Abstract
Increases in cardiac troponin indicative of myocardial injury are common in patients with coronavirus disease-2019 (COVID-19) and are associated with adverse outcomes such as arrhythmias and death. These increases are more likely to occur in those with chronic cardiovascular conditions and in those with severe COVID-19 presentations. The increased inflammatory, prothrombotic, and procoagulant responses following severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection increase the risk for acute nonischemic myocardial injury and acute myocardial infarction, particularly type 2 myocardial infarction, because of respiratory failure with hypoxia and hemodynamic instability in critically ill patients. Myocarditis, stress cardiomyopathy, acute heart failure, and direct injury from SARS-CoV-2 are important etiologies, but primary noncardiac conditions, such as pulmonary embolism, critical illness, and sepsis, probably cause more of the myocardial injury. The structured use of serial cardiac troponin has the potential to facilitate risk stratification, help make decisions about when to use imaging, and inform stage categorization and disease phenotyping among hospitalized COVID-19 patients.
Key Words: cardiac troponin, coronavirus disease 2019, myocardial injury, type 2 myocardial infarction
Abbreviations and Acronyms: COVID-19, coronavirus disease-2019; cTn, cardiac troponin; ECG, electrocardiogram; ICU, intensive care unit; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2; T2MI, type 2 myocardial infarction; URL, upper reference limit
Central Illustration
Increases in cardiac troponin (cTn) indicative of myocardial injury are common in patients with coronavirus disease-2019 (COVID-19), particularly in patients with underlying cardiovascular conditions and severe COVID-19 presentations, and are associated with worse outcomes (1, 2, 3, 4, 5, 6). The purposes of this report are to summarize the evolving understanding of myocardial injury and the use of cTn in COVID-19.
Definition of Myocardial Injury
The Fourth Universal Definition of Myocardial Infarction defines myocardial injury (acute or chronic) as cTn concentrations >99th percentile upper reference limit (URL) (7). Patients with dynamic changes (deltas) have acute injury, and those without changes have chronic injury. Deltas are more precisely measured using high-sensitivity (hs) cTn assays, for which sex-specific thresholds are recommended (7).
Several studies (Table 1 ) have defined the frequency of myocardial injury in COVID-19 (1, 2, 3, 4, 5, 6,8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19). However, as in many cTn studies, how myocardial injury was defined and which cTn assay, thresholds, and sampling intervals were used are often not well defined. Because many studies have not used hs-cTn assays and only used early single time point measurements, the data likely underestimate the frequency and magnitude of myocardial injury in COVID-19. Anticipating more studies addressing this issue, Supplemental Table 1 provides a template for what they should include to make research more interpretable and comparable.
Table 1.
Summary of Studies Addressing Cardiac Troponin and/or Myocardial Injury in Patients With COVID-19
| First Author (Ref. #) | Location | Population | Prevalence of Cardiovascular Disease | Cardiac Troponin Assay and Myocardial Injury Definition | Frequency | Outcomes | Comments |
|---|---|---|---|---|---|---|---|
| Arentz et al. (8) | Evergreen Hospital, Washington, United States | 21 intensive care unit patients | CHF, 42.9% | cTn assay details NR Threshold: >0.3 ng/ml |
3 patients (14%) had cTn concentrations >0.3 ng/ml | NR by cTn results Entire cohort: Cardiomyopathy, 33.3% Death, 52.4% |
BNP mean: 4,720 (69–33,423) Vasopressors: 67% |
| Bhatraju et al. (9) | 9 hospital ICUs in Seattle, Washington, United States | 24 intensive care unit patients | NR | cTn assay details NR Threshold: ≥0.06 ng/ml |
cTn concentrations were elevated (≥0.06 ng/ml) in 2 of 13 (15%) patients early in their ICU course (maximum value, 0.80 ng/dl) | NR by cTn results In-hospital death: 50% |
Echocardiogram completed in 9 of 24 (38%), with none showing left ventricular dysfunction Vasopressors: 71% |
| Chen et al. (3) | Tongji Hospital, Wuhan, China | 799 moderately to severely ill or critically ill patients with confirmed COVID-19 transferred from other hospitals or isolation sites or admitted from fever clinics to Tongji Hospital, of which the final study cohort included 113 who died and 161 who had recovered | Among deaths, CVD (14%) Among recovered patients, CVD (4%) |
cTn assay details NR Threshold: >15.6 pg/ml |
Entire cohort (n = 203): >URL: 41% Deceased cohort Median: 40.8 (14.7–157.8) >threshold: 68 of 94, 72% 8 deceased patients with cTnI >1,000 pg/ml and 2 >10,000 pg/ml Recovered cohort Median: 3.3 (1.9–7.0) >threshold: 15 of 109 (14) |
NR by cTn results Deaths Acute cardiac injury: 72 of 94 (77%) With history of HTN or CVD: 37 of 48 (77%) Without history of HTN or CVD: 35 of 46 (76%) HF: 41 of 83 (49%) With history of HTN or CVD: 21 of 42 (50%) Without history of HTN or CVD: 20 of 41 (49%) Recovered Acute cardiac injury: 18 of 109 (17%) With history of HTN or CVD: 11 of 30 (37%) Without history of HTN or CVD: 7 of 80 (9%) HF: 3 of 94 (3%) With history of HTN or CVD: 2 of 25 (8%) Without history of HTN or CVD: 1 of 68 (1%) |
Chest tightness 49% in deaths and 30% in recovered patients NT-proBNP (threshold ≥285 pg/ml) Deaths: Median: 800.0 (389.8–1,817.5) >threshold: 68 of 80 (85%) Recovered: Median: 72.0 (20.0–185.0) >threshold: 17 of 93 (18%) |
| Cummings et al. (19) | New York Presbyterian, New York, United States | 1,150 adult patients admitted with laboratory-confirmed COVID-19 who were critically ill with acute hypoxemic respiratory failure | Chronic cardiac disease, 19% | hs-cTnT (ng/l) | hs-cTnT measured in 254 of 257 patients Median 19 (IQR: 9–52) ng/l |
NR by cTn results | cTn not included in multivariable Cox model |
| Guo et al. (5) | Seventh Hospital, Wuhan, China | 187 hospitalized patients with COVID-19 at a designated hospital to treat such patients | Normal cTnT HTN: 20.7% CHD: 3.0% Cardiomyopathy, 0% ACE inhibitor/ARB: 5.9% Increased cTnT HTN: 63.5% CHD: 32.7% Cardiomyopathy, 15.4% ACE inhibitor/ARB: 21.1% |
