SARS-CoV-2 and travel. Patient complaints were reported, detailed clinical examination was performed with respiratory precautions. Vital signs, including body temperature, respiratory rate, heart rate and oxygen saturation, were measured. The nasopharyngeal swabs were taken for RT-PCR. The tests were performed by Virological Laboratory of Sanitary-Epidemiological Station in Poznan; Laboratory of University Equipment Center of The Poznan University of Medical Sciences or Microbiological Laboratory of University Hospital of Lord's Transfiguration in Poznan. The test used: CE IVD Bosphore Novel Coronavirus Detection Kit v2. (cut-off value 61.5 copies/mL for gen E; 193.5 copies/mL for gen ORF1ab).
Patients admitted to hospital underwent further laboratory testing involving cellular blood count, blood gases, C-reactive protein (CRP), procalcitonin (PCT), lactate dehydrogenase (LDH), clinical chemistry parameters, high sensitive troponin-I (hsTnI), fibrinogen level, INR, D-dimer, creatinine kinase (CK). The laboratory parameters were evaluated using standard analysers. Chest X-ray and abdominal ultrasound were also performed according to clinical indications. Blood culture was taken in all patients with fever. RT-PCR test for respiratory syncytial virus and influenza virus type A and B were done in all inpatients (Cobas Liat system – Cobas Influenza A/B&RSV). In patients with respiratory tract involvement, PCR panel for additional respiratory pathogens was performed including adenovirus, rhinovirus, bocavirus, parainfluenza virus, coronavirus, Epstein-Barr virus, Mycoplasma pneumoniae, Staphylococcus aureus, Streptococcus pneumoniae.
The total number of 6115 children was tested (age range 3-months-17 years). Eight hundred ninety-one children were tested in the tertiary care centre, remaining 5224 at their homes. The study included all children undergoing tests for SARS-CoV-2 by PCR method between 15.02 and 31.05.2020 in Wielkopolska – the region in western Poland with a population of 3,498,733 (data from 31.12.2019).
Continuous data were presented as mean ± standard deviation (SD) and median. For frequency of categorical data ratio and the percentage was given. Fisher exact test was used for the analysis of categorical data. Detailed statistical analysis was not performed in the group of hospitalised children due to the small number of children in subgroups of patients.
Results
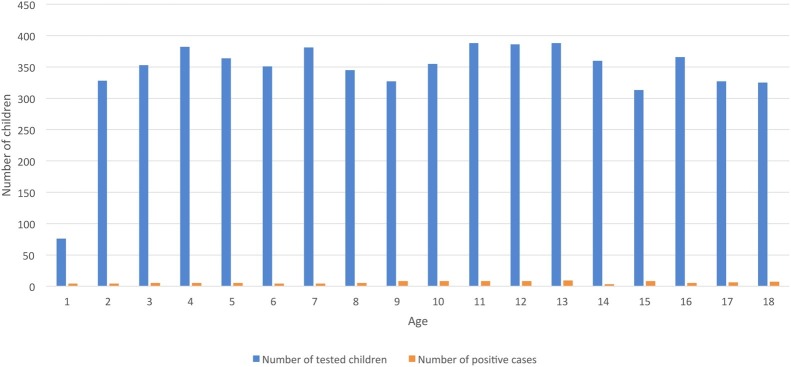
Out of 6115 tested children, 106 were found positive (1.73%), mean age 9.27 ± 4.89 years. The number of 106 pediatric SARS-CoV-2 infections accounts for 4.93% of all diagnosed cases in the region of Wielkopolska. Fig. 1 presents the age of children and the number of positive test outcomes. The tests were performed in all age groups, and positive results were evenly distributed.
Fig. 1.
The number of tested children and positive cases concerning the age.
