Abstract
Background
Cancer services worldwide had to adapt in response to the COVID-19 pandemic to minimise risk to patients and staff. We aimed to assess the national impact of COVID-19 on the prescribing of systemic anticancer treatment in England, immediately after lockdown and after the introduction of new treatments to reduce patient risk.
Methods
We did a retrospective analysis using data from a central National Health Service England web database mandated for clinicians to register intention to start all new systemic anticancer treatments approved for use in England since 2016. We analysed the monthly number of treatment registrations in April, 2020, after the implementation of societal lockdown on March 23, 2020, and after implementation of treatment options to reduce patient risk such as oral or less immunosuppressive drugs, in May and June, 2020. We compared the number of registrations in April–June, 2020, with the mean number of registrations and SD during the previous 6 months of unaffected cancer care (September, 2019, to February, 2020). We calculated the percentage change and absolute difference in SD units for the number of registrations overall, by tumour type, and by type and line of therapy.
Findings
In April, 2020, 2969 registrations were recorded, representing 1417 fewer registrations than in the control period (monthly mean 4386; 32% reduction, absolute difference 4·2 SDs, p<0·0001). In May, 2020, total registrations increased to 3950, representing a 10% reduction compared with the control period (absolute difference 1·3 SDs, p<0·0001). In June, 2020, 5022 registrations were recorded, representing a 15% increase compared with the control period (absolute difference 1·9 SDs; p<0·0001]).
Interpretation
After the onset of the COVID-19 pandemic, there was a reduction in systemic anticancer treatment initiation in England. However, following introduction of treatment options to reduce patient risk, registrations began to increase in May, 2020, and reached higher numbers than the pre-pandemic mean in June, 2020, when other clinical and societal risk mitigation factors (such as telephone consultations, facemasks and physical distancing) are likely to have contributed. However, outcomes of providing less treatment or delaying treatment initiation, particularly for advanced cancers and neoadjuvant therapies, require continued assessment.
Funding
None.
Introduction
Since the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), health-care systems worldwide have adapted to manage the surge in hospital admissions due to COVID-19. Additionally, strategies have been implemented to reduce transmission of SARS-CoV-2 and protect patients and health-care workers.
Patients with cancer can be particularly susceptible to COVID-19 due to several factors, such as advanced age, comorbidities, and the biologically plausible negative effect of the immunosuppressive nature of oncological treatment and cancer itself on the host response to SARS-CoV-2 infection.1, 2 Additionally, relatively frequent clinic visits for treatment and assessments might increase the risk of SARS-CoV-2 exposure in these patients. Evidence on the effect of COVID-19 in patients with cancer has shown adverse outcomes in patients with cancer, such as higher mortality or higher probability of hospital admissions.3, 4, 5, 6, 7, 8, 9, 10, 11 The effect of systemic anticancer treatment on COVID-19 infection has not been fully recognised, although two large studies have reported no link between recent systemic anticancer treatment and increased mortality from COVID-19.12, 13
Many cancer services in England and elsewhere have undergone extensive changes to minimise COVID-19 exposure among patients with cancer and health-care staff. These changes have included delayed surgery and radiotherapy, fewer chemotherapy treatment cycles, reduced outpatient visits (often replaced with telephone assessments) and, where possible, switching from therapies that require intravenous administration to oral drugs. Diagnostic services have also been affected, with a clear reduction in cancer referrals.14, 15
Research in context.
Evidence before this study
Due to the rapid emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in England in 2020, little evidence is available on the use of systemic anticancer treatments during this time. Since the start of the COVID-19 pandemic, cancer services have been reduced to minimise viral exposure among patients with cancer (an inherently susceptible population shown to have poor outcomes following COVID-19 infection) and staff. We searched PubMed from database inception to Aug 30, 2020, for articles published in English on the impact of COVID-19 on patients with cancer using the search terms (“COVID-19” OR “SARS-CoV-2”) AND (“oncology” OR “cancer” OR “malignancy”). Studies have suggested increased risk of hospital admission and higher death rate among patients with cancer. Data published in June and August, 2020, have assessed the impact of systemic anticancer treatment on COVID-19 outcomes; however, evidence on the use and provision of systemic anticancer treatment during the pandemic is scarce.
