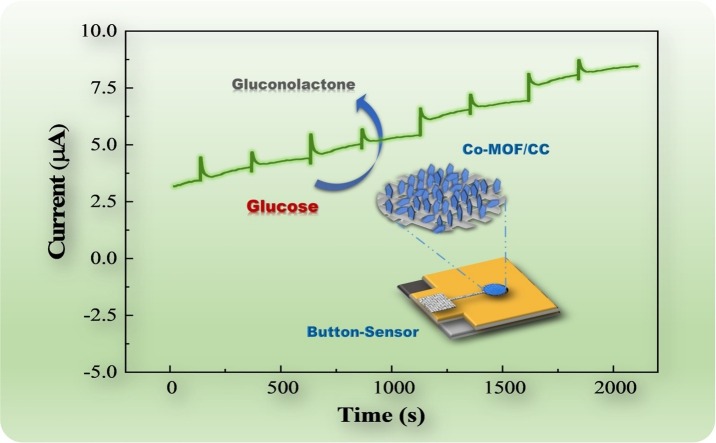
Graphical abstract
Keywords: Metal-organic framework, Carbon cloth, Paper-based device, Nonenzymatic sensor, Glucose diagnostics
Abstract
In the growing pandemic, family healthcare is widely concerned with the increase of medical self-diagnosis away from the hospital. A cobalt metal-organic framework modified carbon cloth/paper (Co-MOF/CC/Paper) hybrid button-sensor was developed as a portable, robust, and user-friendly electrochemical analytical chip for nonenzymatic quantitative detection of glucose. Highly integrated electrochemical analytical chip was successfully fabricated with a flexible Co-MOF/CC sensing interface, effectively increasing the specific area and catalytic sites than the traditional plane electrode. Based on the button-sensor, rapid quantitative detection of glucose was achieved in multiple complex bio-matrixes, such as serum, urine, and saliva, with desired selectivity, stability, and durability. With the advantages of low cost, high environment tolerance, ease of production, our nanozyme-based electrochemical analytical chip achieved reliable nonenzymatic electrocatalysis, has great potential for the application of rapid on-site analysis in personalized diagnostic and disease prevention.
1. Introduction
Global pandemic of COVID-19 has been causing a huge disaster for human beings, remaining thousands of deaths each day in the second quarter of 2020 [1]. Especially for the elderly, American Centers for Disease Control (CDC) statistics showed that lives over the age of 65 account for higher than 80 % of the total deaths in USA [2]. Among them, considerable fatal cases were recorded with comorbidities, such as circulatory diseases, respiratory diseases, and diabetes reaching top three incidences [3]. Facing the pandemic, the public healthcare systems are hardly adequate for regular surveillance of uninfected chronic patients and healthy individuals in low- or lower-middle-income countries. Therefore, the accessible point-of-care diagnostic testing (POCT) becomes extremely significant to support the self-assessment of individual health and prevent the infection of chronic patients, whose infection mortality is alarming. For example, POCTs for heart rate, blood pressure, and glucose can effectively monitor major chronic diseases and reduce severe or accidental damage by the timely diagnosis.
In recent decades, numerous commercial POCTs from the first report [4] for glucose have been employed for the rapid and portable detection of different clinical analytes, such as glucose dehydrogenase (GDH) and glucose oxidase (GOD)-mediated glucose sensing by glucometer [5], enzyme-linked immunoassay for human immunodeficiency virus (HIV)sensing by HIV kit, [6,7] and prostate-specific antigen (PSA)immuno-sensing in cancer analysis, [8,9]etc. Global POCT market analysis [10] shows that the market assessment of POCT reached 28.5 billion US$ in 2019, and predicts a robust increase to 46.7 billion US$ in 2024 at a compound annual growth rate of 10.4 %. With the advantages of high specificity, selectivity, and excellent catalytic performance, biological enzyme-mediated biochemical sensors have been applied in major POCTs with immuno-sensingand DNA-sensingmechanisms. For example, enzyme-catalytic optical methods were developed to construct fluorescent [11], chemiluminescent [12], and colorimetric [13,14] POCTs. The stable optical signal can be quantitatively read by the camera-based smartphone or portable electronic devices, whereas mini-light-source and customized optical components bring the cost issue. Some visual POCTs [15,16] can complete simple qualitative distinguish by naked eyes, but limited sensitivity is hard to fulfill the detection of clinical targets. Besides, enzyme-regulated electrochemical POCTs were proposed based on interfacial functionalization of the traditional electrode [17], and biochemical electrode-chip using novel materials [18]. Sensitive electrical sensing system can be easily assembled in the miniature electrochemical device with equipment-free readout, particularly suitable for the development of wearable POCTs [19]. Additionally, based on original handhold devices with temperature [20], pressure [21], or electrophoretic distance [22] as signal transduction, enzyme-related POCTs were gracefully fabricated with multiple technologies. However, those enzyme-assisted methods require high production cost of natural enzymes, rigorous storage condition to maintain uniform enzyme activity, moderate measuring condition to avoid the influence of temperature and humidity, which greatly limit the promotion of POC application.
In order to solve the inherent shortcomings of natural enzymes, various artificial enzymes, namely called nanozymes, have been developed to hold enduring catalytic property, and robustness under ambient conditions for POC diagnostics. Multiple configurations of nanozymes, such as carbon-, metal-, alloy-, metallic oxide-, polymer-, and composite-based nanomaterials, were successfully synthesized and screened with competent catalytic abilities [23]. Among them, most catalytic performances focus in oxidase-mimicking, peroxidase-mimicking,catalase-mimicking families, and a few of hydrolase-mimicking nanozymes were found. The programmable nanozymes can be easy to manufacture with the cost-effectiveness, more durable and steadier in the catalytical reaction with comparable sensitivity, more diverse via surface and internal functionalization with desired versatility. Besides, the bio-/nano-mixed enzyme-based POCTs were built with a pathway of hybrid catalysis to combine the high selectivity of natural enzyme and the catalytic durability of nanozyme [24,25]. The classic model of glucose detection has been commonly used in the synergistic work of glucose oxidase and horseradish peroxidase-mimicking nanozyme [26]. As an illustration, Qu’s group [27] reported an ultrathin 2D metal-organic framework (MOF)/GOD hybrid nanocatalyst to trigger self-activated cascade reaction for the antibacterial effect in wound healing. The synergistic effect of flaky MOF and GOD fully activated the antibacterial capacity by generating abundant extremely toxic hydroxyl radial, intelligently applying pH reducing to increase the catalytical performance of flaky MOF. Therefore, due to the significant features of nanozymes, POCTs of different diseases can be flexibly integrated to complete the rapid on-site detection in most complex situations.