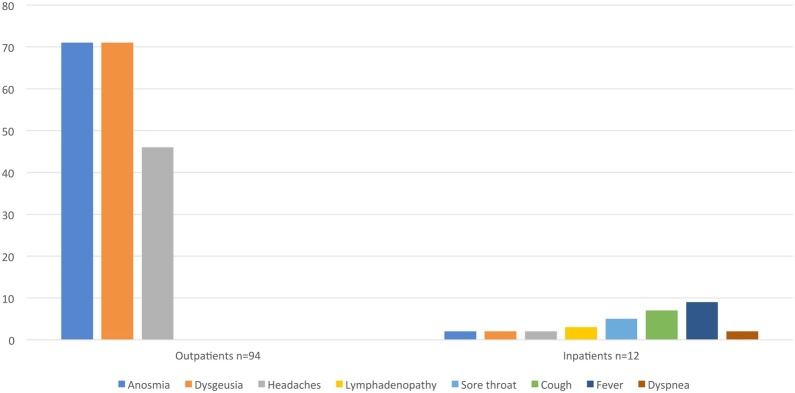
The most common clinical symptoms in the group of outpatients (94 children) included dysgeusia and anosmia, which were present in 71 patients (75.53 patients %) and headaches in 46 children (48.93%). Fig. 2 presents clinical symptoms in the study group.
Fig. 2.
The clinical symptoms in children with SARS-Cov-2 infection, n = 106.
Due to observed clinical symptoms, 891 children were referred to the hospital with a suspicion of COVID-19. Out of this number, the presence of SARS-CoV-2 RNA was confirmed in the nasopharyngeal swab in 12 children - age range 2–17 years (mean 8.15 ± 5.05 years). Contact with an infected family member was reported in 8 children (66,67%), two children travelled to other European countries (16,67%) – Austria and Hungary. In 4 children, underlying disorders were detected, including food allergy, Wilms tumour, acute lymphoblastic leukaemia, spinal cord dissection with hydrocephalus and epilepsy. Table 1 includes the clinical characteristic of hospitalised patients.
Table 1.
The characteristics of hospitalised children with COVID-19, n = 12.
| Parameter | Mean ± SD | Median | Minimum | Maximum |
|---|---|---|---|---|
| Age (years) | 8.79 ± 5.55 | 7 | 2 | 17 |
| Gender M/F | 6/6 | |||
| Contact/Travel/None | 8/2/2 | |||
| Fever Y/N | 9/12 | |||
| Duration of fever (days) | 2.89 ± 1.90 | 2 | 1 | 7 |
| spO2 (%) | 9.75 ± 7.34 | 98.5 | 75 | 100 |
| HR beats/min | 94.17 ± 21.20 | 89 | 65 | 140 |
| RR breath/min | 16 ± 4.5 | 15 | 12 | 35 |
| WBC (G/l) | 6.54 ± 3.79 | 6.38 | 0.61 | 12.47 |
| Neutrophils (G/l) | 3.23 ± 2.36 | 3.40 | 0.10 | 7.15 |
| Limphoctes (G/l) | 2.55 ± 1.70 | 2.29 | 0.38 | 6.44 |
| HGB (g/dl) | 12.53 ± 2.29 | 12.80 | 8.60 | 16.60 |
| PLT G/l | 238.7 ± 123.22 | 275.5 | 7 | 398 |
| CRP (mg/dl) | 2.317 ± 4.89 | 0.30 | 0.2 | 15.98 |
| PCT (ng/mL) | 0.15 ± 0.28 | 0.03 | 0.01 | 0.83 |
| LDH (IU/l) | 249.33 ± 47.53 | 263 | 165 | 318 |
| hsTnI (ng/mL) | 2.25 ± 1.26 | 1.7 | 1.1 | 4.5 |
| Kreat (mg/dl) | 0.41 ± 0.17 | 0.40 | 0.16 | 0.71 |
| Urea (mg/dl) | 23.3 ± 8.98 | 21 | 13 | 38 |
| ALT (IU/l) | 45 ± 73.64 | 16 | 11 | 247 |
| AST (IU/l) | 122 ± 304.33 | 25.5 | 16 | 988 |
| Na (mmol/l) | 136.2 ± 1.62 | 137 | 133 | 138 |
| K (mmol/l) | 4.16 ± 0.35 | 4.11 | 3.50 | 4.61 |
| CK (IU/l) | 119 ± 45s | 112.5 | 58 | 226 |
| Fibrinogen (mg/dl) | 276.2 ± 108.8 | 239 | 186 | 513 |
| INR | 1.17 ± 0.12 | 1.18 | 0.95 | 1.29 |
| APTT (s) | 30.62 ± 4.18 | 31.6 | 21.8 | 36.4 |
| D-dimer (mg/l) | 2.42 ± 2.88 | 1.20 | 0.21 | 7.54 |
| ATIII (%) | 100 ± 18 | 110 | 62 | 118 |
| Ferritin (ng/mL) | 3595.8 ± 3287.19 | 3595.8 | 1271.4 | 5920.2 |
| BNP (ng/mL) | 60.05 ± 35.42 | 60.05 | 35.00 | 85.1 |
| Il-6 (ng/mL) | 46.3 ± 49.07 | 46.3 | 11.6 | 81.0 |
Abbreviations: M- male, F – female, HR – heart rate, RR – respiratory rate, WBC – white blood count, HGB – haemoglobin, PLT platelets, CRP – c-reactive protein, PCT – procalcitonin, LDH – lactate dehydrogenase, hsTnI – high sensitive troponin I, AlT – alanine aminotransferase, AST - aspartate aminotransferase, Na – sodium, K – potassium, CK – creatinine kinase, INR – international normalized ratio, APTT – activated partial thromboplastin time, BNP- brain natriuretic peptide, Il-6 – interleukin 6.