Added value of this study
To our knowledge, this is the first study to assess how prescribing practice for anticancer treatments has changed at a national level since the pandemic began, based on all drugs approved by the National Institute for Health and Care Excellence since 2016. We found that the number of patients who started these systemic therapies substantially reduced in April, 2020, compared with a control period between September, 2019, and February, 2020, but began to increase in May, 2020. The initial reductions were largely reversed by June, 2020, when the number of treatment registrations was significantly higher than those observed during the control period. This pattern was observed for all intents of therapy, with the exception of neoadjuvant therapy, and for most cancer types. These findings can provide some reassurance to patients and clinicians that treatment delays can be minimised or avoided if health-care providers are able to quickly implement guidance on drug prescribing. Such guidance involves providing more treatment options via temporary approval of oral drug alternatives and the use of less immunosuppressive drugs in earlier lines of therapy, which are currently only licensed for use later in the treatment pathway. Cytotoxic chemotherapies approved before 2016 and hormonal therapies are not included in our findings, so we are unable to comment on their prescribing patterns.
Implications of all the available evidence
In England, which is a high-income country with an established health-care system, many patients did not start their expected systemic anticancer treatment in the early phase of the pandemic, although recovery of cancer therapy initiation in England was rapid. The impact of not prescribing or delaying systemic anticancer treatment on patient outcomes needs to be monitored in the forthcoming months, especially for non-curative therapies used for advanced disease (some of which only extend survival by a few months), and for neoadjuvant treatment. Considering the substantial impact estimated as a result of the second wave of COVID-19, health-care systems should implement measures to ensure that patients with cancer can safely start their systemic anticancer treatment on time.
Guidance from the UK National Health Service (NHS) and the National Institute for Health and Care Excellence (NICE) issued on March 20, 2020, aimed to aid clinical decision making by prioritising the need to retain systemic anticancer treatment services.16 Clinicians were advised to categorise patients into six priority levels on the basis of their cancer stage and treatment: patients having highly curative treatment were assigned the highest level (level 1), and those being treated with non-curative therapies with an intermediate or low chance of palliation were assigned the lowest level (level 6). Hospitals in England were tasked to rapidly translate this guidance into service provision. Considering the global nature of the pandemic and its effect on all health-care services, similar guidance had also been published elsewhere, including by the European Society of Medical Oncology.17
In this study, we aimed to assess the impact of COVID-19 on the initiation of systemic anticancer treatment at a national level, during the first peak of the pandemic in April, 2020, following societal lockdown on March 23, 2020, and after the NHS implemented additional and specific treatment options in April and May, 2020, to mitigate the risk of COVID-19 in patients requiring systemic anticancer treatment.
Methods
Data sources
We did a retrospective analysis using data from the NHS England Prior Approval system, which is a central web database mandated for clinicians to register intention for all patients commencing systemic anticancer treatment (except hormone therapies) for all drug indications recommended by NICE since April, 2016. For each patient, clinicians select the appropriate indication, which includes cancer type, drug name, specific line of therapy in the treatment pathway, and mode of administration (oral or intravenous). We extracted all available data for the period April 1, 2019, to June 30, 2020, and one author (JJC) used the indication descriptions to categorise intent of therapy (curative or non-curative intent for advanced disease, and neoadjuvant or adjuvant intent for early stage disease), which was confirmed by a second clinician (PC). All currently approved indications, including all treatment criteria that have to be satisfied for each indication, and the new COVID-19-specific measures are included in the National Cancer Drug Fund list.
We classified all immunotherapies for solid tumours as non-curative in intent unless these were delivered with the specific indications of maintenance therapy (eg, the potentially curative treatment of durvalumab following radical chemoradiotherapy in lung cancer) or as adjuvant therapy in melanoma. The NHS England Prior Approval system captures all immunotherapies, all monoclonal antibodies (excluding rituximab and trastuzumab), all antibody–drug conjugates, and the majority of approved tyrosine kinase inhibitors. Most cytotoxic chemotherapies are not included in the system because they were approved before 2016.
Data analysis
The main study outcome was the number of systemic anticancer treatment registrations recorded per month. In England, societal lockdown was formalised on March 23, 2020; therefore registrations during March reflect both normal use and the start of the impact of the pandemic in the health-care system. Thus, we extracted the number of registrations recorded per calendar month in April, May, and June, 2020. Sept 1, 2019, to Feb 29, 2020, was selected as the control period (ie, the 6 months preceding April, 2020, that were unaffected by the pandemic) because these months were the closest in chronological time. The use of April to June, 2019, as the control period would have been inappropriate because the number of systemic anticancer treatment registrations is affected by the total number of newly approved drug indications, which differs for a particular month, from year to year. However, we also extracted data for the period of April 1 to June 30, 2019, to provide a secondary comparison.