MOF nanomaterial as one of the typical nanozymes has been widely applied for anti-bacteria, bio-sensing, therapeutics, and bio-imaging, etc. [28]. MOF-based nanozyme with considerable merits, including large specific surface area, abundant catalytic sites, and tailorable multi-dimensional configurations shows great potential for the point of care. Multitudinous primeval MOFs, MOF-based derivatives, or composites have been compliantly constructed according to different enzyme-mimicking performances. For instance, Ling’s group [29] constructed a Pt@ metalloporphyrin-MOF integrated electrocatalytic interface to achieve catalase- and peroxidase-like multi-enzymes catalysis, successfully replacing two common natural enzymes by the MOF-based composite. Wu’s group [30] reported an intensive and persistent chemiluminescent system relying on iron porphyrin MOF/GOD composite for the dual nano-/bio-enzyme catalysis. Moreover, part of fluorescent sensor [31] and electrochemical sensor [32]were proposed by MOF-mediated catalytical sensing. Most applications were reported based on bulky instruments and professional operations, whereas few MOF-nanozyme-based POCTs were established. Given the urgent demand for family healthcare in the era of pandemic, MOF-based nanozymes should be broadly employed in portable biochemical sensors for the development of more POC applications in resource-limited regions.
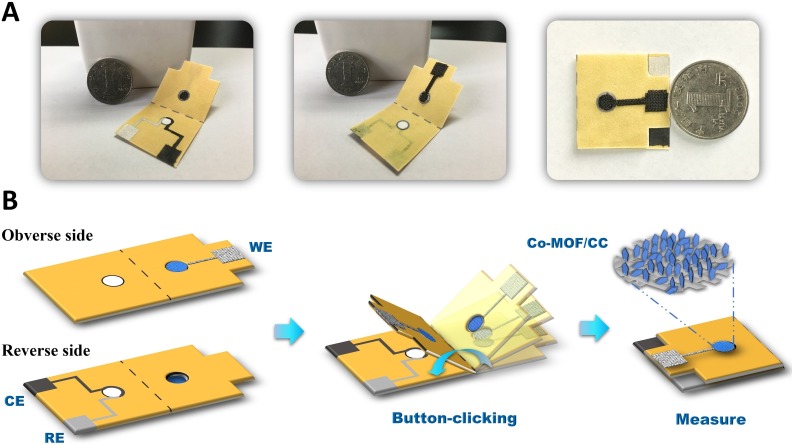
In this work, we fabricated a cobalt-MOF (Co-MOF) modified carbon cloth/paper (CC/Paper) hybrid button-sensor as portable, robust, and user-friendly electrochemical analytical chip for nonenzymatic quantitative detection of glucose. Cobalt-MOF moderately crystalized on the CC at ambient state and assembled with the patterned paper electrode to form a hybrid button-sensor as shown in Fig. 1 . The introduced button-like architecture can effectively isolate the working electrode from sample region, where simple filtration or incubation can be executed for the complex biomatrix [33]. Glucose, as a classical biomarker of diabetes, was successfully detected on the button-sensor, achieving high selective and stable quantification in multiple complicated biosystems. Flexible CC eligibly provides a programmable working electrode with a sufficient conductive area in limited mini-device, where the patterned CC film can be easily fabricated by the laser cutter. Compared with the traditional glass carbon electrode, the porous cellulose structure of CC enables to amplify the specific surface for more crystallization, and its ductile character reserves the possibility as a wearable sensor. Furthermore, pentagon-like Co-MOFs as the nanozyme of our sensor densely grew on the CC to maximize the catalytic sites and promote the sensitivity. Considering the diversity of MOFs, metal centers, ligands, and framework structures can be modulated to screen out that highly selective and stable alternatives. Without the bio-enzyme, synthesized Co-MOF/CC didn’t need to be preserved under constant temperature, and maintained a robust activity in 2 months at ambient surroundings, meeting the critical requirement as POCT. Finally, on the basis of previous paper-based POCTs for colorimetric analysis [34], cheap and portable cellulose carrier was fast wax-/screen-printed to fabricate the electrochemical button-sensor, which can be obediently incorporated with microfluidic and origami process for the multi-steps reaction. This highly integrated CC/Paper hybrid button-sensor not only can achieve largescale production but also easy to burn for the prevention of biochemical pollution, given as an important criterion in pandemic. Due to above significant features, our nanozyme-based hybrid electrochemical button-sensor owns great prospects for portable detection of clinical biomarkers in family diagnostics, personalized healthcare, and disease prevention.
Fig. 1.
Photographs (A) of button-sensor and 3D schematic (B) of the assay procedure.
2. Experimental section
2.1. Reagents and materials
Cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O), 2-methylimidazole (C4H6N2), ascorbic acid (AA), Urea, sucrose (Suc), fructose (Fru), Lactose (Lac), glucose, and l-tryptophan (L-Trp) were all purchased from J&K Chemical (Shanghai, China). Potassium chloride (KCl), sodium hydroxide (NaOH), sulfuric acid (H2SO4), 30 % H2O2, and other reagents were applied from Sinopharm Chemical Reagent (Shanghai, China). All reagents were of analytical grade and were used without further purification. Body fluids, including serum, urine, saliva samples from healthy individuals, were provided by the affiliated hospital (group) of Putian University. The aqueous solutions were prepared with deionized water obtained from a Milli-Q Plus system (18.2 MΩ cm; Millipore Inc., USA).