cTn assay details NR Patients were considered to have acute myocardial injury if serum levels of cTnT were above the 99th percentile URL |
Myocardial injury: 52 of 187 (27.8%) | Death Normal cTnT: 12 (8.9%) Increased cTnT: 31 (59.6%) Death according to CVD: Normal cTnT/without CVD: 8 of 105 (7.6%) Normal cTnT/with CVD: 4 of 30 (13.3%) Increased cTnT/without CVD: 6 of 16 (37.5%) Increased cTnT/with CVD: 69.44% (25 of 36) |
Patients with underlying CVD were more likely to exhibit elevation of cTnT (54.5%) compared with patients without CVD (13.2%) Both cTnT and NT-proBNP levels increased significantly during the course of hospitalization in those who ultimately died, but no such dynamic changes were evident in survivors |
| Han et al. (10) | Renmin Hospital, Wuhan University, China | 273 patients with SARS-CoV-2 infection | NR | Ultra-TnI measured in serum using Siemens ADVIA Centaur XP URL: 0.04 ng/ml |
>URL (0.04 ng/ml) in 27 of 273 (9.9%) By disease severity: Mild: 10 of 198 (5%) Severe: 14 of 60 (23%) Critical: 3 of 15 (20%) |
Cases in abnormal parameters group (i.e., increased CK-MB, myoglobin, cTnI, and NT-proBNP) had a case fatality rate of 22.8% (13 of 57) compared with a rate of 5.1% (11 of 216) in normal parameters group | Increase in cTnI showed significant difference between mild and severe cases |
| He et al. (11) | Sino-French New City Campus of Tongji Hospital, Tongji Medical College, and Huazhong University of Science and Technology | Retrospective analysis of 54 confirmed cases of severe/critical COVID-19 | HTN: 44% CHD: 15% DLD: 7.4% |
Troponin >34.2 ng/l was considered abnormal Study defined myocardial damage as cTn concentrations ≥3× ULN |
24 of 54 (44%) had cTn ≥3× ULN Deaths Myocardial injury: 69% (18 of 26) Survivors Myocardial injury: 21% (6 of 28) |
In-hospital mortality: Myocardial injury: 75% (18 of 24) Without injury: 26.7% (8 of 30) |
None |
| Huang et al. (1) | Jin Yintan Hospital, Wuhan, China | 41 patients with COVID-19 admitted to designated hospital | Hypertension: 15% CVD: 15% |
hs-cTnI, threshold >28 ng/l (99th percentile). Other details NR Cardiac injury was defined as 1 or more of the following: Blood levels of cardiac biomarkers (cTnI or CK-MB) >99th percentile URL New abnormalities in electrocardiography, including supraventricular tachycardia, ventricular tachycardia, atrial fibrillation, ventricular fibrillation, bundle branch block, ST-segment elevation/depression, T-wave flattening/inversion, and QT interval prolongation New abnormalities in echocardiography, including decreased EF value (<50%) or a worsening of the underlying state, regional/global ventricular wall motion abnormalities, the presence of pericardial effusion, and pulmonary arterial hypertension |
Hs-cTnI was substantially increased in 5 of 41 (12%) patients in whom the diagnosis of virus-related cardiac injury was made Hs-cTnI >99th URL All: 5 of 41 (12%) ICU: 4 of 13 (31%) Non-ICU: 1 of 28 (4%) Median hs-cTnI (pg/ml) All: 3.4 (1.1-9.1) ICU: 3.3 (3.0-163.0) Non-ICU: 3.5 (0.7–5.4) |
NR by cTn results Acute cardiac injury: All: 5 of 41 (12%) ICU: 4 of 13 (31%) Non-ICU: 1 of 28 (4%) Shock: All: 3 of 41 (7%) ICU: 3 of 12 (23%) Non-ICU: 0 of 28 (0%) Death: All: 6 of 41 (15%) ICU: 5 of 13 (38%) Non-ICU: 1 of 28 (4%) |
None |
| Hui et al. (12) | Beijing Youan Hospital, China | 41 consecutive COVID-19 patients at Beijing Hospital | 9 of 41 patients had cardiac-related chronic disease | cTn assay details NR cTnI (ng/ml) was available in 20 of 41 patients |
cTnI increased: 4 of 20, including 1 severe patient and 3 critical patients cTnI according to disease severity: Light: 0.01 ng/ml Mild: 0.01 ng/ml Severe 0.1 ng/ml Critical: 0.54 (0.05–5.90) ng/ml |
Major clinical outcomes NR | SpO2 was lower in severe/critical patients, and 2 patients in the critical group had onset of atrial fibrillation |
| Lala et al. (13) | Mount Sinai System Hospitals, New York City, United States | 2,736 patients with COVID-19 with cTnI measured within 24 h of admission | cTnI 0–0.03 ng/ml (normal) CAD: 9.8% CHF: 4.3% HTN: 34% cTnI 0.03–0.09 ng/ml (mildly elevated) CAD: 21.3% CHF: 14.7% HTN: 45.1% cTnI >0.09 ng/ml (elevated) CAD: 34.9% CHF: 25.3% HTN: 50.0% |
Abbott ARCHITECT cTnI 99th percentile 0.028 ng/ml URL: 0.03 ng/ml |
1,751 (64%) had initial cTnI within normal range Admission cTnI Normal: 1,751 of 2,736 (64%) Mildly elevated: 455 of 2,736 (16.6%) Elevated: 530 of 2,736 (19.4%) Overall injury: 36% |
Mildly elevated cTnI: adjusted HR: 1.77, 95% CI: 1.39–2.26 Elevated cTnI: adjusted HR: 3.23, 95% CI: 2.59–4.02 |
None |
| Liu et al. (14) | Shenzhen Third People’s Hospital, China | 12 patients with COVID-19 | CHD: 4 of 12 HTN: 3 of 12 |
cTn assay details NR cTnI (ug/ml): normal range 0–0.1 |
1 of 12 patients had concentrations exceeding URL, with a concentration of 11.37 ug/ml | NR by cTn results Cardiac failure, 1 of 12 Shock, 1 of 12 |
None |
| Shi et al. (6) | Renmin Hospital of Wuhan University, Wuhan, China | 416 consecutive patients admitted to hospital with laboratory-confirmed COVID-19. Cases without cardiac biomarkers, including values of cTnI and CK-MB were excluded | With cardiac injury: HTN: 59.8% CHD: 29.3% CHF: 14.6% Without injury: HTN: 23.4% CHD: 6.0% CHF: 1.5% |
cTn assay details NR cTnI (Siemens ADVIA Centaur); lowest measurable value <0.006 ng/ml; >0.04 ng/ml was considered indicative of cardiac injury and >0.78 ng/ml suggesting myocardial infarction possible Cardiac injury was defined as blood levels of cardiac biomarkers (hs-cTnI) above the 99th percentile upper reference limit, regardless of new abnormalities in electrocardiography and echocardiography |
Cardiac injury: 82 of 416 (19.7%) cTnI (median) All: <0.006 (IQR: <0.006–0.02) Cardiac injury: 0.19 (IQR: 0.08–1.12) Without injury: <0.006 (IQR: <0.006–0.009) |
Death Cardiac injury, 51.2% Without injury, 4.5% Mortality rate increased in association with the magnitude of the reference value of cTnI Cox proportional hazard regression model showed significant higher risk of death in patients with cardiac injury than in those without, either during time from symptom onset (HR: 4.2; 95% CI: 1.92–9.49) or time from admission to study endpoint (HR: 3.41; 95% CI: 1.62–7.16) |
Chest pain: Cardiac injury, 13.4% Without injury, 0.9% |