We calculated the mean number of monthly registrations and accompanying SDs for the control period. The mean value was an appropriate comparator because the observed monthly number was stable during this period. We compared the mean number of monthly registrations in the control period with the number of registrations recorded per calendar month in April, May, and June 2020, to calculate relative changes and absolute differences. We analysed data for April, May, and June, 2020, separately because we had no expectations about any patterns and wanted to examine each month, and not fit trends. We calculated the relative reduction in monthly registrations as the percentage change. The absolute difference was calculated as the difference in SDs from the monthly mean for the control period, to reflect the usual variability between the control months and to provide a standardised parameter considering that the number of registrations varies by tumour type, and line and type of therapy. A difference greater than 2 SDs from the control value was considered to indicate a large absolute difference. We used the χ2 test to compare differences in registration counts for April, May, and June, 2020, with the control period. A p value of less than 0·05 denoted statistical significance. The summated data used to produce the results are provided in the appendix (pp 10–11). All statistical analyses were done using GraphPad Prism (version 6.0).
Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. JJC, DD, PC, and AH had access to the raw data, and the corresponding author had final responsibility to submit for publication.
Results
159 separate indications were recorded in the registration system for March, 2020, 204 for April, 2020, 211 for May, 2020, and 211 for June, 2020. The number of indications for the period April to June, 2020, included the new COVID-19-specific measures introduced by NHS England in April and May, 2020. NICE recommendations added two new indications in April, 2020, (larotrectinib and lorlatinib, both indications for a small number of patients) and three new indications in May, 2020 (atezolizumab for advanced triple-negative breast cancer and small-cell lung cancer and trastuzumab emtansine as adjuvant breast cancer therapy, all for potentially larger patient populations). Other new treatment options in response to the pandemic were introduced on April 12, 14, and 28, 2020, and May 4, 5, and 22, 2020 (appendix pp 8–9), which included oral drug alternatives and the earlier use of less immunosuppressive drugs only licensed for use later in the treatment pathway.
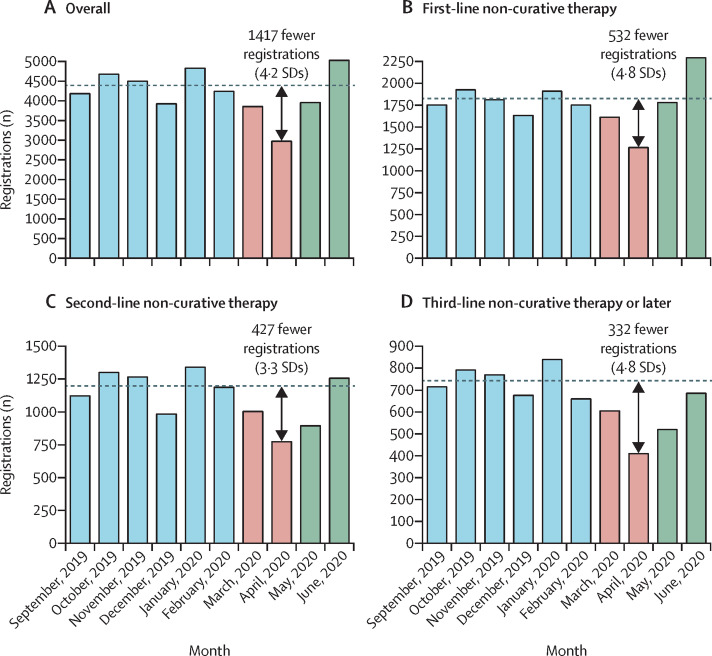
In April, 2020, 2969 registrations were recorded, which was lower than that in any month during the control period (range 3922–4819). Compared with the mean number of registrations per month for the control period (4386 [SD 335]), 1417 fewer registrations were recorded in April, 2020, representing a relative reduction of 32%, and an absolute difference of 4·2 SDs (figure 1 , figure 2A ), both of which were statistically significant (p<0·0001). During May, 2020, the total number of registrations increased to 3950, representing a 10% reduction compared with the control period (1·3 SD difference, p<0·0001). In June, 2020, 5022 registrations were recorded, a 15% increase compared with the control period (1·9 SD difference, p<0·0001).