Carbon cloth (WOS1009) was purchased from CeTech (Taiwan, China). Whatman No.1 filter paper (size, 46 × 57 cm2) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Carbon ink (CH-8(MOD2)) and silver/silver chloride (Ag/AgCl) ink (C2130809D5) were respectively obtained from JuJo printing supplies & technology (Pinghu, China) and Gwent Group (Torfaen, United Kingdom).
2.2. Instrumentation and apparatus
All electrochemical measurements were performed on a CHI660E electrochemical workstation (Shanghai Chenhua Instrument Co., Ltd.). A standard three-electrode system was used by a circular Co-MOF/CC spot (∅ =5.6 mm) as the working electrode, a carbon screen-printed semi-loop as the counter electrode, and a Ag/AgCl screen-printed semi-loop as the reference electrode. All morphological analysis of nanomaterial and CC was investigated by a SU8010 field-emission scanning electron microscope (FE-SEM, Hitachi, Japan). XRD patterns and absorption spectrum were separately taken on an X-ray diffractometer (Rigaku, Japan) and a Thermo Scientific microplate reader (Waltham, MA, USA).
2.3. Preparation of Co-MOF/CC working interface
The bare CC was dipped in the mixed solution (1:1, v/v) of concentrated sulfuric acid and 30 % H2O2 with an ultrasonic treatment at room temperature for 2 h., getting the activated CC after rinse with water and ethanol for three times. Meanwhile, Co(NO3)2 solution (10 mL, 125 mM) and 2-methylimidazole solution (10 mL, 1.0 M) were quickly mixed and diluted to 50 mL by water as Mixture-1. The circular section of pretreated CC was immersed into Mixture-1 for 4 h. at room temperature, and then was cleaned with water and dried overnight in the air. The capacity of Co-MOFs on CC is approximately 10.0 mg/cm2.
2.4. Fabrication of Co-MOF/CC/Paper hybrid button-sensor
A wax patterning method was applied to fabricate the paper-based carrier of button-sensor. The pattern of carrier was designed using Adobe Illustrator2018 software. Paper patterns were fabricated on filter paper using a Xerox Colorqube 8580 wax printer and then placed in a thermostat at 110 °C for 1.5 min. The final paper carrier was obtained by natural cooling to ambient temperature. Carbon ink and Ag/AgCl ink were screen-printed onto the sample reservoir (∅ =6 mm) in the reserve side, while Co-MOF/CC was glued to cover the hollow-spot in the observe side for constructing a three-electrode system. This integrated hybrid chip introduced the medium dotted line and two carved squares to assist the operation in the measurement, finally folded to be a ready Co-MOF/CC/Paper hybrid button-sensor.
2.5. Electrochemical measurement
The electrochemical performance of Co-MOF/CC/Paper hybrid button-sensor was investigated by electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV). Both of them were executed in 0.1 M NaOH solution (20 μL) as the electrolyte. The frequency range in EIS was set from 0.1 Hz to 100 kHz with a response value by the Zview2 software. The potential range in CV was selected between 0.0 to +0.7 V with a scan rate of 50 mV/s. For the quantitative detection of glucose, amperometric response was measured in 0.1 M NaOH (20 μL) at the constant potential of 0.45 V over 50 s for a saturation current. Similarly, the amperometric responses of glucose spiked samples were detected to evaluate the stability and recovery of button-sensor in different body fluids, where the serum (5-fold dilution), urine (4-fold dilution), saliva (4-fold dilution) samples were diluted in 0.1 M NaOH as electrolytes.
3. Results and discussion
3.1. Characterization of Co-MOF/CC
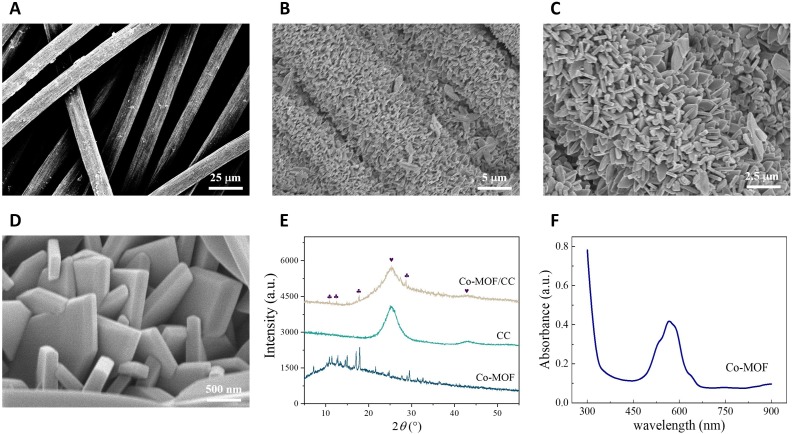
The key catalytical interface of button-sensor was characterized to validate the effective synthesis of Co-MOF crystals on the CC fibers. FE-SEM was first applied to inspect the morphological feature of CC and crystal structure of Co-MOF. As shown in Fig. 2 A, micronsized carbon fibers intertwined randomly with large specific surface area, which can support the anticipative conductance.After a facile modification under the ambient condition, tremendous angular Co-MOFs densely grew along the fiber bundle with the stuffed gap among fibers (Fig. 2B), and their uniformity was also acceptable with few bigger foliate forms (Fig. 2C). The major Co-MOFs showed a pentagon shape at sub-micron level with the non-oriented array (See Fig. 2D). Compared with the flat interface of traditional electrodes, the abundant contacting sites of Co-MOF/CC, as potential catalytic sites were successfully obtained in stereoscopic space. Additionally, XRD patterns of Co-MOF, CC, and their composite are exhibited in Fig. 2 E to confirm the actual crystallization. For the pattern of Co-MOF/CC, two specific broad peaks (marked with the heart) were measured at 2θ 26.5°, and 44.0° ascribing to CC, and other observed peaks (marked with the club) at 2θ 11.6°, 12.4°, 18.2°, and 30.0° neatly aligned with those from pristine Co-MOF, meanwhile matching with the XRD pattern of reported ZIF-L [35]. The XRD results verified the pentagonal Co-MOF with well crystallinity, while many sprawling weak peaks arose on account of diverse crystal orientation. Furthermore, the absorbance spectrum of Co-MOF solution was investigated as seen in Fig. 2 F. The maximum absorbance peak located at 570 nm in line with the similar spectral pattern of ZIF-L [36]. Taken together, Co-MOF/CC was successfully fabricated with a pure crystalline phase through a straightforward ambient incubation.