| Shi et al. (16) | Renmin Hospital, Wuhan University, China | 671 hospitalized patients with severe COVID-19 Cases missing cardiac biomarkers, including cTnI were excluded | With myocardial injury: HTN: 59.4% CHD: 27.4% CHF: 12.3% CVD: 10.4% AF: 0.9% Without injury; HTN: 24.1% CHD: 5.5% CHF: 1.6% CVD: 1.9% AF: 1.1% |
Myocardial injury was defined as blood levels of cTnI increased above the 99th percentile Siemens ADVIA Centaur XP Immunoassay system, cTnI; range: 0–0.04 ng/ml |
Myocardial injury: 106 of 671 (15.8%) Median: 0.159 (IQR: 0.075–0.695) ng/ml |
Median cTnI by patient status: All: 0.006 ng/ml Death: 0.235 ng/ml Survivors: 0.005 ng/ml Myocardial injury by alive/death status: Nonsurvivor: 75.8% Survivor: 9.7% |
cTnI of 0.026 ng/l was identified as the concentration predictive of in-hospital mortality HR: 4.56; 95% CI: 1.28–16.28, p = 0.019 cTnI (ln-transformed): HR 1.90, 95% CI: 1.44-2.49 for in-hospital mortality Predictors of myocardial injury: age, hypertension, coronary heart disease, chronic renal disease, chronic obstructive pulmonary disease, and C-reactive protein |
| Ruan et al. (15) | Jin Yin-Tan Hospital and Tongji Hospital, China | Retrospective, multicenter study of 68 death cases and 82 discharged cases with laboratory confirmed SARS-CoV-2 | Among deaths: HTN: 43% CVD: 19% Among discharged: HTN: 28% CVD: 0% |
cTn assay details NR cTn (pg/ml) normal range: 2.0–28.0 |
cTn (pg/ml), mean ± SD Deaths: 30.3 ± 151.0 Discharged: 3.5 ± 6.2 |
NR by cTn results Patients with CVD had a significantly increased risk of death when infected with SARS-CoV-2 (p < 0.001) |
None |
| Wang et al. (4) | Zhongnan Hospital of Wuhan University, China | 138 consecutive hospitalized patients with confirmed coronavirus-infected pneumonia | Hypertension All: 31.2% ICU: 58.3% Non-ICU: 21.6% CVD All: 14.5% ICU: 25.0% Non-ICU: 10.8% |
cTn assay details NR hs-cTnI (“hypersensitive”) (pg/ml), threshold 26.2 pg/ml Cardiac injury was defined if the serum levels of cardiac biomarkers (e.g., troponin I) were above the 99th percentile URL or new abnormalities in electrocardiography and echocardiography |
hs-cTnI (pg/ml), median (IQR) All: 6.4 (2.8–18.5) ICU: 11.0 (5.6–26.4) Non-ICU: 5.1 (2.1–9.8) |
NR by cTn results Acute cardiac injury All: 7.2% ICU: 22.2% Non-ICU: 2.0% Shock All: 8.7% ICU: 30.6% Non-ICU: 1.0% Arrhythmia All: 16.7% ICU: 44.4% Non-ICU: 6.9% |
None |
| Yang et al. (17) | Jin Yin-tan Hospital, Wuhan, China | Retrospective, observational study of 52 critically ill adult patients with SARS-CoV-2 pneumonia admitted to the ICU | Chronic cardiac disease All: 10% Survivors: 10% Nonsurvivors: 9% |
cTn assay details NR Cardiac injury was diagnosed if the serum concentration of hs-cTnI (“hypersensitive”) was above the upper limit of the reference range, >28 pg/ml |
Cardiac injury All, 12 of 52 (23%) Survivors, 3 of 20 (15%) Nonsurvivors, 9 of 32 (28%) Median hs-cTnI was 161.0 (IQR: 41.8–766.1) pg/ml |
NR by cTn results 32 (61.5%) had died at 28 days |
None |
| Zhou et al. (2) | Jinyintan Hospital and Wuhan Pulmonary Hospital, Wuhan, China | Retrospective study including 2 cohorts of adult inpatients diagnosed with COVID-19 Enrolled all adult inpatients who were hospitalized for COVID-19 and had a definite outcome (dead or discharged) | HTN All: 30% Nonsurvivor: 48% Survivor: 23% CHD All: 8% Nonsurvivor: 24% Survivor: 1% |
cTn assay details NR hs-cTnI (pg/ml), threshold >28 pg/ml Acute cardiac injury was diagnosed if serum levels of cardiac biomarkers (e.g., high-sensitivity cardiac troponin I) were above the 99th percentile upper limit, or if new abnormalities were shown in electrocardiography and echocardiography Routine blood examination included myocardial enzymes |
hs-cTnI (pg/ml), median (IQR) All: 4.1 (2.0–14.1) Nonsurvivor: 22.2 (5.6–83.1) Survivor: 3.0 (1.1–5.5) hs-cTnI > threshold All: 24 of 145 (17%) Nonsurvivor: 23 of 50 (46%) Survivor: 1 of 95 (1%) |
NR by cTn results Heart failure All: 23% Nonsurvivor: 52% Survivor: 12% Acute cardiac injury All: 17% Nonsurvivor: 59% Survivor: 1% hs-cTnI >28 pg/ml was identified as a risk factor associated with in-hospital death on univariable analysis (OR: 80.07; 95% CI: 10.34–620.36) |
hs-cTnI was not included in multivariate logistic regression model as it was deemed that it “might be unavailable in emergency circumstances.” In nonsurvivors, hs-cTnI increased rapidly from day 16 after disease onset Median onset of acute cardiac injury: All, 15 days (10.0–17.0) Nonsurvivors, 14.5 days (9.5–17.0) |
| Zhou et al. (18) | West District of Union Hospital of Tongji Medical College, China | 34 patients admitted to hospital | cTn assay details NR; cTnI URL: <26.2 ng/l |
cTnI >URL All: 9 of 34 (26%) Severe: 1 of 26 (3.8%) Very severe: 8 of 8 (100%) cTnI median (IQR) (ng/l) Severe: 4.8 (2.5–8.4) Very severe: 46.8 (34.2–299.8) |
NR | None |
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; BNP = B-type natriuretic peptide; CAD = coronary artery disease; CHD = chronic heart disease; CHF = coronary heart failure; CI = confidence interval; CK-MB = creatine kinase myocardial band; COVID-19 = coronavirus disease-2019; cTn = cardiac troponin; CVD = cardiovascular disease; DLD = dyslipidemia; EF = ejection fraction; HF = heart failure; HR = hazard ratio; hs-cTnT = high-sensitivity cardiac troponin T; HTN = hypertension; ICU = intensive care unit; IQR = interquartile range; NR = not reported; NT-proBNP = N-terminal pro–B-type natriuretic peptide; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; URL = upper reference limit.
It should also be noted that many studies have used nonguideline definitions of myocardial injury based on electrocardiographic (ECG) or echocardiographic abnormalities (1,2,4) or use thresholds other than the 99th percentile (11).
Clinical Characteristics and Epidemiology
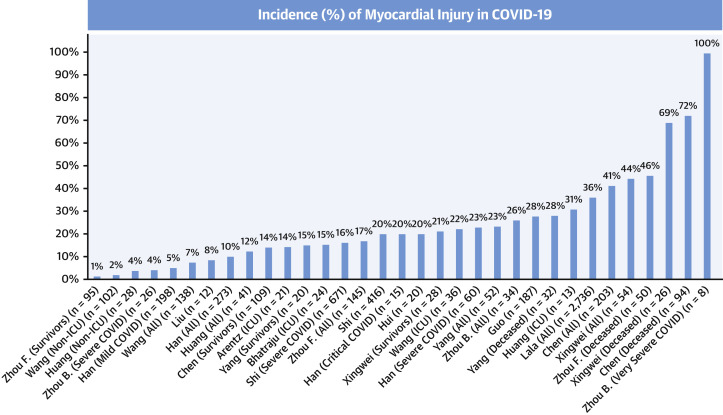
The frequency of myocardial injury in COVID-19 varies widely (Table 1, Figure 1 ), in part because of variations in definitions, populations studied, timing of sampling, and cTn assays/thresholds. There is a continuum in the relationship between myocardial injury and illness severity, with a higher frequency of myocardial injury in critically ill patients and nonsurvivors. The range of myocardial injury in COVID-19, however, is wide (1, 2, 3, 4), with cTn concentrations often considerably lower than often observed for acute MI, including in severe COVID-19 cases (19).