The course of the disease was mild in 3 patients (25%) (mean age 13.67 ± 5.77 years; median 17 years), moderate in 6 patients (50%) (mean age 8.33 ± 5.46 years; median 7.5 years) and severe in 3 cases (25%) (mean age 4.83 ± 1.26 years; median 5 years).
The most common clinical symptoms in hospitalised children include fever in 9 (75%), cough in 7 (58,33%), sore throat in 5 (41,6%) and lymphadenopathy in 3 children (25%). Headaches, dysgeusia and anosmia, dyspnea were noted in 2 children. The fever lasted 2–7 days before admission to the hospital (Fig. 2). Anosmia and dysgeusia, as well as headaches, were significantly more common in outpatients (p = 0.0001 and 0.0345, respectively).
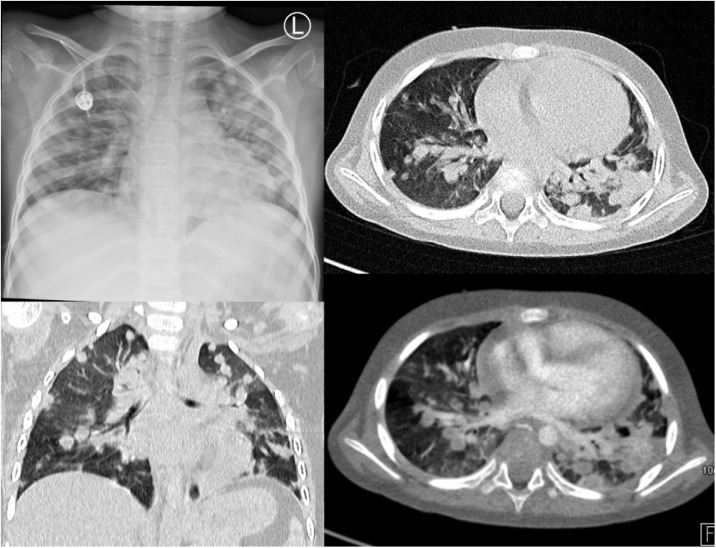
In children with respiratory symptoms, chest X-rays were performed, which were without abnormalities in 5 cases. Typical results included disseminated consolidations (2 cases) or interstitial pneumonia (2 cases). One patient had repeated CT scans revealing disseminated nodular changes with interstitial infiltrates and ground-glass opacities in both lungs (Fig. 3 ).
Fig. 3.
The chest X-ray (A) and CT scan (B,C,D) of a 5-year-old boy. In both lungs, numerous, dotted nodules are visible, the largest of 10-15 mm. Nodules merge in places and give a picture of consolidations; around nodules and interstitial lesions visible areas of ground-glass opacities. Besides, the banded interstitial consolidations and the thickening of the interlobular septa are visible. The lesions more severe in the left lung.