Fig. 2.
Material characterization: SEM images of (A) CC, and (B to D) Co-MOF. (E) XRD patterns of Co-CMOF, CC and their composite. (F) Absorbance spectrum of Co-MOF solution.
3.2. Feasibility of glucose determination based on button-sensor
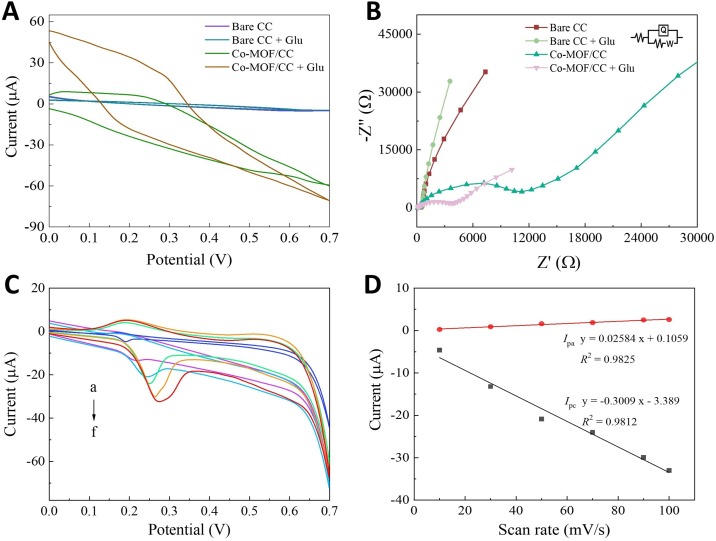
To investigate the feasibility of glucose determination, some electrochemical experiments based on the Co-MOF/CC/paper hybrid button-sensor were conducted by cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). Fig. 3 A shows the CVs of bare CC and Co-MOF/CC on the button-sensor in 0.1 M NaOH solution at a scanning rate of 50 mV/s. It’s clearly defined that no redox peaks are observed for the bare CC (purple curve), indicating an electrochemical silence in the selected potential range. As a catalysis-free control, 1 mM glucose sample was measured on the bare CC with the negligible current change (dark-cyan curve), which means no redox reaction occurs in the absence of the catalyst. While one couple of redox peaks can be measured on the Co-MOF/CC (green curve), because redox reaction of some Co ions close to the CC in alkaline electrolyte [37]. However, their current intensities of redox peaks were still faint, hard to read the cathodic peak with inferior symmetry. With the adding of glucose, the redox peaks (red curve) have an obvious increase of current intensity and about 100 mV potential separation as a quasi-reversible electron-transport process. This result proved the feasibility of glucose determination based on the Co-MOF/CC/paper hybrid button-sensor. Similar to previous reports of cobalt nanomaterials, [37,38] the possible redox mechanism of Co-MOF/CC in alkaline condition toward glucose can be described as follows:
| Coδ+ + nOH¯ + H2O ↔ CoOOH + ne¯ | (1) |
| CoOOH + H2O ↔ Co4+ + 3OH¯ + e¯ | (2) |
| Co-MOF + glucose ↔ CoOOH + gluconolactone + H2O | (3) |
Fig. 3.
(A) CV and (B) EIS plots of bare CC, Co-MOF/CC as the working electrode on button-sensor recorded in 0.1 M NaOH aqueous solution. EIS plots were measured in the range of 0.1 Hz to 100 kHz at a potential of 0.25 V and an alternating-current amplitude of 5.0 mV. (C) CV curves of Co-MOF/CC with different scan rates in 0.1 M NaOH containing 1.0 mM Glucose. Curves a-f are obtained at 10, 30, 50, 70, 90, and 100 mV/s, respectively. (D) The plot of peak current vs scan rate.
Moreover, EIS analysis also conformed an effective surface modification of Co-MOF and expectant glucose sensing performance. As displayed in Fig. 3B, the fitted charge-transfer resistance (R ct) of bare CC in 0.1 M NaOH was too weak to be considered by reason of the excellent conductance. Conversely, a significant increase up to 11000 Ω of R ct was measured after the assembling of Co-MOF/CC, suggesting that a robust deposition of MOFs was formed to hinder the conductivity by multiple insulating organic ligands. In the presence of glucose (1 mM), no detectable change of R ct happened on the bare CC, indicating no effect by the absorbance of glucose on the carbon fibers. However, R ct of Co-MOF/CC dramatically reduced to about 4000 Ω. We inferred this result was caused by two reasons: one is the catalytic property of Co-MOF, and the other is the extensive adsorption of glucose on the surface or in the porous gap of Co-MOF/CC. They together promoted efficient charge-transferring via the electrocatalytic reaction of glucose. Further to verify our hypothesis, the influence of scan rates (v) for the anodic and catholic peak current (I pa and I pc) of glucose was investigated on Co-MOF/CC via CV. As seen in Fig. 3C&D, both peak currents increase linearly along with the v in the range of 10–100 mV/s. Thus, the electrochemical reaction of glucose on the Co-MOF/CC was concluded as a typical surface-controlled process, which can be one reason for the significant drop of R ct.