Figure 1.
Incidence of Myocardial Injury in COVID-19
Frequency of myocardial injury increases with greater severity of illness. COVID-19 = coronavirus disease-2019.
Non-COVID studies have demonstrated that myocardial injury is more likely to occur in critically ill older patients and in those with comorbidities (20). Previous data demonstrated that cTn increases are frequent (42% using fourth-generation cTnT, median 0.2 ng/ml = 200 ng/l with fifth-generation cTnT) and prognostic in those with acute respiratory disease (21). Increases occur in SARS-CoV-1, MERS-CoV, and influenza (22,23).
COVID-19 studies show similar findings. Using cTnT among hospitalized patients with COVID-19, Guo et al. (5) demonstrated that compared with patients without myocardial injury, patients with cTnT >99th percentile (27.8%, 52 of 187) were older and had higher prevalence of chronic cardiovascular conditions (5). In a larger single-sample study using cTnI, Shi et al. (6) demonstrated that 19.7% (82 of 416) of hospitalized COVID-19 patients had cTnI >99th percentile (6). Those patients had a higher prevalence of hypertension, coronary heart disease, and heart failure. The incidence of cTn increases is much higher with severe COVID-19 presentations. Data from Chen et al. (3) demonstrated that 41% (83 of 203) of moderately to severely ill or critically ill patients had myocardial injury (3). Those dying had a median hs-cTnI of 40.8 ng/l (URL 15.6 ng/l) compared with 3.3 ng/l in those who survived.
Mechanisms and Classification
Except for rare analytical confounds, cTn increases >99th percentile URL are indicative of myocardial injury (7). Although these increases are cardiospecific, there are a myriad of conditions that can cause them. Despite reports of myocarditis with COVID-19 (24,25), which can cause increases in cTn, elevations in COVID-19 patients should not always be considered to be due to myocarditis or direct severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) injury. The etiology of all cTn increases, including those in COVID-19, depends on clinical context and collaborating information, and not cTn alone.
The risk for acute myocardial injury, ischemia, and infarction following acute infection (viral or bacterial) is well established and is thought to be related to the increased inflammatory, prothrombotic, and procoagulant state (26). Laboratory data from COVID-19 studies confirm the relationship between illness severity, an increased inflammatory and prothrombotic state, and myocardial injury. Patients with more severe COVID-19 presentations have greater increases in inflammatory markers such as C-reactive protein (3,5,6,15), ferritin (2,3,15), interleukin-6 (2,3,15), tumor necrosis factor-α (1,3), and thrombotic markers such as D-dimer (1, 2, 3, 4, 5). In some (3,5,6) but not all (1,2) studies, procalcitonin is also increased. It is challenging, however, to discern whether inflammation is always a cause or a response of acute myocardial injury.
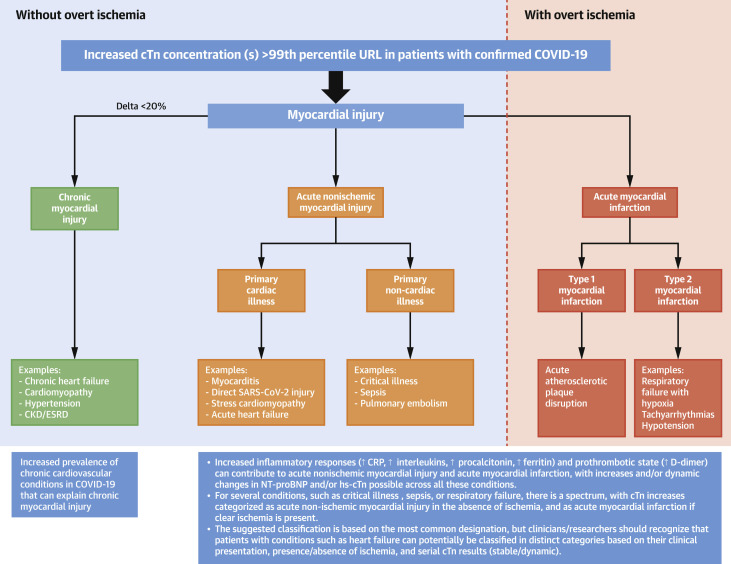
Figure 2 illustrates the potential mechanisms that can lead to myocardial injury in COVID-19. In general, for any patient with cTn increases >99th percentile, elevations should be broadly classified as: 1) chronic myocardial injury; 2) acute nonischemic myocardial injury; or 3) acute myocardial infarction (MI).
Figure 2.
Classification of Myocardial Injury in COVID-19
Increases in cTn should be categorized as chronic myocardial injury, acute nonischemic myocardial injury, or acute myocardial infarction. For several conditions, there is a spectrum and the most common category is indicated, but can present in other ways. CKD = chronic kidney disease; COVID-19 = coronavirus disease-2019; CRP = C-reactive protein; cTn = cardiac troponin; ESRD = end-stage renal disease; NT-proBNP = N-terminal pro–B-type natriuretic peptide; URL = upper reference limit.
Chronic myocardial injury
For those with chronic conditions and comorbidities, chronic “stable” (<20% change) cTn increases are categorized as chronic myocardial injury (7). This is likely the etiology for many COVID-19 patients because of the high prevalence of chronic cardiovascular conditions (5,6). These elevations are true positives for myocardial injury and are associated with an adverse prognosis (27) even without intercurrent disease. Such increases are often observed in patients with renal disease. Although SARS-CoV-2 appears to have renal tropism (28) and COVID-19 patients with increased cTn concentrations have higher rates of kidney injury (5), it is important to note that at high concentrations, cTn is mainly cleared through extrarenal mechanisms (29). There appears to be a small component related to renal clearance at low concentrations (29) and no apparent difference in clearance between cTnI and cTnT once released to the circulation. Overall, in patients with renal impairment, marked and/or dynamic cTn should not be assumed to be due to renal clearance issues, but likely reflective of myocardial injury.
Acute nonischemic myocardial injury
Multiple mechanisms can lead to acute nonischemic myocardial injury. Common cardiac etiologies include myocarditis, stress cardiomyopathy, and acute heart failure due to either systolic or diastolic dysfunction (9,24,25,30,31). Critical illness, acute pulmonary embolism, and sepsis can also cause cTn increases without overt myocardial ischemia that are categorized as acute nonischemic myocardial injury.
Abnormalities of systolic function and impaired myocardial relaxation may both develop in patients with sepsis. hs-cTnT and N-terminal pro–B-type natriuretic peptide (NP) increases best correlate with diastolic abnormalities and right ventricular enlargement (30). Inflammatory cytokines do not correlate with either systolic or diastolic abnormalities (31). COVID-19 studies have shown marked increases in NPs in patients with myocardial injury (5,6). These increases have prognostic implications in COVID-19, with Chen et al. (3) reporting mean N-terminal pro–B-type NP concentrations of 72 pg/ml in patients who recovered compared with 800 pg/ml in those who died (3). Although elevated, these are considerably lower than what has been seen in other studies of critical illness/ARDS (acute respiratory distress syndrome) (32). A number of COVID-19 reports also suggest that acute nonischemic myocardial injury might also be associated with a form of stress cardiomyopathy that is similar in presentation and course to that of Takotsubo syndrome (25,33).
