Abstract
Background
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has raised concern for the health of immunocompromised individuals, who are potentially at higher risk of more severe infection and poorer outcomes. As a large London transplant center serving a diverse patient population, we report the outcomes of SARS-CoV-2 infection in our cohort of 2848 kidney and/or pancreas transplant patients.
Methods
Data were obtained retrospectively for all transplant patients who attended hospital during the peak of the pandemic and had a positive nasopharyngeal SARS-CoV-2 test.
Results
Sixty-six patients were found to be positive for SARS-CoV-2. Twenty percent were treated as outpatients, 59% were admitted to the general ward, and 21% required intensive care. Treatment consisted of reduced immunosuppression, antibiotics for pneumonia or sepsis, and other supportive treatments. Within our cohort, 12 patients died (18%), with an overall mortality of 0.4%. Predictive risk factors for COVID-19 severity were explored.
Conclusions
Severe disease was associated with lower hemoglobin prior to COVID-19 diagnosis and lower lymphocyte count at the time of diagnosis but not age, sex, ethnicity, or preexisting comorbidities. Lower glomerular filtration rate and higher C-reactive protein were associated with more severe disease. Despite no use of hydroxychloroquine, azithromycin, antiviral, or immunomodulatory medications, our mortality rate (kidney and pancreas transplant patients) is similar to current international rates.
Since first recognition of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in December 2019, coronavirus disease 2019 (COVID-19) has spread to all countries, with over 6 million cases of infection globally and in excess of 350,000 deaths [1]. In late May 2020, the death toll from COVID-19 in the United Kingdom was the highest in Europe [1], with London being the epicenter of infections [2]. Reports from China, where the disease was first identified, demonstrate that 14% of patients with COVID-19 suffer disease at the severe end of the spectrum, with 5% progressing to critical disease, and an overall case fatality rate of 2.3%, reaching 49% in critical cases [3].
Severe infections and case fatality rates were notably higher in patients with coexisting premorbid conditions such as diabetes, hypertension, active cancer, morbid obesity, and chronic kidney disease [[3], [4], [5]].
Immunosuppression in patients who have had a solid organ transplant is also thought to be a risk factor for severe infection and therefore poses an increased risk of graft dysfunction, multiorgan failure, and mortality.
We report the outcomes of 66 patients with a functioning kidney transplant, all of whom were transplanted at a single London center, Guy’s & St. Thomas’ Hospitals (GSTT), and who were positive for SARS-CoV-2 confirmed by reverse transcription-polymerase chain reaction (RT-PCR) on nasal and throat swab samples. These were among a cohort of 2848 kidney transplant recipients followed up at this same center, in conjunction with 2 partner hospitals within the region: King’s College Hospital (KCH) and Kent & Canterbury Hospitals (K&C).
Methods
All recipients aged >18 years with a functioning kidney and/or pancreas transplant from the cohort undergoing follow-up at GSTT, KCH, and K&C and with a positive SARS-CoV-2 test were assessed. Each case of COVID-19 was defined by a positive RT-PCR result for SARS-CoV-2, with all nasopharyngeal swab tests performed between March 10 and May 3, 2020. Data were extracted from the electronic medical record systems of all 3 hospitals and assessed retrospectively. Although all 66 patients were under regular specialty follow-up at 1 of the 3 hospitals, data were also obtained from the hospitals to which each patient presented, within and outside the region. This study was approved by GSTT as a service evaluation and clinical audit project (Project No. 10823).
Baseline blood markers were obtained from each patient’s most recent outpatient visit, prior to diagnosis of SARS-CoV-2. All patients underwent routine blood tests on the day of diagnosis of SARS-CoV-2. Depending on the clinical picture, a chest radiograph was also undertaken. Patients who were treated on an outpatient basis received welfare phone calls from a member of the transplant nursing staff and subsequently returned for follow-up blood tests. Based on these results, virtual or face-to-face consultation was held by a clinician.
A small subgroup of patients are likely to have suffered nosocomial infection with the virus, on the basis of an existing inpatient stay preceding the positive RT-PCR swab result. For these patients, baseline blood markers prior to development of symptoms consistent with COVID-19 were recorded. Furthermore, length of hospital stay for this group was calculated from the date of the positive RT-PCR swab result. Length of stay (LOS) was otherwise calculated from the date of admission to the date of death or discharge from hospital or May 23, 2020 (the time of writing), for those patients still admitted to hospital.
Patients were categorized according to the mainstay of care during their COVID-19 illness:
-
•
Group 1: Outpatient care only.
-
•
Group 2: Admission to hospital with ward-based care (which included patients eligible for intensive care unit [ICU] care and those for whom a ceiling of care was instituted in ward-based care).
-
•
Group 3: Admission to an ICU.
At the time of admission to hospital and diagnosis of COVID-19, all patients underwent a triage process taking into account factors such as age, comorbidities, and level of frailty. Based on this assessment, each patient had a decision in place with regard to the ceiling of care, and those thought to have a poor survival prognosis would not receive escalation of care within the setting of an ICU regardless of the severity of COVID-19 illness.