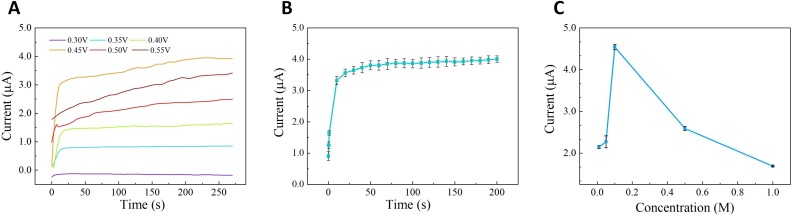
3.3. Condition optimization of button-sensor
Three major parameters of a button-sensor in the electrocatalytic assay were optimized for a stronger current response of 1 mM glucose, namely, the constant potential, the catalytic time, and the concentration of electrolyte. Considering the main impact of current from the constant potential in amperometric method, the saturated intensity, and stability of I-T curve were comprehensively evaluated in the potential range from 0.30 to 0.55 V. As shown in Fig. 4 A, the saturated current intensity increased with an increase of the constant potential from 0.30 to 0.45 V and then decreased after 0.45 V. Meanwhile, the platform of I-T curve emerged later after 0.45 V with more fluctuation of signals, which reduced the stability and testing efficiency in one assay. So, 0.45 V of constant potential was selected as the optimal working potential in amperometric measurement. To reduce the evaporation of loaded sample and ambient influence, the reaction time of integrated system on the portable chip should be controlled as short as possible. The catalytic time, occupying the main time of detection process, was inspected for the dynamic change of current response against the time (See Fig. 4B). The current signal increased remarkably in the first 10 s and then maintained a slow increase to 50 s. No significant current change was observed after 50 s, reaching a signal platform. Herein, 50 s was picked as the optimal catalytic time on the button-sensor. Except for the optimization of reaction setting, the electrocatalytic reaction should stably work in the proper electrolyte. Different concentration of NaOH solution was investigated for the change of saturated current. Fig. 4 C shows that the current increased up to a peak value with an increase of NaOH concentration from 0.01 to 0.1 M and then decreased after 0.1 M. Their relative standard deviations (RSDs) were calculated out under 5% with an acceptable level, apart from the result in 0.05 M NaOH. As a result, 0.1 M NaOH was chosen as the supporting electrolyte for the button-sensor.
Fig. 4.
Optimization of the (A) constant potential, (B) catalytic time, (C) concentration of electrolyte. The error bars represent standard deviations from three parallel measurements.
3.4. Quantitative detection of glucose and selectivity
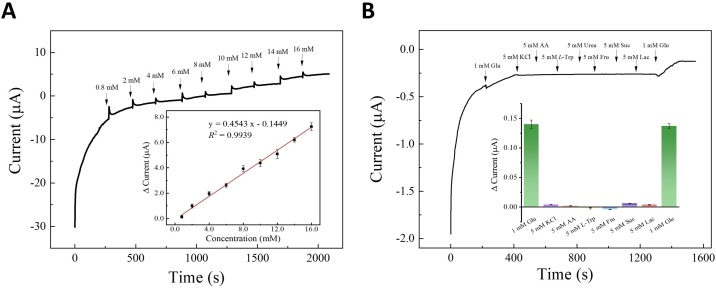
The performance of the portable sensor for the quantification of analyte and selectivity is significant as a practical analytical chip. The sensing sensitivity and detection range of proposed button-sensor were investigated in the detection of glucose. Glucose samples with different concentrations from (0 ∼ 16 mM) were tested in three parallel measurements. As shown in Fig. 5 A, the current intensity increased with the increase of the concentration of glucose from 0.8–16 mM. Taking the initial current without glucose as the blank, the Δcurrents after adding the glucose in series were calculated and plotted versus the concentration of glucose. A desired linear calibration curve was obtained between the mean Δcurrent and glucose concentration in the range of 0.8 mM to 16 mM with the R 2 value of 0.9939. The LOD of 0.15 mM glucose was achieved based on 3σb/slope, where 3σb means 3-folds of the standard deviation vs the blank signal. Compared with enzyme-based commercial glucometers [39], our nonenzymatic button-sensor has comparable detection sensitivity by the robust nanozyme-based catalysis. Moreover, based on the programable working electrode of button-sensor, higher sensitivity required in different bio-matrixes can be accessed by multiple approaches. First, the electrosensing area enables to be simply extended by larger CC spot or other CC types with higher fiber-density. Second, MOF-nanozyme can be easy to composite with common electroactive components, such as graphene-based nanofilms, and metal-based nanoparticles. The conductance of sensing interface on the button-sensor can be flexibly regulated without the concern of compatibility in the enzyme-catalytic system. Overall, the experiment results established that the button-sensor can be employed for portable quantitative detection of glucose.
Fig. 5.
(A) Amperometric I-T curve of Co-MOF/CC to the successive addition of glucose from 0.8 mM to 16 mM in continuously stirred 0.1 M NaOH at 0.45 V (Inset: corresponding calibration curve of current vs glucose concentration). (B) Amperometric response of Co-MOF/CC to different possible distractors from bio-matrix (Inset: related histograms of current vs analyte). The standard deviations were obtained from three parallel measurements.
Considering the application of a button-sensor in complicated biochemical matrixes, the selectivity of Co-MOF/CC sensing interface for glucose (Glu) was evaluated and exhibited in Fig. 5 B. Several probable coexistences, including metal ion, small molecule, amino acid in serum and urine, and structural analogues of glucose were tested in the electrolyte with 5-fold concentration of glucose. As presented, only the specific target, glucose (1 mM) showed an effective increase of current signal near 0.15 μA at the first and last adding, whereas negligible responses were observed from 5 mM KCl, AA, L-Trp, urea, Fru, Suc, and Lac. This result confirmed the high selectivity of button-sensor for glucose detection.
3.5. Real sample analysis
To validate the effectivity of button-sensor in complex bio-matrixes, glucose spiked samples in multiple body fluids, including serum, urine, and saliva, were measured and listed in Table 1 . All recoveries from different concentrations of glucose were identified at a reasonable level between 87 % and 109 %, and all the coefficients of variation (CVs) are less than 12 %. Therefore, the button-sensor possesses the desired applicability and vast potential of clinical application in complicated body fluids.
Table 1.