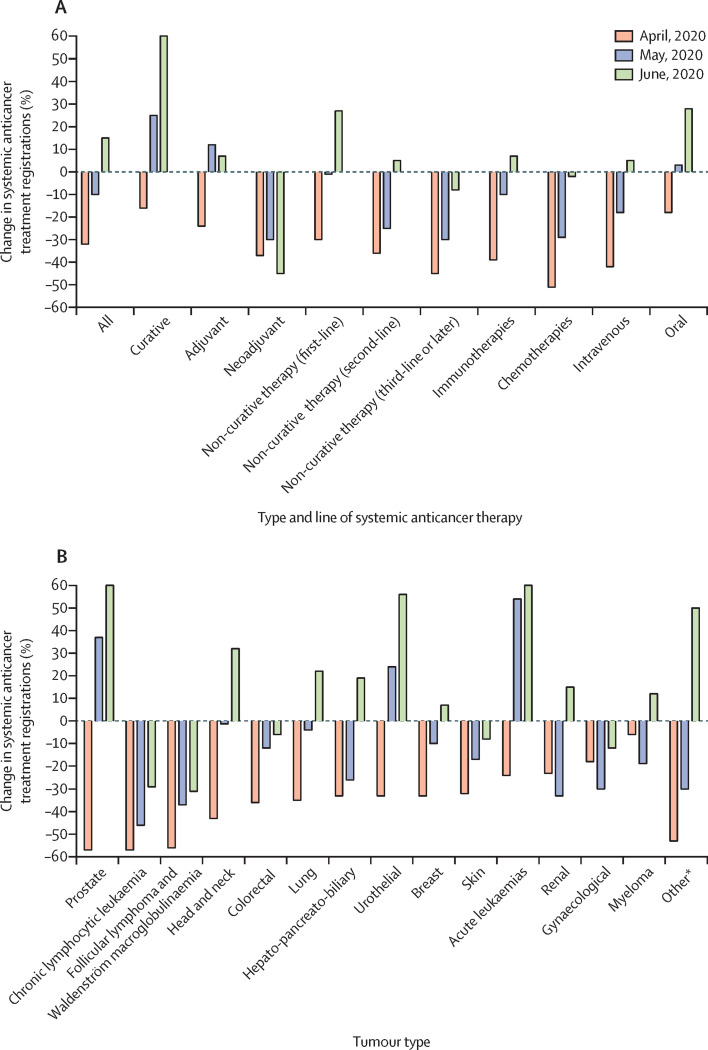
Figure 1.
Change in systemic anticancer treatment registrations for April–June, 2020, by intended treatment type and line of therapy (A) and tumour type (B)
The number of registrations for April, May, and June, 2020, were compared against the mean number for the control period. *Includes neuroendocrine tumours, thyroid tumours, sarcomas, gastrointestinal stromal tumours, gestational trophoblastic disease, and CNS tumours.
Figure 2.
The number of systemic anticancer treatment registrations observed per month, overall (A) and for first-line non-curative therapy (B), second-line non-curative therapy (C), and third-line or later non-curative therapy (D)
The dashed horizontal line shows the mean number of registrations for the control period.
For first-line non-curative therapies, the number of registrations was significantly lower in April, 2020 (relative reduction 30%; absolute difference 4·8 SDs, p<0·0001), similar in May, 2020 (relative decrease 1%; absolute difference −0·2 SDs, p=0·94), and higher in June, 2020 (relative increase 27%; absolute difference 4·5 SDs, p<0·0001), compared with the control period (figure 2B). For second-line non-curative therapies, the number of registrations were significantly lower in April, 2020 (relative reduction 36%; absolute difference 3·3 SDs, p<0·0001) and May, 2020 (relative reduction 25%; absolute difference 2·3 SDs, p<0·0001), and similar in June, 2020 (relative increase 5%; absolute difference 0·4 SDs, p=0·21), compared with the control period (figure 2C). The pattern was similar for third-line non-curative therapies or later (figure 2D).