Pulmonary embolism is another consideration in COVID-19, given data that procoagulant activity is increased (34, 35, 36). In a study of 184 intensive care unit (ICU) patients with COVID-19 pneumonia, Klok et al. (35) showed that despite standard thromboprophylactic doses, 31% developed thrombotic complications. The majority (81%) were due to pulmonary embolism; none were due to MI. Pathological data from COVID-19 patients has showed thrombotic microangiopathy is usually restricted to the lungs (36). These data have led to the recommendation to use prophylactic anticoagulation (34).
Myocarditis and myopericarditis are causes of acute nonischemic myocardial injury that warrant particular concern in COVID-19 (24,25). A shared feature in most but not all cases has been the presence of normal/nonobstructive coronaries despite ST-segment elevation (24,25,37).
Another potential mechanism leading to acute nonischemic myocardial injury is direct injury by SARS-CoV-2 as angiotensin-converting enzyme 2 (ACE2) receptors are present in myocardium and ACE2 is a functional receptor for SARS-CoV-2 (38). Pericytes have a high expression of ACE2, and their injury by SARS-CoV-2 can result in capillary endothelial cell and microvascular dysfunction (38). Patients with heart failure have a higher expression of ACE2 (38), which may explain their increased risk for myocardial injury from SARS-CoV-2.
Type 1 MI
There is a theoretical increased risk for acute atherothrombosis (type 1 MI) due to the inflammatory responses evoked by infection that can directly affect atherosclerotic plaques and increase procoagulant and prothrombotic activity (26). This has not been reported yet for COVID-19, and if anything, recently published data suggest a reduction in ST-segment elevation MI frequency (39). Concerns exist that symptomatic patients may not be seeking attention or present late given social distancing measures and concerns about nosocomial infection (39,40). Those who do present to the cardiac catheterization laboratory often present late (40). This could cause lower cTn concentrations due to the lack of washout of marker, or present with an unchanging pattern where cTn may have plateaued (41).
Type 2 MI
A higher risk for myocardial oxygen supply-demand mismatch (type 2 myocardial infarction [T2MI]) exists due to the responses to acute infection, including the release of interleukins, tumor necrosis factor-α, and catecholamines, as well as the consequences of hypoxia, acidosis, and hypotension or hypertension (26,42). All of these factors contribute to acute myocardial injury and ischemia if pronounced and/or sustained. Compared with COVID-19 patients without myocardial injury, those with myocardial injury have worse hypoxemia (5). It is tempting to label patients with such disturbances as having T2MI but without convincing clinical evidence of myocardial ischemia; this is a mistake (7). Clinicians should not diagnose T2MI unless the Universal Definition of Myocardial Infarction criteria are met. Recognizing the challenges in distinguishing injury from T2MI in those with ambiguous presentations, including those who are intubated or critically ill, we favor the use of objective features of clinical myocardial ischemia before deploying the diagnosis (42).
Critically, in some patients, a hyperinflammatory response and “cytokine storm” can ensue (43). Increases in certain cytokines and chemokines have been reported in COVID-19 ICU patients compared with non-ICU patients (1). This may contribute to the development of myocardial injury and, if ischemia is present, T2MI.
There is a paucity of data on MI subtypes in patients with acute infection (26). Data from the French regional RICO (obseRvatoire des Infarctus de Cote d’Or) (44) and UTROPIA (Use of TROPonin I in Acute coronary syndromes) (45) studies suggests that concomitant acute infection is frequent among patients with MI (RICO 10%; UTROPIA 29%). Most of the infections are respiratory (RICO 67% and UTROPIA 59%) and primarily among patients with T2MI.
The incidence of primary acute coronary etiologies in T2MI is uncertain and likely less common, but coronary thrombosis without atherosclerotic plaque disruption and microvascular dysfunction have been reported in patients with COVID-19 (46).
Pathological Studies
There are limited cardiac pathological data (25,36,47, 48, 49). The viral proteome has been detected in the myocardium using polymerase chain reaction (28). This indicates the presence of SARS-CoV-2 but not necessarily active infection. In an autopsy series, Fox et al. (36) reported cardiomegaly and right ventricular dilatation. None of the cases showed significant coronary artery stenosis or acute thrombus. Microscopic findings include cardiomyocyte hypertrophy; degeneration; necrosis; interstitial hyperemia; edema; and infiltration of lymphocytes, monocytes, and neutrophils (25,36,47,48). Notably, pathological examinations have failed to show the typical pattern of viral myocarditis (36), and several studies have been unable to detect SARS-CoV-2 in myocardium (25,36,47). The first case of biopsy-proven myocardial localization of viral particles with a morphology and size typical of coronavirus was recently reported in a patient confirmed to have COVID-19 (49).
Imaging
Given heterogeneous mechanisms leading to myocardial injury, in normal circumstances, imaging is used to clarify the etiologies of these morbid events. The logistics for imaging during the COVID-19 pandemic are problematic given the risk for infection among health care personnel. Because of its portability, echocardiography is often the initial preferred step. Point-of-care ultrasound use has grown substantially in COVID-19 and can also be considered a first-line option. Invasive coronary angiography or coronary computed tomography angiography should be reserved for when there is strong suspicion for obstructive CAD. Cardiac magnetic resonance imaging would be ideal as it can clarify more precisely the nature of acute myocardial injury. Cardiac magnetic resonance imaging, however, may not be available 24/7 and it may not be practical for imaging critically ill patients.
For the COVID-19 pandemic, there are both national and international recommendations for the selective use of noninvasive and invasive cardiac imaging modalities (50). All emphasize that for patients with suspected or confirmed COVID-19, cardiac imaging requires strong clinical information that the results will affect patient management. Given these constraints, serial cardiac biomarker measurements may be valuable in helping to decide when to employ imaging in patients with COVID-19.
Critically, not everyone with cTn increases >99th percentile (or even with an increasing pattern) requires imaging. If the increases are mild or modest, stable over time, and comporting to underlying comorbidities, imaging may not be needed. Likewise, imaging can likely be avoided or deferred in those with normal hs-cTn and NP, particularly if accompanied by a normal ECG. Selective imaging can be considered in COVID-19 patients with very marked cTn increases, as such are often observed in those with acute MI and myocarditis. If serial measurements are used, more advanced imaging can be considered in those with rising cTn and/or NP, particularly if accompanied by hemodynamic instability, unequivocal ischemic symptoms, ECG abnormalities, or left ventricular dysfunction on point-of-care ultrasound.
Future studies, such as the EACVI (European Association of Cardiovascular Imaging) echocardiography in COVID-19 registry, may help clarify who benefits the most from imaging. Further, an emerging role may exist for novel approaches, such as the use of ECG-based artificial intelligence algorithms that detect left ventricular dysfunction (51).
Prognostic Implications and Use of Cardiac Troponin for Risk Stratification
Cardiac troponin is a robust, continuous prognostic marker that has been demonstrated to be a reliable measure of short- and long-term cardiovascular risk, including in the setting of acute respiratory failure (21). In this context, data from COVID-19 studies demonstrating that increases in cTn are associated with adverse outcomes are not surprising (5,6), but further support the fact that cTn is a robust prognostic marker across a myriad of circumstances.