Detection of Glucose Spiked in Serum, Urine, and Saliva Samples.a
| Real samples | Spiked concn of glucose (mM) | Av measurement ± SD (mM) | CV (%) | Recovery (%) |
|---|---|---|---|---|
| Serum | 0.6 | 0.56 ± 0.04 | 7.14 % | 93.33 % |
| 5 | 4.36 ± 0.37 | 8.49 % | 87.20 % | |
| Urine | 1.5 | 1.43 ± 0.13 | 9.09 % | 95.33 % |
| 10 | 10.55 ± 1.22 | 11.56 % | 105.50 % | |
| Saliva | 7 | 7.6 ± 0.51 | 6.71 % | 108.57 % |
| 14 | 13.80 ± 0.86 | 6.23 % | 98.57 % |
Standard deviations (SDs) and coefficients of variation (CVs) were obtained from three parallel measurements.
3.6. Comparison with glucometer and stability investigation
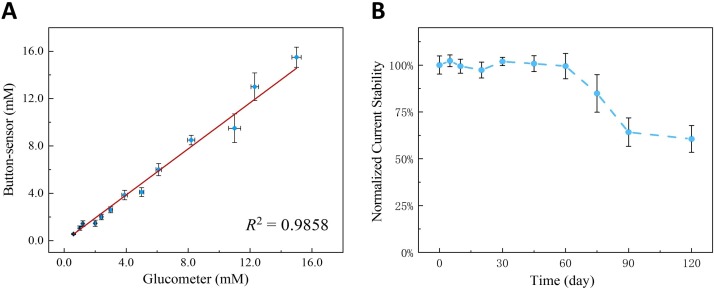
The rapid quantification of glucose is usually executed for diabetics by the commercial glucometer as personalized surveillance. To validate the credibility of the portable button-sensor, the glucometer was utilized as a comparison for glucose detection in serum. 13 samples in serum were evaluated by two kinds of devices. Their results were fitted as a calibration curve in Fig. 6 A, and the error bars of each measurement were obtained from three parallel assays on different button-sensors (y-axis) or glucose-strips (x-axis). An obvious positive relationship between the two readouts was found, with a slope of 0.974 ± 0.0353 and a correlation coefficient of 0.9858. Considering the experiment error, these results from the two devices matched very well. The result sufficiently indicated that the accuracy of our button-sensor was comparable to that of a commercial glucometer. Besides, the reproducibility of the entire detection process was determined for 1 mM glucose by 5 independent button-sensors. The RSD of current change was calculated to be 9.1 %, showing an acceptable reproducibility as a portable analyzer. The error was presumably ascribed to the difference of ablated CC size and evaporation in some extent. At the ambient condition without special storage, the long-term stability of button-sensors was researched for the periodic trial over 4 months. The time-dependence evolution in Fig. 6 B shows the normalized current maintains at a stable platform in 60 days, and then gradually decreased to about 60 % after 120 days. The electrocatalytic performance of Co-MOF/CC can be kept in an optimum state without the storing cost, whereas the special enveloping to avoid deactivation and the strip-coding due to the difference in enzymatic activity have to be required for glucose strips. Consequently, the result verified that our nonenzymatic electrochemical method on the button-sensor is trustworthy as an alternative test with satisfactory durability.
Fig. 6.
(A) Comparison of the button-sensor with the commercial glucometer based on 13 samples in serum. (B) Time-dependence evolution of normalized current stability for glucose detection on the button-sensor. The markers and error bars reflect the average and standard deviations of three measurements.
4. Conclusions
In conclusion, we have developed a novel Co-MOF/CC/Paper hybrid button-sensor as the simple and portable electrochemical analytical chip to achieve the nonenzymatic quantification of glucose. The flexible Co-MOF/CC sensing interface not only provides adequate catalytic sites and high specific area but also effectively integrated with the patterned paper to form an electrochemical sensing coin-chip. Rapid quantitative detection of glucose on-chip demonstrated the reliable sensitivity and selectivity of Co-MOF/CC, meanwhile exhibited the excellent robustness and durability in multiple complex bio-matrixes. The commonly used enzyme in traditional glucose testing was successfully replaced by artificial nanozyme, Co-MOF with low cost, high environment tolerance, and ease of production. Additionally, abundant MOFs and related composites present a promising platform for different biochemical analytes, and MOF-based electrochemical chips own great potential for the application of rapid on-site analysis in terms of home healthcare and disease control.
CRediT authorship contribution statement
Xiaofeng Wei: Conceptualization, Methodology, Validation, Formal analysis, Writing - review & editing, Funding acquisition. Jialei Guo: Validation, Formal analysis, Writing - original draft. Huiting Lian: Methodology, Writing - review & editing. Xiangying Sun: Supervision, Project administration. Bin Liu: Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We would like to acknowledge the financial support from the National Natural Science Foundation of China (Grant no. 21904043 and 21575044), Natural Science Foundation of Fujian Province of China (Grant no. 2015J01054 and 2016J01062), Foundation of Graphene Powder & Composite Research Center of Fujian Province (2017H2001).
Biographies
Xiaofeng Wei* received his Ph.D. degree in analytical chemistry from Fuzhou University in 2016. Now he is working as a lecturer in College of Materials Science and Engineering of Huaqiao University. His academic interests involve microfluidics, biomimetic application of nanomaterials, biochemical sensors, point-of-care diagnostics and bioanalytics.
Jialei Guo received her M.S. degree in analytical chemistry from Huaqiao University in 2020. She finished the main academic research in chiral electrochemical sensor and novel metal-organic framework-based sensing interface.
Huiting Lian is associate professor in College of Materials Science and Engineering of Huaqiao University. She owned her M.S. degree from East China Normal University in 1998. Her research interests include analysis of chiral drug, electrochemical analysis, and nanomaterial-based sensing interface.
Xiangying Sun is full professor in College of Materials Science and Engineering of Huaqiao University. She received her Ph.D. degree in analytical chemistry from Xiamen University in 2002. Her current academic research interests involve supramolecular self-assembled film, photoelectric sensing interface, and surveillance and analysis of environmental pollutants.
Bin Liu* is full professor in College of Materials Science and Engineering of Huaqiao University. He earned his M.S. degree from East China Normal University in 1989. His current research interests include chiral electrochemical recognition, electrochemical sensor, and electro-catalysis of nanomaterials.