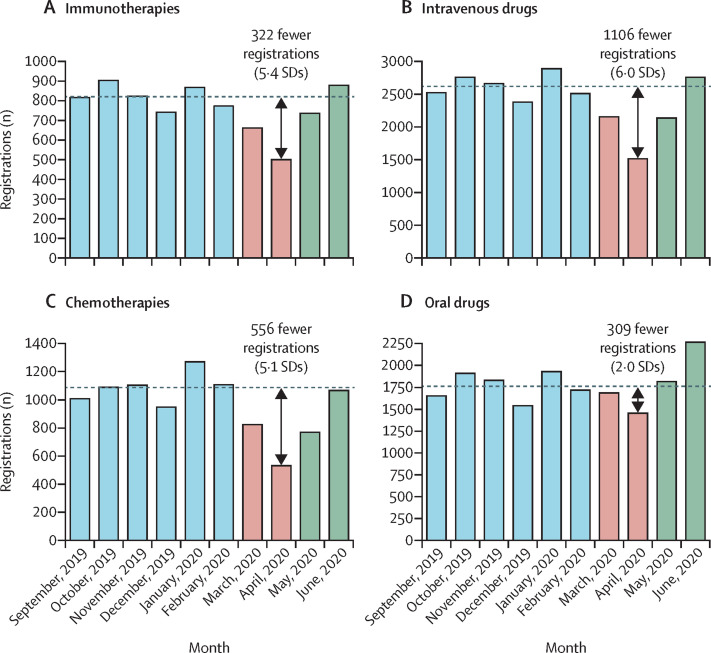
Compared with the control period, the number of registrations for immunotherapies decreased significantly during April, 2020 (relative reduction 39%; absolute difference 5·4 SDs, p<0·0001) and May, 2020 (relative reduction 10%; absolute difference 1·4 SDs, p<0·0001), but returned to typical levels in June, 2020 (relative increase 7%; absolute difference 1·0 SDs, p=0·052; figure 3A ). This pattern was similar for intravenous drugs (figure 3B) and chemotherapies (figure 3C). For oral drugs, the number of registrations in April, 2020, was significantly lower than the control period (relative reduction 18%; absolute difference 2·0 SDs, p<0·0001), similar to the control period in May, 2020 (relative increase 3%; absolute difference 0·3 SDs, p=0·49), and significantly higher than the control period in June, 2020 (relative increase 28%; absolute difference 3·3 SDs, p<0·0001; figure 3D).
Figure 3.
The number of systemic anticancer treatment registrations observed per month, for immunotherapies (A), intravenous drugs (B), chemotherapies (C), and oral drugs (D)
The dashed horizontal line shows the mean number of registrations for the control period.
The reductions in the number of registrations in April, 2020, were consistent across all groups, including intent of therapy, tumour type, line of non-curative therapy, and mode of administration (the results and categories were not necessarily mutually exclusive; appendix pp 10–11). For the four most common solid tumours, registrations were significantly lower in April, 2020, than the mean for the control period for each tumour type (p<0·0001; appendix p 4). The number of registrations decreased by 33% (absolute difference 4·2 SDs) for breast cancer, by 57% (absolute difference 4·8 SDs) for prostate cancer, by 36% (absolute difference 5·2 SDs) for lung cancer, and by 32% (absolute difference 3·4 SDs) for skin cancers. During June, 2020, the number of registrations for breast and skin cancer returned to typical levels, but were significantly higher for prostate and lung cancer (p<0·0001), with relative increases of 83% and 22%, respectively.
Compared with the control period, the number of registrations remained significantly lower in May, 2020, for neoadjuvant therapies, non-curative second-line therapies, non-curative third-line therapies, cytotoxic chemotherapies, and intravenous drugs (appendix p 10).
In June, 2020, the number of registrations remained significantly lower than in the control period for neoadjuvant therapies, chronic lymphocytic leukaemia, and follicular lymphoma and Waldenström macroglobulinaemia. For all other tumour types, the registrations were similar to or exceeded those in the control period.
The COVID-19-specific drug indications introduced by NHS England in April and May, 2020, contributed substantially to the increase in the number of registrations observed in May and June, 2020. Compared with April, 2020, 981 additional registrations were observed in May, 2020, of which 795 (81%) were for COVID-19-specific drug indications. Compared with April, 2020, 2053 additional registrations were observed in June, 2020, of which 896 (44%) were for COVID-19-specific drug indications.
We also assessed the total number of monthly registrations between April 1 and June 30, 2019 (appendix p 7). Despite the usual variability in the number of new drug approvals per month and the expected seasonal decline in approvals in December, 2019, the decreases between two adjacent months was larger between March and April, 2020, than any other time period in the previous year; and the increase between April and June, 2020, was larger than that observed across any 2-month period in the previous year (appendix p 7). If April to June, 2019, had been used as the comparison period (mean number of registrations 3868), the number of registrations observed in April, 2020 (2969) and June, 2020 (5022) would have remained significantly different (p<0·0001; appendix p 7).
Discussion
Our study provides a national assessment of the effect of the COVID-19 pandemic on patients starting their systemic anticancer treatment, which is likely to be observed in other countries, particularly those with similar health-care structures. We found that the COVID-19 pandemic had a negative impact on prescribing patterns in April, 2020, at the start of the pandemic, followed by a quick recovery in most clinical scenarios by June, 2020.