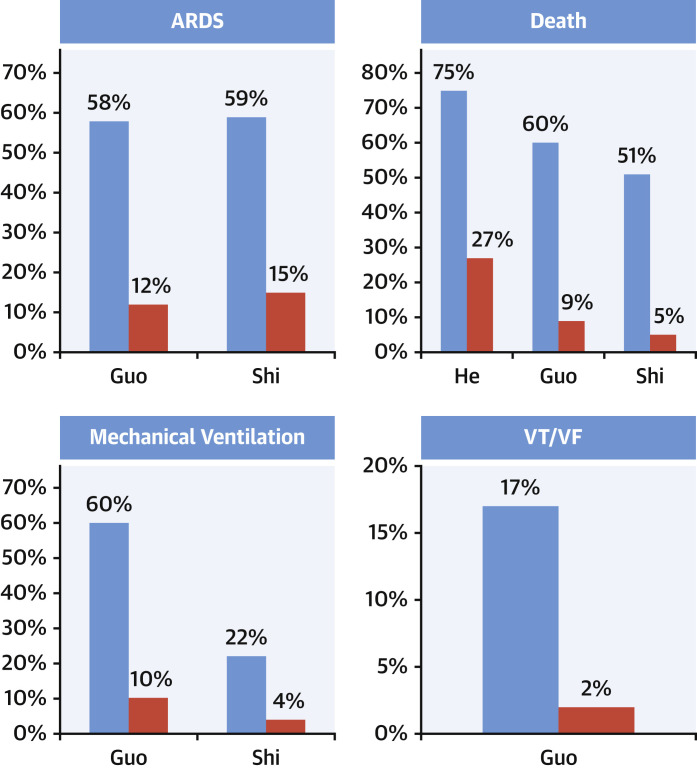
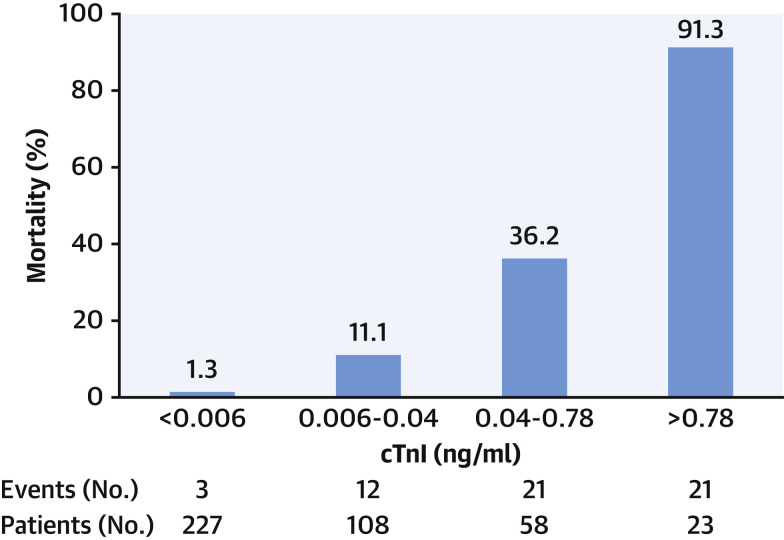
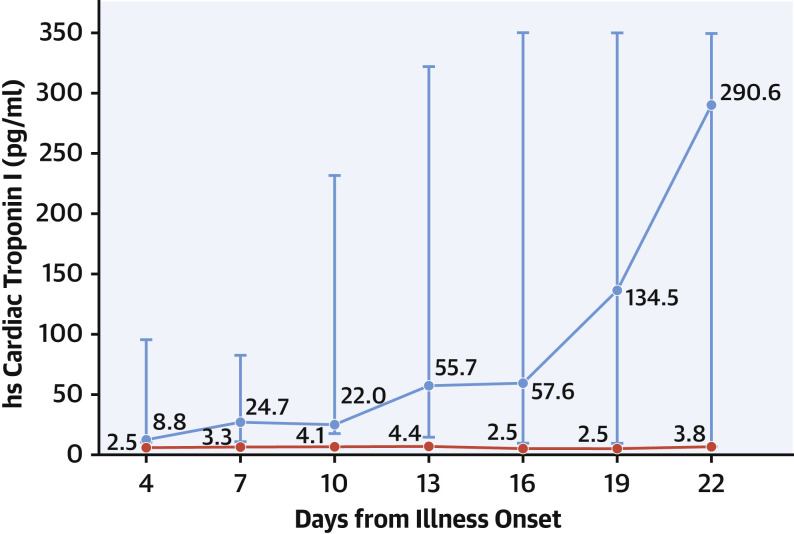
Several important messages have been reinforced by recent COVID-19 studies. First, a clear association exists between myocardial injury and adverse outcomes, including a higher risk for death and arrhythmias (Figure 3 ). Second, there is a continuous relationship between cTn concentrations and outcomes, with data from Shi et al. (6) showing that mortality rates increase with higher hs-cTn concentrations (Figure 4 ). Third, patients with chronic cardiovascular conditions diagnosed with COVID-19 are not only at higher risk for developing acute myocardial injury, but are at a higher mortality risk (5). Last, COVID-19 studies underscore the use of serial measurements to detect changing patterns of cTn (2,5) (Figure 5 ) and/or NP (5) that augment the distinction between survivors and nonsurvivors, and facilitate the identification of patients in whom further evaluation or interventions may be needed.
Figure 3.
Outcomes
Patients with myocardial injury (blue bars) have a higher risk of death, arrhythmias, ARDS, and mechanical ventilation as compared with those without injury (red bars). ARDS = acute respiratory distress syndrome; VF = ventricular fibrillation; VT = ventricular tachycardia.
Figure 4.
Relationship Between cTn and Mortality in COVID-19
Data from Shi et al. (6) showing that the higher the cardiac troponin (cTn), the worse the outcome, and vice versa. Abbreviations as in Figure 2.
Figure 5.
Serial Cardiac Troponin Measurements to Distinguish Survivors From Nonsurvivors
Data from Zhou et al. (2) showing stable (red line, survivors) versus dynamic (blue line, nonsurvivors) high-sensitivity (hs) cardiac troponin patterns.
Tension, however, exists in the preferred approach to testing, with some favoring a restrictive approach to testing that focuses on measuring cTn only if the diagnosis of acute MI or myocarditis is being considered on clinical grounds (52), whereas others advocate for more liberal testing for diagnosis and prognosis (53). There are advantages and disadvantages to each approach (Table 2 ). It is necessary to concede there are no data yet available demonstrating that specific treatment strategies should be employed (or avoided) in COVID-19 patients with myocardial injury.
Table 2.
Potential Advantages and Disadvantages of Systematic Cardiac Troponin Measurement in Patients With COVID-19
| Potential Advantages | Potential Disadvantages |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
ARDS = acute respiratory distress syndrome; other abbreviations as in Table 1.
Clinicians are often tempted to react to cTn increases, which can lead to potentially unnecessary evaluations and health care personnel exposure during the COVID-19 pandemic. In this context, we do not advocate for the liberal, unstructured use of cTn tests if such a strategy is not accompanied by clear education about the goals and potential implications and responses to cTn results prior to obtaining the test. However, if it is clearly understood that the role of cTn measurements is for risk-stratification purposes and to assist in defining when imaging studies may be needed, the structured use of cTn should not result in unnecessary or inappropriate downstream testing.