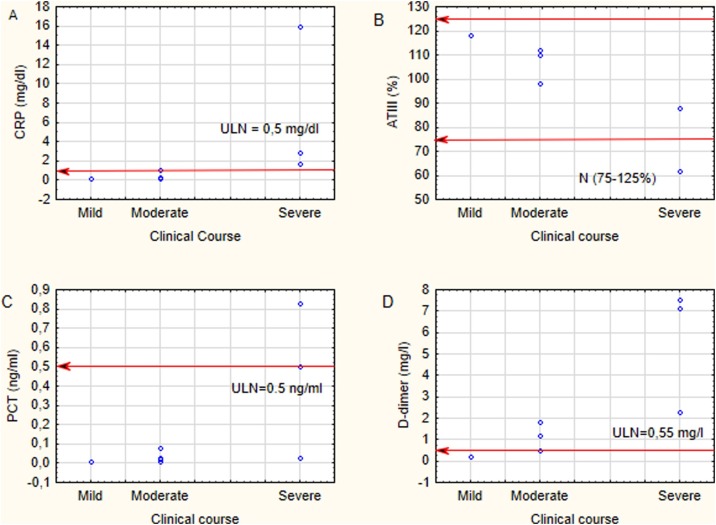
Laboratory findings were within reference values in the majority of children (Table 1), which was concurrent with a moderate course of infection. Children with a severe progression of disease developed higher inflammatory markers (CRP, PCT) and D-dimer, lower haemoglobin level (Fig. 4 ). In this group of patients, ferritin and interleukin-6 levels were tested and found significantly increased (Table 2 ).
Fig. 4.
The inflammatory markers – CRP (A), PCT (C) and D-dimer (B) and ATIII (D) in children regarding the clinical course of COVID-19.
Table 2.
The clinical picture of severe cases of COVID-19 in hospitalised children.
| Initials | Age | Gender | Underlying disorder | Epidemiology | Symptoms on admission | Laboratory abnormalities | SRS-CoV-2 positivity by PCR | Coinfection | Complication | Treatment | Duration of hospital treatment and outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LG | 3.5 | F | spinal dissection, hydrocephalus, ventriculoperitoneal shunt, epilepsy, neurogenic bladder | Household contact with an infected family member | Fever, cough, seizures Dyspnea |
CRP 15,98 mg/dl D-dimer 7,54 mg/l |
26 days | Streptococus pneumoniae in blood and respiratory secretion culture | Severe pneumonia | Cefotaxim (22 days)+ vancomycin (12 days) with alteration to meropenem on fever recurrence (10 days); azytromycin (15 days) | 30 days Recovery |
| MB | 5 | M | Acute lymphoblastic leukemia | None | Fever, cough, sore throat dyspnea |
WBC 0,61 G/l PLT 52 G/l ALT 77 IU/l, D-dimer 2,61 mg/l, Ferritin 1271,4 ng/mL, IL-6 81 ng/mL |
7 days however clinical symptoms persisted for 45 days | Epstein-Barr virus, Aspergillus spp. | Severe pneumonia, Encephalitis | Ceftriaxon + Piperacylin + tazobactam (16 days) altered to Cefepime + Vancomycin (23 days) Lopinavir + ritonavir, Amphotericin (21 days) altered to voriconazole (21 days) |
53 days Undergoing treatment of aspergillosis |
| HS | 6 | F | None | None | Fever, sore throat, petechiae | WBC 1,2 G/l PLT 5 G/l, ALT 247 IU/l, AST 98 IU/l, LDH 318 IU/l CRP Fibrinogen 422 mg/dl D-dimer 5,22 mg/l, Ferritin 5920,2 ng/mL, IL-6 11,6 ng/mL |
43 days | None | Aplastic anaemia | IVIG (2 g/kg) 2 x, metyprdnisolone, ceftriakson, azytromycine, Pipracylin + tazobactam, Lopinavir/ritonavir Convalescent serum |
56 days Scheduled for bone-marrow transplant |
Children with a moderate course of COVID-19 were diagnosed with the upper respiratory tract infection (4 patients) and bronchopneumonia (2 patients). In two patients, interstitial pneumonia was observed. No child with COVID-19 had coexistent respiratory syncytial virus or influenza virus infection. Single cases of Epstein-Barr virus, Streptococcus pneumoniae and Aspergillus spp. coinfections were diagnosed.
Clinical characteristic of cases with a severe course of COVID-19 was presented in Table 2. There were two cases of acute lower respiratory tract infection (one with CNS involvement). One child suffered from aplastic anaemia. In all patients, the treatment of coinfections was introduced according to the obtained results of the microbiological assessment. Azithromycin (10 mg/k for 10 days) was used in patients with a moderate and severe course of COVID-19. Administration of antiviral therapy was based on individual decisions regarding clinical findings. Two children were treated with lopinavir/ritonavir (lopinavir component 230 mg/m2). One received the regimen for 10 days. In the other, the regimen was withdrawn in the 3rd day due to maculopapular rash on the trunk and limbs.