The initial substantial reduction in April, 2020, in patients starting recently approved systemic anticancer treatment drugs was probably due to several factors, including patient choice, clinical advice on the benefit and risks of starting treatment, and a reduction in referrals and subsequent diagnoses as a result of fewer patient presentations or reduced service capacity. However, delayed diagnosis would have only affected patients having curative, adjuvant, neoadjuvant, and non-curative first-line treatments, which together comprised 56% of the population commencing registered systemic anticancer treatments in the control period. Additionally, we do not consider the changes observed between April and June, 2020, to reflect seasonal variation since the only significant seasonal decline observed annually in registrations in the NHS occurs in December, which is modest.
Overall, the number of registrations significantly reduced at the start of the pandemic (32% reduction in April, 10% reduction in May), and the effect was greater for non-curative indications, particularly for later lines of therapy. Compared with April, 2020, marked increases in prescribing were observed in May, 2020, particularly for curative and adjuvant treatments, immunotherapies, and first-line non-curative therapies, but reductions persisted by comparison to the control period, particularly for neoadjuvant therapies. By June, 2020, the number of registrations was higher than the control period, a pattern that was observed for most intents of therapy and tumour types, with the exception of neoadjuvant treatments and low-grade lymphoid malignancies.
The increases in registrations observed in May, 2020, and particularly in June, 2020, are likely to reflect both delayed initiation of treatment and an increase in referrals and diagnoses following the peak of the pandemic. Treatments given temporary approval by the NHS to reduce the risk to patients also contributed substantially to the increases observed in May (81% of the increase) and June (44% of the increase). By June, a greater proportion of the increase was in routinely available treatments, signifying a substantial shift towards pre-pandemic functioning with hospital systems making adjustments using physical distancing, telephone consultations, face masks, and routine SARS-CoV-2 testing with designated so-called clean treatment areas. These changes in prescribing reflect a health-care system that can adapt quickly to the provision of new treatment options, many of which only became available in late April and early May, 2020.
The number of registrations for tumour subtypes such as prostate cancer and chronic lymphocytic leukaemia decreased substantially in April, 2020. The typically older age of patients with these types of cancer, which has been associated with higher COVID-19 risk, and the less aggressive biology of these more indolent cancers, were likely to be factors when considering treatment delays. The availability of new oral treatment options with substantially lower immunosuppressive risk proved beneficial. For example, for prostate cancer, the increase in registrations observed in May and June (compared with the control period) were likely to have been influenced by the introduction of first-line enzalutamide instead of chemotherapy for metastatic hormone-sensitive prostate cancer. By contrast, initiation of treatments for chronic lymphocytic leukaemia in May and June, 2020, remained much lower than that in the control period. A reluctance to initiate therapy in lymphoid malignancy probably reflects the additional immunosuppression imposed by the disease itself and the reported high mortality among such patients with COVID-19 infection.18, 19
A reduction in immunotherapy registrations was observed in April, 2020. Although immunotherapy is not generally considered to be immunosuppressive for most patients, it is likely that clinicians were reluctant to expose patients to multiple hospital visits and sought to avert treatment toxicity. Immunotherapies also have the additional potential risk of causing pneumonitis, which is of particular concern during the COVID-19 pandemic. Such concerns have not been corroborated in published reports, which have shown no association between PD-1 blockade and COVID-19 severity.20
In April, 2020, a larger reduction in intravenous treatment registrations was observed than in oral therapy registrations, which reflects a strategy to maintain treatment where possible, especially considering that many oral treatments (eg, tyrosine kinase inhibitors) are not strongly immunosuppressive and these drugs could be delivered to patients' homes. A marked increase (28%) was observed in June, 2020, for oral drugs.
Some of the smallest reductions in registrations in April were observed for treatments deemed curative and adjuvant, reflecting their high long-term benefit–risk ratio. Examples of curative therapies that had fewer registrations include durvalumab after chemoradiotherapy for non-small cell lung cancer, and recently approved additions to chemotherapy for acute leukaemia, such as gemtuzumab ozogamicin. The increase in curative treatments observed in May and June, 2020, were likely to have been influenced by the provision of venetoclax combinations and gilteritinib for acute myeloid leukaemia. The adjuvant treatment registrations were limited to HER2-positive breast cancer and melanoma, although the decrease in adjuvant therapies during April, 2020, was largely observed for melanoma due to a decrease in adjuvant immunotherapy. One explanation for this reduction could be more aggressive risk stratification, with less adjuvant treatment for patients deemed at lower risk of relapse, combined with a strategy to avoid potentially toxic treatment or hospital visits. The use of these adjuvant therapies increased quickly in May and June, 2020, with the number of registrations increasing to numbers higher than that in the control period.