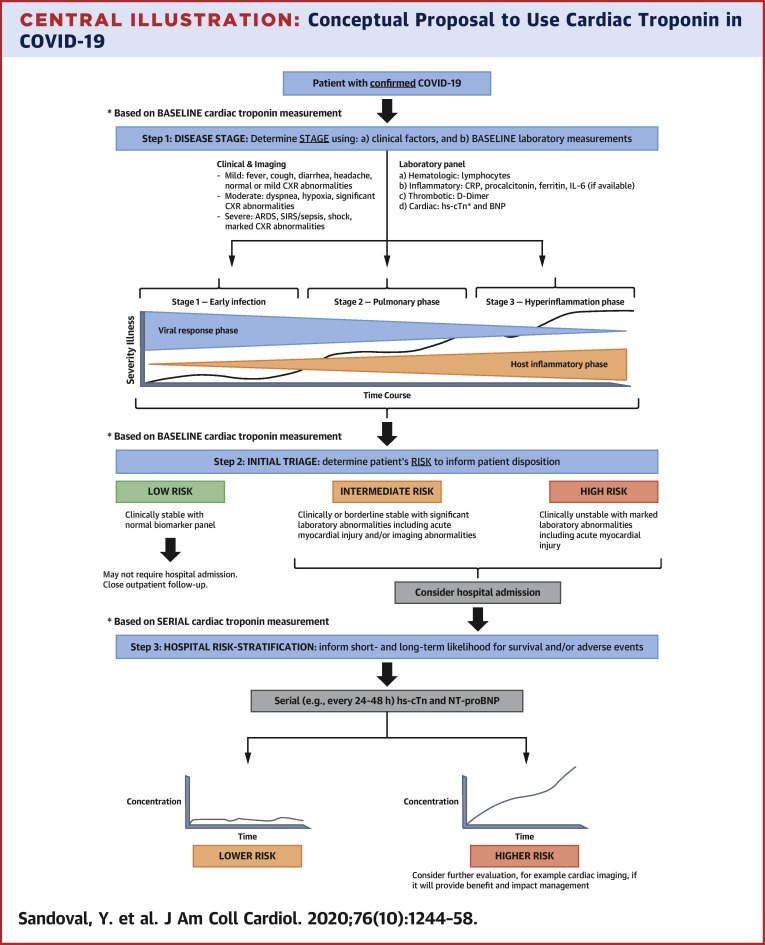
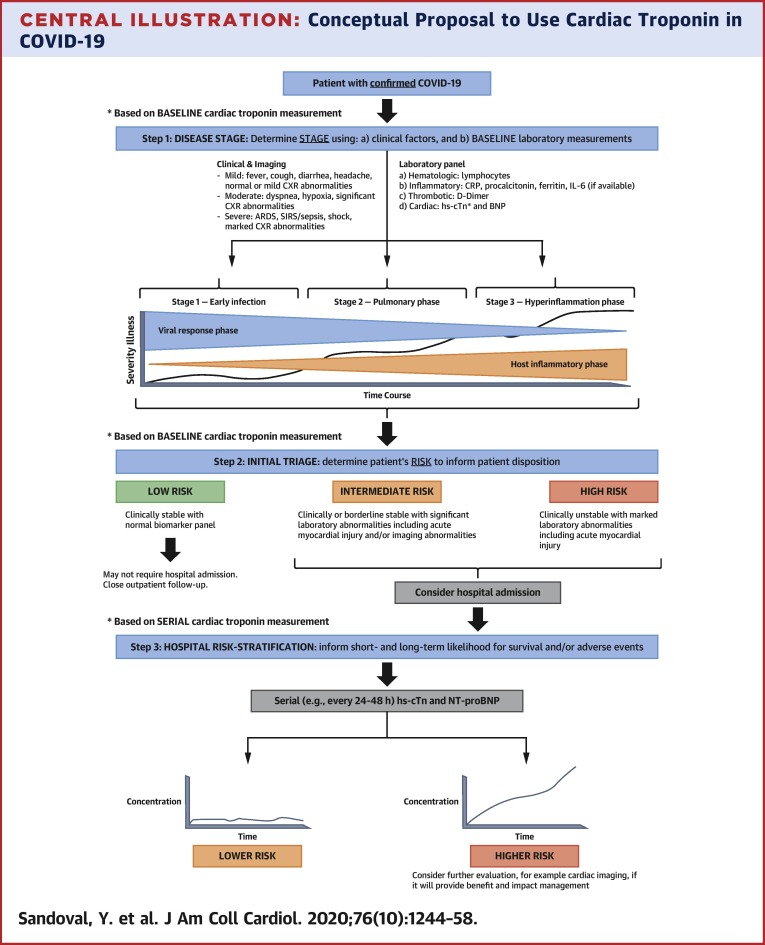
Serial cTn measurements facilitate the understanding of results compared with single-point measurements without a baseline sample. Some centers have incorporated cTn into their routine laboratory panel for COVID-19 patients. In the context of upfront clinical judgment and with the understanding that abnormal cTn results do not equate to acute MI, incorporation of cTn measurement to a set of other inflammatory and thrombotic markers to facilitate the understanding of COVID-19 stages (54), risk profiles, and disease phenotypes is reasonable (Central Illustration ). This information is likely to be most beneficial in those in whom disease stage and risk status is uncertain, where cTn can help with decisions about triage and level of care.
Central Illustration.
Conceptual Proposal to Use Cardiac Troponin in COVID-19
Baseline measurements can facilitate stage classification (52) and initial triage (steps 1 and 2), and serial measurements help with short- and long-term risk stratification (step 3). This information is likely to be most beneficial in those in whom disease stage and risk status is uncertain, where cTn can help with decisions about triage and level of care. ARDS = acute respiratory distress syndrome; CRP = C-reactive protein; CXR = chest x-ray; NT-proBNP = N-terminal pro–B-type natriuretic peptide.
Management
No established therapies exist for myocardial injury associated with COVID-19. For patients with myocardial injury, in general, the same concepts apply for patients with and without COVID-19. Management decisions should be based on serial measurements that facilitate decision-making. Patients with cTn increases that are not marked and stable (without a significant rise and/or fall) likely reflect chronic myocardial injury; particularly if there is a facile explanation such as chronic heart failure, cardiomyopathy, or chronic kidney disease. These patients do not require additional therapies except for treatment of their underlying conditions. Patients with acute nonischemic myocardial injury require individualized care for the specific conditions that caused the cTn increase. For those with cardiovascular etiologies, such as stress cardiomyopathy, myocarditis, or acute heart failure, usual clinical practice guideline recommendations apply. This also applies to those with arrhythmias that can also be seen with COVID-19, including cautions concerning QTc prolongation. For patients with overt myocardial ischemia in whom acute MI is diagnosed, those whose clinical presentation is thought to be most consistent with type 1 MI should be managed accordingly and receive evidence-based care. Those in whom acute atherothrombosis is thought to be less likely and T2MI is suspected can be managed conservatively. In general, the underlying trigger(s) should be corrected, and for those in whom underlying bystander concomitant CAD is present or suspected, therapies such as aspirin and statins are reasonable. The role of anticoagulation for cardiac indications in patients with COVID-19 is undefined, but prophylactic anticoagulation is recommended due to the increased risk for other thrombotic complications such as pulmonary embolism (34).
Conclusions
Cardiac troponin increases indicative of acute or chronic myocardial injury are frequent in patients with COVID-19 and associated with adverse outcomes. The increased inflammatory and thrombotic responses following COVID-19 infection increase the risk for both acute nonischemic myocardial injury and acute MI, particularly T2MI. To facilitate the detection of myocardial injury and risk stratification, as well as to potentially facilitate COVID-19 stage categorization and disease phenotyping, within the context of clinical judgment, a role may exist for serial cTn measurements among hospitalized patients with COVID-19.