Statistical Approach and Analysis
Sixty-six patients were classifed in 3 groups in the methods set out based on where they were cared for: outpatient vs ward-based vs ICU. For the purposes of performing statistical analysis, we compared 2 groups: mild-to-moderate and life-threatening illness as there was a ceiling of care defined for a subgroup of patients which meant that they received only ward-based care as they were not eligible for ICU care. Using this classification, patients in group 3 and those in group 2 who died were deemed to have had life-threatening disease. All other patients were considered to have mild-to-moderate COVID-19.
Associations between disease severity and categorical variables were performed using chi-square or Fisher’s exact test; comparisons of continuous variables between the 2 groups were performed with the Mann-Whitney U test. Multivariable binary regression analysis was undertaken to assess the association of variables with disease severity and results were expressed as odds ratios (ORs) with 95% confidence intervals (CIs). A 2-tailed P value of <.05 was considered significant. All statistical analyses were performed using IBM SPSS version 26.0 (IBM, Armonk, NY, United States).
Results
Patient Characteristics
Sixty-six recipients with functioning kidney and/or pancreas grafts, all transplanted at GSTT, tested positive for SARS-CoV-2 during the period March 10 to May 3, 2020. Of these patients, 37 were undergoing regular transplant follow-up at GSTT, 21 at KCH, and 8 at K&C.
Twenty-six patients were admitted directly to a tertiary hospital with a specialist renal transplant service. Nine patients were admitted to another hospital within the region and subsequently transferred to either GSTT, KCH, or K&C, and 13 were admitted to other hospitals but were not transferred. Five patients were already in hospital at the time of SARS-CoV-2 diagnosis.
The overall median age of the cohort was 58 (interquartile range [IQR] = 48-64); 43 were male (65%) and 23 were female (35%). Ethnic distribution within the cohort is as follows: 30 were black (46%), 27 were white (41%), 6 were Asian (9%), and 3 were other ethnicities (5%). Fifty-six patients had received single kidney transplants (85%), 2 had dual kidney transplants (3%), and 8 had combined kidney and pancreas transplants (12%).
Median time from transplantation to the time of infection was 66 months (IQR = 17-115), with 12 patients having received a transplant in the 12 months preceding COVID-19 infection (18%). Eight patients had been transplanted between 12 and 24 months prior to infection (12%), and the remaining 46 patients were at least 2 years out of transplantation at the time of COVID-19 infection (70%).
Of this cohort, 54 patients were on at least 1 agent for hypertension (82%), 28 had diabetes (42%), 10 had a history of cancer (15%), and 23 were classified as obese with a body mass index >30 kg/m2 (35%). Of the 10 patients with a history of cancer, 7 had active disease, of whom 2 were receiving treatment for posttransplant lymphoproliferative disorder. The baseline characteristics of the entire cohort are summarized in Table 1 .
Table 1.
Baseline Demographics of Entire Cohort
| All (n = 66) | Group 1 (n = 13) | Group 2 (n = 39) | Group 3 (n = 14) | |
|---|---|---|---|---|
| Age in years, median (IQR) | 58 (48-64) | 48 (41-57) | 60 (52-67) | 57 (46-63) |
| Age >60 (%) | 29 (44) | 2 (15) | 20 (51) | 6 (43) |
| Sex (%) | ||||
| Male | 43 (65) | 7 (54) | 27 (69) | 9 (64) |
| Female | 23 (35) | 6 (46) | 12 (31) | 5 (36) |
| Race (%) | ||||
| White | 27 (41) | 4 (31) | 18 (46) | 5 (36) |
| Black | 30 (46) | 5 (39) | 15 (41) | 9 (64) |
| Asian | 6 (9) | 2 (15) | 4 (10) | |
| Other | 3 (5) | 2 (15) | 1 (3) | |
| Organ transplant (%) | ||||
| Single kidney | 56 (85) | 10 (77) | 33 (85) | 13 (93) |
| Dual kidneys | 2 (3) | 2 (5) | ||
| Kidney-pancreas | 8 (12) | 3 (23) | 4 (10) | 1 (7) |
| Donor type (%) | ||||
| DBD | 34 (52) | 6 (46) | 20 (51) | 8 (57) |
| DCD | 16 (24) | 3 (23) | 9 (23) | 4 (29) |
| LD | 16 (24) | 4 (31) | 10 (26) | 2 (14) |