The reduced number of registrations for neoadjuvant treatment is likely to reflect patients proceeding straight to surgery rather than risking chemotherapy complications and subsequent delays, and this pattern continued in May and June.
Our study has some limitations. First, the NHS registration system records an intention to treat that might not necessarily result in treatment itself, although previous audits have shown that 92–95% of registrations result in actual treatment (Dominguez-Lezcano J, NHS England, personal communication). Second, this analysis only covers drugs approved for use since 2016, and does not include hormone therapies or free-of-charge drugs from companies. Most adjuvant and neoadjuvant treatment for solid malignancies is conventional cytotoxic chemotherapy, which is not captured by the national registration system. Similarly, many curative treatments were established before 2016, including those for germ-cell tumours, lymphoma, and acute leukaemia, so data on these treatments are not recorded. The NHS systemic anticancer treatment registration data therefore does not represent the total change in prescribing practice for all treatments that occurred during the pandemic. Because most standard cytotoxic chemotherapies are administered intravenously, we expect that there was a corresponding reduction in such treatment, but we cannot quantify this. Third, the registration system does not indicate whether patients who started systemic anticancer treatment had reduced or delayed doses to minimise clinic visits. Future patient-level analyses might also show how age, sex, geographical location, tumour factors, and intent of therapy together affected the initiation of systemic anticancer treatment.
The therapies listed on the national systemic anticancer treatment registration system have proven benefits for patients in terms of longer survival, or reduced risk of cancer progression or recurrence. Failing to initiate or delaying these treatments by 1–2 months, particularly for patients with advanced cancers for whom approved drugs might improve survival by only a few months, could have negative consequences for patients. Clinical outcomes such as survival need to be carefully examined in these patients for the next year at least to quantify such effects. Delays in treatment have been shown to lead to worse outcomes in some,21, 22, 23 but not all malignancies.24 Similarly, the reduction in use of neoadjuvant therapies also requires assessment with regards to its effects on longer-term outcomes.
Our study shows the consequences of NHS England offering clinicians and patients a wide range of treatment options including drugs not yet appraised by NICE or which are off-label, but are likely to result in less risk to patients from the pandemic. The evidence from our study shows that these additional options contributed to the greater number of registrations for new patients starting systemic anticancer treatment in May and June, 2020.
In conclusion, our study has four key messages. First, we showed that at the height of the pandemic significantly fewer patients with cancer started systemic anticancer treatment than expected, but services recovered within 2 months. Second, clinicians and health-care providers can act quickly to provide treatment options considered to confer a lower risk to patients while maintaining efficacy. Third, although it is important to consider a risk–benefit balance for each patient when determining the initiation of anticancer therapies, the emerging data on outcomes for patients receiving systemic anticancer treatment who contract COVID-19 highlight the need for continued scrutiny and discussion regarding overall gains of systemic anticancer treatment. Fourth, it will be important to audit the outcomes of systemic anticancer treatment on patients during the pandemic including those given the newly permitted treatment options. Many of these options were off-label and the effects of these options will provide important data for health-care providers.
Data sharing
The summated data used in the analyses are provided in the appendix (pp 10–11). The original source data was obtained from the NHS England central database. Total numbers of registrations for the initiation of systemic anticancer therapies approved for use since July, 2016 funded by the Cancer Drugs Fund are published by drug, indication, and month, and can be found online. Such data provision has an open government licence and hence can be used for any type of analysis. At present, NHS England does not routinely publish registration data for drug indications that are routinely commissioned. Registration data for drugs funded by the Cancer Drugs Fund and by routine commissioning are subject to the rules governing Freedom of Information requests and NHS England can provide such data. The authors will not provide any raw data.
Acknowledgments
Acknowledgments
This work was partly supported by Cancer Research UK; AH is supported by a Cancer Research UK trials centre core grant to University College London (C444/A15953).
Contributors
JJC, AH, and PC created the initial concept and study design. DD and NP were responsible for data collection and verification of the raw data. AH and JJC did statistical analyses. JJC, AH, PC, and PJ interpreted the data. JJC, DD, NP, PC, PJ, and AH were involved in writing the manuscript and the decision to submit for publication.
Declaration of interests
JJC reports personal fees from Pfizer, outside the submitted work. PJ reports grants from Epizyme and Janssen; and personal fees from Takeda, Bristol-Myers Squibb, Novartis, Celgene, Boehringer Ingelheim, Kite Pharmaceuticals, and Genmab and Incyte, outside the submitted work. AH reports personal fees from Abbvie, Boehringer Ingelheim, Takeda, AstraZeneca, Daiichi Sankyo, Merck Serono, UCB, and Roche, outside the submitted work. All other authors declare no competing interests.