Footnotes
Dr. Sandoval has served on the Advisory Boards for Roche Diagnostics (past) and Abbott Diagnostics without personal compensation; and has served as a speaker without personal financial compensation for Abbott Diagnostics. Dr. Januzzi is a Trustee of the American College of Cardiology; has received grant support from Novartis Pharmaceuticals and Abbott Diagnostics; has received consulting income from Abbott Diagnostics, Janssen, MyoKardia, Novartis, and Roche Diagnostics; and has participated in clinical endpoint committees/data safety monitoring boards for Abbott, AbbVie, Amgen, Bayer, CVRx, Janssen, and Takeda. Dr. Jaffe has consulted for or presently consults for most of the major diagnostics companies, including Beckman, Abbott, Siemens, ET Healthcare, Roche, Quidel, Sphingotec, Brava, Blade, and Novartis. Kuang-Yuh Chyu, MD, served as Guest Editor for this paper. P.K. Shah, MD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACCauthor instructions page.
Appendix
For a supplemental table, please see the online version of this paper.
Appendix
References
- 1.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1–8. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 8.Arentz M., Yim E., Klaff L., et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatraju P.K., Ghassemieh B.J., Nichols M., et al. Covid-19 in critically ill patients in the Seattle region – case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han H., Xie L., Liu R., et al. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J Med Virol. 2020;92:819–823. doi: 10.1002/jmv.25809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He X.W., Lai J.S., Cheng J., et al. Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 patients. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E011. doi: 10.3760/cma.j.cn112148-20200228-00137. [DOI] [PubMed] [Google Scholar]
- 12.Hui H., Zhang Y., Yang X., et al. Clinical and radiographic features of cardiac injury in patients with 2019 novel coronavirus pneumonia. medRxiv. 2020 Feb [E-pub ahead of print] [Google Scholar]
- 13.Lala A., Johnson K.W., Russak A.J., et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. medRxiv. 2020 Apr doi: 10.1016/j.jacc.2020.06.007. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y., Yang Y., Zhang C., et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi S., Qin M., Cai Y., et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41:2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou B., She J., Wang Y., Ma X. The clinical characteristics of myocardial injury 1 in severe and very severe patients with 2019 novel coronavirus disease. J Infect. 2020;81:147–178. doi: 10.1016/j.jinf.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cummings M.J., Baldwin M.R., Abrams D., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCarthy C.P., Raber I., Chapman A.R., et al. Myocardial injury in the era of high-sensitivity cardiac troponin assays: a practical approach for clinicians. JAMA Cardiol. 2019;4:1034–1042. doi: 10.1001/jamacardio.2019.2724. [DOI] [PubMed] [Google Scholar]
- 21.Vasile V.C., Chai H.S., Khambatta S., Afessa B., Jaffe A.S. Significant of elevated cardiac troponin T levels in critically ill patients with acute respiratory disease. Am J Med. 2010;123:1049–1058. doi: 10.1016/j.amjmed.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Kwong J.C., Schwartz K.L., Campitelli M.A., et al. Acute myocardial infarction after laboratory-confirmed Influenza infection. N Engl J Med. 2018;378:345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 23.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1286. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Inciardi R.M., Lupi L., Zaccone G., et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1096. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sala S., Peretto G., Gramegna M., et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musher D.M., Abers M.S., Corrales-Medina V.F. Acute infection and myocardial infarction. N Engl J Med. 2019;380:171–176. doi: 10.1056/NEJMra1808137. [DOI] [PubMed] [Google Scholar]
- 27.Kadesjö E., Roos A., Siddiqui A., Desta L., Lundbäck M., Holzmann M.J. Acute versus chronic myocardial injury and long-term outcomes. Heart. 2019;105:1905–1912. doi: 10.1136/heartjnl-2019-315036. [DOI] [PubMed] [Google Scholar]
- 28.Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fridén V., Starnberg K., Muslimovic A., et al. Clearance of cardiac troponin T with and without kidney function. Clin Biochem. 2017;50:468–474. doi: 10.1016/j.clinbiochem.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Landesberg G., Jaffe A.S., Gilon D., et al. Troponin elevation in severe sepsis and septic shock:the role of left ventricular diastolic dysfunction and right ventricular dilatation. Crit Care Med. 2014;42:790–800. doi: 10.1097/CCM.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 31.Landesberg G., Levin P.D., Gilon D., et al. Myocardial dysfunction in severe sepsis and septic shock:no correlation with inflammatory cytokines in real-life clinical setting. Chest. 2015;148:93–102. doi: 10.1378/chest.14-2259. [DOI] [PubMed] [Google Scholar]
- 32.Bajwa E.K., Januzzi J.L., Gong M.N., Thompson B.T., Christiani D.C. Prognostic value of plasma N-terminal probrain natriuretic peptide levels in the acute respiratory distress syndrome. Crit Care Med. 2008;36:2322–2327. doi: 10.1097/CCM.0b013e318181040d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hendren N.S., Drazner M.H., Bozkurt B., Cooper L.T., Jr. Description and proposed management of acute COVID-19 cardiovascular syndrome. Circulation. 2020;141:1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tachil J., Tang N., Gando S., et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bangalore S., Sharma A., Slotwiner A., et al. ST-segment elevation in patients with Covid-19– a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L., Li X., Chen M., Yen Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia S., Albaghdadi M.S., Meraj P.M., et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tam C.F., Cheung K.S., Lam S., et al. Impact of coronavirus disease 2019 (COVID-19) outbreak on ST-segment elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13 doi: 10.1161/CIRCOUTCOMES.120.006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bjurman C., Larsson M., Johanson P., Petzold M., Lindahl B., Fu M.L.X., Hammarsten O. Small changes in troponin T levels are common in patients with non-ST-segment elevation myocardial infarction and are linked to higher mortality. J Am Coll Cardiol. 2013;62:1231–1238. doi: 10.1016/j.jacc.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 42.Sandoval Y., Jaffe A.S. Type 2 myocardial infarction: JACC review topic of the week. J Am Coll Cardiol. 2019;73:1846–1860. doi: 10.1016/j.jacc.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 43.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 44.Putot A., Chaque F., Manckoundia P., Cottin Y., Zeller M. Post-infectious myocardial infarction: new insights for improved screening. J Clin Med. 2019;8:E827. doi: 10.3390/jcm8060827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandoval Y., Smith S.W., Schulz K., Sexter A., Apple F.S. Incidence and prognostic impact of infection among patients with type 1 and 2 myocardial infarction. Clin Chem. 2020 July 26 doi: 10.1093/clinchem/hvaa138. [E-pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 46.Dominguez-Erquicia P., Dobarro D., Raposeiras-Roubín S., Bastos-Fernandez G., Iñiguez-Romo A. Multivessel coronary thrombosis in a patient with COVID-19 pneumonia. Eur Heart J. 2020;41:2132. doi: 10.1093/eurheartj/ehaa393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao X.H., Li T.Y., He Z.C., et al. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 48.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tavazzi G., Pellegrini C., Maurelli M., et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur Heart J. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skulstad H., Cosyns B., Popescu B.A., et al. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging. 2020;21:592–598. doi: 10.1093/ehjci/jeaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Attia Z.I., Kapa S., Yao X., et al. Prospective validation of a deep learning electrocardiogram algorithm for the detection of left ventricular systolic dysfunction. J Cardiovasc Eletrophysiol. 2019;30:668–674. doi: 10.1111/jce.13889. [DOI] [PubMed] [Google Scholar]
- 52.Januzzi J., Jr. Troponin and BNP use in COVID-19. https://www.acc.org/latest-in-cardiology/articles/2020/03/18/15/25/troponin-and-bnp-use-in-covid19 Available at:
- 53.Chapman A.R., Bularga A., Mills N.L. High-sensitivity cardiac troponin can be an ally in the fight against COVID-19. Circulation. 2020;141:1733–1735. doi: 10.1161/CIRCULATIONAHA.120.047008. [DOI] [PubMed] [Google Scholar]
- 54.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.