The child with severe pneumonia and increased Il-6 level (81 ng/mL) was prepared to receive tocilizumab (antibody against the Il-6 receptor). However, the spontaneous decline of the parameter and improvement of the clinical course was observed. Therefore the drug was finally not introduced in this case. In one child with bone marrow aplasia treatment with convalescent serum was administered with the approval of the local Ethical Committee and favourable outcome [10].
No child required mechanical ventilation; no fatal cases were noted.
Discussion
According to available data, all age groups are susceptible to COVID-19, and there is no significant age difference. Children may also serve as reservoirs of viral transmission [7].
There is an increasing number of published reports concerning the clinical course of COVID-19 in children [[5], [6], [7], [8], [9]]. The spectrum of disease ranges from mild to severe. The most common course is relatively mild and does not involve an exaggerated immune response. The form associated with the febrile inflammatory state is less common. Usually, mild to moderate symptoms are observed, elevated inflammatory markers, but signs of multisystem involvement are lacking [[8], [9], [10], [11]].
Similar observations were made in our study group, where the majority of children who did not require admission to the hospital, developed anosmia and dysgeusia or headaches. In children from the hospitalised group, febrile illness was present as the most common symptom with signs of respiratory tract involvement. Inflammatory indexes were elevated proportionally to the severity of the clinical course with the highest values in children with severe complications of the disease.
The explanation for a less severe clinical course of COVID-19 in children compared to adults is not fully understood. Some hypotheses are explaining this phenomenon, including lower maturity and function of angiotensin-converting enzyme II (ACEII) in children, which is a receptor for SARS-CoV-2 [12]. Specific regulatory mechanisms in the respiratory immune system and cross-protection of antibodies against common viral infections may also play an important role. Moreover, general immaturity of the immune system of children may explain the lower potential to develop exaggerated immune response and cytokine storm [13].
On the other hand, severe cases are observed in various age groups of children with COVID-19. In our study patients with a severe course of the disease were 3.5, 5 and 6 years old (median 5). Although the groups were too small to perform statistical analysis, it was visible that moderate and mild progression was observed in older children (median 7.5 and 17 years old, respectively). Chinese data suggest the highest incidence of critical cases in infants (10.6%), children 1–5 years old (7.3%) with decreasing prevalence in older age groups [6,14]. These findings suggest that younger children are vulnerable to SARS-CoV-2 infection. Italian study reports 40% rate of hospital admissions in children below 1-year-old, which may also indicate higher tendency to seek medical help for infants and the higher propensity of clinicians to admit such children to hospitals [15].
Underlying illnesses may also play an essential role in the course of COVID-19 in children. In our group, two children with moderate and 2 with severe progression had underlying illnesses (33.33%). The same Italian study describes 33 children with underlying diseases in a group of 168 COVID-19 patients (19.6%) [15].
It also remains unclear how frequently children progress from mild to more severe manifestations, and what the risk factors are for such progression.
Our study is the first to our knowledge regarding the clinical course of COVID-19 in Polish children. The group of hospitalised children is relatively small and does not allow to perform detailed statistical analysis. Nevertheless, the study contains valuable information regarding a large group of outpatients and their symptoms. The clinical course of the disease in children was generally mild. Yet, the severe course of the disease and less typical signs as aplastic anaemia in a previously healthy child may be developed.
It has to be mentioned that other consequences related to SARS-CoV-2 infection in children were also described. An increasing number of complete or incomplete Kawasaki disease were reported in various countries during SARS-CoV-2 epidemics. Some patients do not develop shock and multisystem involvement. There is, however, a group of patients with markedly elevated inflammatory markers and multisystem involvement, including shock and cardiovascular complications. In this group of patients, PCR results from nasopharyngeal swabs may negative with the presence of anti-SARS-Cov-2 antibodies, which indicates that the virus triggers an immune response in the pathogenesis of the disease [[16], [17], [18]].
There is no established antiviral treatment of SARS-CoV-2 infection in children. Due to relatively mild disease progression in children, supportive therapy may be sufficient. Antiviral drugs without clear evidence of safety and efficiency are not recommended in pediatric patients. There may be, however, a strong need to introduce antiviral therapy in the severe progression of COVID-19 [19]. The use of remdesivir and tocilizumab in the treatment of SARS-CoV-2 infection in children requires further clinical trials [20]. In our study, a patient with persisting SARS-CoV-2 infection obtained convalescent serum with good clinical outcome [10].