References
- 1.2020. World Health Organization.https://covid19.who.int/ [Google Scholar]
- 2.2020. Centers for Disease Control and Prevention.https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/index.htm [Google Scholar]
- 3.2020. Centers for Disease Control and Prevention.https://data.cdc.gov/d/hk9y-quqm/visualization [Google Scholar]
- 4.Clark L.C., Jr, Lyons C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N.Y. Acad. Sci. 1962;102:29–45. doi: 10.1111/j.1749-6632.1962.tb13623.x. [DOI] [PubMed] [Google Scholar]
- 5.Sehit E., Altintas Z. Significance of nanomaterials in electrochemical glucose sensors: an updated review (2016-2020) Biosens. Bioelectron. 2020;159:112165. doi: 10.1016/j.bios.2020.112165. [DOI] [PubMed] [Google Scholar]
- 6.Liu D., Zhang Y., Zhu M., Yu Z., Ma X., Song Y., et al. Microfluidic-integrated multicolor immunosensor for visual detection of HIV-1 p24 antigen with the naked eye. Anal. Chem. 2020;10 doi: 10.1021/acs.analchem.0c02091. 1021/acs.analchem.0c02091. [DOI] [PubMed] [Google Scholar]
- 7.Saylan Y., Erdem O., Unal S., Denizli A. An alternative medical diagnosis method: biosensors for virus detection. Biosensors (Basel) 2019;9 doi: 10.3390/bios9020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen M., Li N., Lu Y., Cheng J., Xu Y. An enhanced centrifugation-assisted lateral flow immunoassay for the point-of-care detection of protein biomarkers. Lab Chip. 2020;20:2626–2634. doi: 10.1039/d0lc00518e. [DOI] [PubMed] [Google Scholar]
- 9.Barbosa A.I., Castanheira A.P., Edwards A.D., Reis N.M. A lab-in-a-briefcase for rapid prostate specific antigen (PSA) screening from whole blood. Lab Chip. 2014;14:2918–2928. doi: 10.1039/c4lc00464g. [DOI] [PubMed] [Google Scholar]
- 10.Marketsandmarkets . 2019. Point of Care Diagnostics Market by Product, Platform, Mode, End-User.https://www.marketsandmarkets.com/Market-Reports/point-of-care-diagnostic-market-106829185.html [Google Scholar]
- 11.Wu H., Ma Z., Wei C., Jiang M., Hong X., Li Y., et al. Three-dimensional microporous hollow Fiber membrane microfluidic device integrated with selective separation and capillary self-driven for point-of-Care testing. Anal. Chem. 2020;92:6358–6365. doi: 10.1021/acs.analchem.9b05342. [DOI] [PubMed] [Google Scholar]
- 12.Han G.R., Ki H., Kim M.G. Automated, universal, and mass-producible paper-based lateral flow biosensing platform for high-performance point-of-care testing. ACS Appl. Mater. Interfaces. 2020;12:1885–1894. doi: 10.1021/acsami.9b17888. [DOI] [PubMed] [Google Scholar]
- 13.Shen L., Jia K., Bing T., Zhang Z., Zhen X., Liu X., et al. Detection of circulating tumor-related materials by aptamer capturing and endogenous enzyme-signal amplification. Anal. Chem. 2020;92:5370–5378. doi: 10.1021/acs.analchem.0c00051. [DOI] [PubMed] [Google Scholar]
- 14.Han G.R., Koo H.J., Ki H., Kim M.G. Paper/Soluble polymer hybrid-based lateral flow biosensing platform for high-performance point-of-Care testing. ACS Appl. Mater. Interfaces. 2020;10 doi: 10.1021/acsami.0c07893. 1021/acsami.0c07893. [DOI] [PubMed] [Google Scholar]
- 15.Wang H., Wang H., Jia Y., Sun R., Hong W., Zhang M., et al. visual detection of fusion genes by ligation-triggered isothermal exponential amplification: a point-of-care testing method for highly specific and sensitive quantitation of fusion genes with a smartphone. Anal. Chem. 2019;91:12428–12434. doi: 10.1021/acs.analchem.9b03061. [DOI] [PubMed] [Google Scholar]
- 16.Tian T., Wei X., Jia S., Zhang R., Li J., Zhu Z., et al. Integration of target responsive hydrogel with cascaded enzymatic reactions and microfluidic paper-based analytic devices (microPADs) for point-of-care testing (POCT) Biosens. Bioelectron. 2016;77:537–542. doi: 10.1016/j.bios.2015.09.049. [DOI] [PubMed] [Google Scholar]
- 17.Miao P., Tang Y. Dumbbell hybridization chain reaction based electrochemical biosensor for ultrasensitive detection of exosomal miRNA. Anal. Chem. 2020;10 doi: 10.1021/acs.analchem.0c02654. 1021/acs.analchem.0c02654. [DOI] [PubMed] [Google Scholar]
- 18.Zhu L., Liu X., Yang J., He Y., Li Y. Application of multiplex microfluidic electrochemical sensors in monitoring hematological tumor biomarkers. Anal. Chem. 2020;10 doi: 10.1021/acs.analchem.0c02430. 1021/acs.analchem.0c02430. [DOI] [PubMed] [Google Scholar]
- 19.Sempionatto J.R., Jeerapan I., Krishnan S., Wang J. Wearable chemical sensors: emerging systems for on-body analytical chemistry. Anal. Chem. 2020;92:378–396. doi: 10.1021/acs.analchem.9b04668. [DOI] [PubMed] [Google Scholar]
- 20.Liu X., Zou L., Yang X., Wang Q., Zheng Y., Geng X., et al. Point-of-Care assay of alkaline phosphatase enzymatic activity using a thermometer or temperature discoloration sticker as readout. Anal. Chem. 2019;91:7943–7949. doi: 10.1021/acs.analchem.9b01883. [DOI] [PubMed] [Google Scholar]