Supplementary Material
References
- 1.Kamboj M, Sepkowitz KA. Nosocomial infections in patients with cancer. Lancet Oncol. 2009;10:589–597. doi: 10.1016/S1470-2045(09)70069-5. [DOI] [PubMed] [Google Scholar]
- 2.Sica A, Massarotti M. Myeloid suppressor cells in cancer and autoimmunity. J Autoimmun. 2017;85:117–125. doi: 10.1016/j.jaut.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 4.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang F, Shi S, Zhu J, Shi J, Dai K, Chen X. Clinical characteristics and outcomes of cancer patients with COVID-19. J Med Virol. 2020;92:2067–2073. doi: 10.1002/jmv.25972. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-COV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aries JA, Davies JK, Auer RL, et al. Clinical outcome of coronavirus disease 2019 in haemato-oncology patients. Br J Haematol. 2020 doi: 10.1111/bjh.16852. published online May 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He W, Chen L, Chen L, et al. COVID-19 in persons with haematological cancers. Leukemia. 2020;34:1637–1645. doi: 10.1038/s41375-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma J, Yin J, Qian Y, Wu Y. Clinical characteristics and prognosis in cancer patients with COVID-19: a single center's retrospective study. J Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang K, Sheng Y, Huang C, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee LY, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinato DJ, Zambelli A, Aguilar-Company J, et al. Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients. Cancer Discov. 2020;10:1465–1474. doi: 10.1158/2159-8290.CD-20-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinmohamed AG, Visser O, Verhoeven RHA, et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21:750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodkin H. The Telegraph; London: April 15, 2020. Cancer referrals down by 80 per cent in some areas as coronavirus fears keep patients from hospitals.https://www.telegraph.co.uk/news/2020/04/15/cancer-referrals-80-per-cent-areas-coronavirus-fears-keep-patients/ [Google Scholar]
- 16.NHS England Clinical guide for the management of noncoronavirus patients requiring acute treatment: cancer. 2020. https://www.nice.org.uk/media/default/about/covid-19/specialty-guides/cancer-and-covid-19.pdf
- 17.European Society for Medical Oncology Cancer patient management during the COVID-19 pandemic. 2020. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic
- 18.Mato AR, Roeker LE, Lamanna N, et al. Outcomes of COVID-19 in patients with CLL: a multicenter, international experience. Blood. 2020;136:1134–1143. doi: 10.1182/blood.2020006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scarfò L, Chatzikonstantinou T, Rigolin GM, et al. COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia. 2020;34:2354–2363. doi: 10.1038/s41375-020-0959-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo J, Rizvi H, Egger JV, Preeshagul IR, Wolchok JD, Hellmann MD. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020;10:1121–1128. doi: 10.1158/2159-8290.CD-20-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raphael MJ, Biagi JJ, Kong W, Mates M, Booth CM, Mackillop WJ. The relationship between time to initiation of adjuvant chemotherapy and survival in breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2016;160:17–28. doi: 10.1007/s10549-016-3960-3. [DOI] [PubMed] [Google Scholar]
- 22.Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011;305:2335–2342. doi: 10.1001/jama.2011.749. [DOI] [PubMed] [Google Scholar]
- 23.Brooks EG, Connors JM, Sehn LH, et al. Impact of time from diagnosis to initiation of curative-intent chemotherapy on clinical outcomes in patients with classical Hodgkin lymphoma. Leuk Lymphoma. 2016;57:872–879. doi: 10.3109/10428194.2015.1086919. [DOI] [PubMed] [Google Scholar]
- 24.Perri T, Issakov G, Ben-Baruch G, et al. Effect of treatment delay on survival in patients with cervical cancer: a historical cohort study. Int J Gynecol Cancer. 2014;24:1326–1332. doi: 10.1097/IGC.0000000000000211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The summated data used in the analyses are provided in the appendix (pp 10–11). The original source data was obtained from the NHS England central database. Total numbers of registrations for the initiation of systemic anticancer therapies approved for use since July, 2016 funded by the Cancer Drugs Fund are published by drug, indication, and month, and can be found online. Such data provision has an open government licence and hence can be used for any type of analysis. At present, NHS England does not routinely publish registration data for drug indications that are routinely commissioned. Registration data for drugs funded by the Cancer Drugs Fund and by routine commissioning are subject to the rules governing Freedom of Information requests and NHS England can provide such data. The authors will not provide any raw data.