The knowledge concerning the clinical spectrum of COVID-19 in children extends with the number of reported cases. Case definitions described so far may evolve in the future, as other reports are published. Since all aspects concerning COVID-19 are not fully elucidated, all reports add additional information regarding the subject.
Funding
No funding sources.
Conflict of interest
None declared.
Ethical approval
The study was approved by the Ethical Committee of the Poznan University of Medical Sciences No 298/20 and 376/20.
Informed consent
All legal guardians signed informed consent form to participate in the study.
Acknowledgements
Members of the team of Department of Infectious Diseases and Child Neurology: Małgorzata Borowiak, Zuzanna Lewandowska, Ewa Bućko, Paweł Małecki, Cezary Witczak, Kamil Faltin, Agnieszka Myszkowska-Torz, Agnieszka Cwalińska, Marta Budzyn, Karolina Partyka, Paweł Grobelski, Gabriela Gray, Valeria Hyrhoruk, nurses and students volounteers;
Prof. Danuta-Lewandowska-Januszkiewicz MD, PhD, Department of Pediatric Oncology, Hematology and Transplantology, Poznan University of Medical Sciences, Poland
References
- 1.World Health Organization. Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020. (Accessed 31 May 2020).
- 2.World Health Organization (WHO). WHO Director-General’s opening remarks at the media briefing on COVID-19 – 11 March 2020. Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020. (Accessed 30 May 2020).
- 3.Centers for Disease Control and Prevention . 2019. Novel coronavirus, Wuhan, China. Information for healthcare professionals.https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html [Google Scholar]
- 4.Polish National Institue of Health. COVID-19 Pandemia. The current situation for 23.05.2020.
- 5.Zimmermann P., Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39:355. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong Y., Mo X., Hu Y. Epidemiology of COVID-19 among children in China. Paediatrics. 2020 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 7.Lu X., Zhang L., Du H. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:1663. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 9.Rehman S., Majeed T., Azam Ansari M. Current Scenario of COVID-19 in Pediatric Age Group and Physiology of Immune and Thymus response. Saudi J Biol Sci. 2020;27:2567–2573. doi: 10.1016/j.sjbs.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figlerowicz M., Mania A., Lubarski K., Lewandowska Z., Służewski W., Derwich K. First case of convalescent plasma transfusion in a child with COVID-19-associated severe aplastic anemia. Transfus Apher Sci. 2020;59 doi: 10.1016/j.transci.2020.102866. 102866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmermann P., Curtis N. COVID-19 in children, pregnancy and neonates. Pediatric Infect Dis J. 2020;39(June (6)):469–477. doi: 10.1097/INF.0000000000002700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee P.I., Hu Y.L., Chen P.Y., Huang Y.C., Hsueh P.R. Are children less susceptible to COVID-19? J Microb Immunol Infect. 2020:S1684–1182. doi: 10.1016/j.jmii.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130(5):2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei M., Yuan J., Liu Y. Novel coronavirus infection in hospitalised infants under 1 year of age in China. JAMA. 2020 doi: 10.1001/jama.2020.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garazzino S., Montagniaani C., Dona D. Multicenter Italian study of SARS-CoV-2 infection in children and adolescents, preliminarydata as at 10 April 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.18.2000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CDC COVID-19 Response Team Coronavirus disease 2019 in children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godfred-Cato S., Bryant B., Leung J. COVID-19-Associated Multisystem Inflammatory Syndrome in Children - United States, March-July 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okarska-Napierała M., Ludwikowska K., Szenborn L. Pediatric Inflammatory Multisystem Syndrome (PIMS) Did Occur in Poland during Months with Low COVID-19 Prevalence. Preliminary Results of a Nationwide Register. J Clin Med. 2020;9:3386. doi: 10.20944/preprints202009.0435.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng H., Gao P., Xu Q. Coronavirus disease 2019 in children: characteristics, antimicrobial treatment, and outcomes 2020. J Clin Virol. 2020;128:104425. doi: 10.1016/j.jcv.2020.104425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko W.C., Rolain J.M., Lee N.Y. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105933. [DOI] [PMC free article] [PubMed] [Google Scholar]