- 21.Li Y., Xuan J., Xia T., Han X., Song Y., Cao Z., et al. Competitive volumetric bar-chart chip with real-time internal control for point-of-care diagnostics. Anal. Chem. 2015;87:3771–3777. doi: 10.1021/ac504301y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong F.Z., Jahan S., Zhong R., Cao X.Y., Li W.L., Wang Y.X., et al. Electrophoresis titration model of a moving redox boundary chip for a point-of-Care test of an enzyme-linked immunosorbent assay. ACS Sens. 2019;4:126–133. doi: 10.1021/acssensors.8b01017. [DOI] [PubMed] [Google Scholar]
- 23.Huang Y., Ren J., Qu X. Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 2019;119:4357–4412. doi: 10.1021/acs.chemrev.8b00672. [DOI] [PubMed] [Google Scholar]
- 24.Farka Z., Jurik T., Kovar D., Trnkova L., Skladal P. Nanoparticle-based immunochemical biosensors and assays: recent advances and challenges. Chem. Rev. 2017;117:9973–10042. doi: 10.1021/acs.chemrev.7b00037. [DOI] [PubMed] [Google Scholar]
- 25.Mahmudunnabi R.G., Farhana F.Z., Kashaninejad N., Firoz S.H., Shim Y.B., Shiddiky M.J.A. Nanozyme-based electrochemical biosensors for disease biomarker detection. Analyst. 2020;145:4398–4420. doi: 10.1039/d0an00558d. [DOI] [PubMed] [Google Scholar]
- 26.Sahar S., Zeb A., Ling C., Raja A., Wang G., Ullah N., et al. A hybrid VOx incorporated hexacyanoferrate nanostructured hydrogel as a multienzyme mimetic via cascade reactions. ACS Nano. 2020;14:3017–3031. doi: 10.1021/acsnano.9b07886. [DOI] [PubMed] [Google Scholar]
- 27.Liu X., Yan Z., Zhang Y., Liu Z., Sun Y., Ren J., et al. Two-dimensional metal-organic Framework/Enzyme hybrid nanocatalyst as a benign and self-activated cascade reagent for in vivo wound healing. ACS Nano. 2019;13:5222–5230. doi: 10.1021/acsnano.8b09501. [DOI] [PubMed] [Google Scholar]
- 28.Wang D., Jana D., Zhao Y. Metal-organic framework derived nanozymes in biomedicine. Acc. Chem. Res. 2020;53:1389–1400. doi: 10.1021/acs.accounts.0c00268. [DOI] [PubMed] [Google Scholar]
- 29.Ling P., Cheng S., Chen N., Qian C., Gao F. Nanozyme-modified metal-organic frameworks with multienzymes activity as biomimetic catalysts and electrocatalytic interfaces. ACS Appl. Mater. Interfaces. 2020;12:17185–17192. doi: 10.1021/acsami.9b23147. [DOI] [PubMed] [Google Scholar]
- 30.Dang P., Liu X., Ju H., Wu J. Intensive and persistent chemiluminescence system based on nano-/Bioenzymes with local tandem catalysis and surface diffusion. Anal. Chem. 2020;92:5517–5523. doi: 10.1021/acs.analchem.0c00337. [DOI] [PubMed] [Google Scholar]
- 31.Wang L., Chen Y. Luminescence-sensing Tb-MOF nanozyme for the detection and degradation of estrogen endocrine disruptors. ACS Appl. Mater. Interfaces. 2020;12:8351–8358. doi: 10.1021/acsami.9b22537. [DOI] [PubMed] [Google Scholar]
- 32.Zhang T., Xing Y., Song Y., Gu Y., Yan X., Lu N., et al. AuPt/MOF-graphene: a synergistic catalyst with surprisingly high peroxidase-like activity and its application for H2O2 detection. Anal. Chem. 2019;91:10589–10595. doi: 10.1021/acs.analchem.9b01715. [DOI] [PubMed] [Google Scholar]
- 33.Ruecha N., Shin K., Chailapakul O., Rodthongkum N. Label-free paper-based electrochemical impedance immunosensor for human interferon gamma detection. Sens. Actuators B Chem. 2019;279:298–304. [Google Scholar]
- 34.Wei X., Tian T., Jia S., Zhu Z., Ma Y., Sun J., et al. Microfluidic distance readout sweet hydrogel integrated paper-based analytical device (muDiSH-PAD) for visual quantitative point-of-Care testing. Anal. Chem. 2016;88:2345–2352. doi: 10.1021/acs.analchem.5b04294. [DOI] [PubMed] [Google Scholar]
- 35.Wang M., Liu J., Guo C., Gao X., Gong C., Wang Y., et al. Metal–organic frameworks (ZIF-67) as efficient cocatalysts for photocatalytic reduction of CO2: the role of the morphology effect. J. Mater. Chem. A. 2018;6:4768–4775. [Google Scholar]
- 36.Han H., Yuan X., Zhang Z., Zhang J. Preparation of a ZIF-67 derived thin film electrode via electrophoretic deposition for efficient electrocatalytic oxidation of vanillin. Inorg. Chem. 2019;58:3196–3202. doi: 10.1021/acs.inorgchem.8b03281. [DOI] [PubMed] [Google Scholar]
- 37.Sun Q.Q., Wang M., Bao S.J., Wang Y.C., Gu S. Analysis of cobalt phosphide (CoP) nanorods designed for non-enzyme glucose detection. Analyst. 2016;141:256–260. doi: 10.1039/c5an01928a. [DOI] [PubMed] [Google Scholar]
- 38.Xie F., Cao X., Qu F., Asiri A.M., Sun X. Cobalt nitride nanowire array as an efficient electrochemical sensor for glucose and H2O2 detection. Sens. Actuators B Chem. 2018;255:1254–1261. [Google Scholar]
- 39.Tonyushkina K., Nichols J.H. Glucose Meters: A Review of Technical Challenges to Obtaining Accurate Results. J. Diabetes Sci. Technol. 2009;3:971–980. doi: 10.1177/193229680900300446. [DOI] [PMC free article] [PubMed] [Google Scholar]