| Months since transplant, median (IQR) | 66 (17-115) | 61 (14-157) | 72 (26-106) | 44 (13-103) |
| <12 (%) | 12 (19) | 3 (23) | 6 (15) | 3 (21) |
| 12-24 (%) | 8 (12) | 1 (8) | 4 (10) | 3 (21) |
| 24-60 (%) | 8 (12) | 2 (15) | 3 (8) | 3 (21) |
| >60 (%) | 38 (58) | 7 (54) | 26 (67) | 5 (36) |
| Comorbidities (%) | ||||
| HTN | 54 (82) | 11 (85) | 32 (82) | 11 (79) |
| Diabetes | 28 (42) | 4 (31) | 19 (49) | 5 (36) |
| Cancer | 10 (15) | 0 | 7 (18) | 3 (21) |
| Chronic lung disease | 5 (8) | 1 (8) | 3 (8) | 1 (7) |
| Obesity | 23 (35) | 1 0(8) | 14 (36) | 8 (57) |
| HIV | 2 (3) | 1 (3) | 1 (7) | |
| Body mass index (kg/m2) | ||||
| <20 | 2 (3) | 0 | 1 (3) | 1 (7) |
| 20-24.9 | 16 (24) | 4 (31) | 12 (31) | 0 |
| 25-29.9 | 25 (38) | 8 (62) | 12 (31) | 5 (36) |
| 30-34.9 | 19 (29) | 1 (8) | 11 (28) | 7 (50) |
| >35 | 4 (6) | 0 | 3 (8) | 1 (7) |
| Underlying cause of ESRF (%) | ||||
| Diabetes | 16 (24) | 3 (23) | 11 (28) | 2 (14) |
| Hypertension | 10 (15) | 0 | 6 (15) | 4 (29) |
| Autoimmune disease | 14 (21) | 5 (39) | 6 (15) | 3 (21) |
| Polycystic kidney disease | 6 (9) | 1 (8) | 4 (10) | 1 (7) |
| Other causes | 20 (30) | 4 (31) | 12 (31) | 4 (29) |
Abbreviations: DBD, donation after brainstem death; DCD, donation after circulatory death; ESRF, end-stage renal failure; HIV, human immunodeficiency virus; HTN, hypertension; IQR, interquartile range; LD, living donor.
Baseline Immunosuppression and Graft Function
All patients had at least 1 functioning graft at the time of infection. With the exception of 1 patient who was on a single immunosuppression agent only following a recent diagnosis of posttransplant lymphoproliferative disorder, all patients had ongoing maintenance immunosuppression of 2 (30%) or 3 (68%) agents, as per the unit’s protocol.
Five patients within this cohort had at least 1 episode of biopsy-proven acute rejection within 1 year preceding infection with SARS-CoV-2, 4 of whom were diagnosed within the month prior to infection. Three of these patients were treated with high-dose steroids, of whom 1 also received treatment with intravenous immunoglobulin and plasma exchange. One patient did not receive any treatment for rejection or modification of his immunosuppression regimen in view of concurrent active renal cell carcinoma in his native kidney and infection with SARS-CoV-2. The fifth patient had received a course of antithymocyte globulin 1 year prior to COVID-19 infection.
The baseline immunosuppression agents, graft function, and basic blood markers prior to COVID-19 infection are summarized in Table 2 .
Table 2.
Baseline Immunosuppression, Graft Function, and Blood Counts Prior to Infection With SARS-CoV-2
| All (n = 66) | Group 1 (n = 13) | Group 2 (n = 38) | Group 3 (n = 14) | |
|---|---|---|---|---|
| Baseline IS (%) | ||||
| CNI | 59 (89) | 13 (100) | 35 (90) | 12 (86) |
| MMF | 52 (79) | 11 (85) | 30 (77) | 11 (79) |
| Azathioprine | 2 (30) | 0 | 2 (5) | 0 |
| Steroids | 57 (86) | 10 (77) | 33 (85) | 14 (100) |
| mTORi | 4 (6) | 1 (8) | 2 (5) | 1 (7) |
| Graft function, median (IQR) | ||||
| Creatinine (μmol/L) | 153 (111-202) | 119 (91-167) | 167 (117-195) | 163 (125-232) |
| eGFR (mL/min) | 38.5 (27-53) | 49 (40-55) | 36 (25-50) | 36.5 (25-47) |
| Blood counts, median (IQR) | ||||
| Hb (g/dL) | 119 (104-133) | 127 (112-139) | 116 (104-133) | 116 (98-127) |
| WCC (×109) | 6.3 (5-8.5) | 7.1 (5.7-8.5) | 6.3 (5-8.6) | 6.3 (4.8-7.9) |
| Lymphocytes (×109) | 1.2 (0.8-1.6) | 1.3 (0.8-1.5) | 1.1 (0.9-1.7) | 1.4 (0.9-1.7) |
Abbreviations: CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; IQR, interquartile range; IS, immunosuppression; MMF, mycophenolate mofetil; mTORi, mammalian target of rapamycin inhibitors; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WCC, white cell count.
Clinical Presentation
The most common presenting symptoms were fever (68%) and a cough (68%), either dry or productive. Other common symptoms include dyspnea (50%), fatigue (44%), and myalgia (30%). Two patients were asymptomatic at the time of positive SARS-CoV-2 swab test. Both of these patients had been admitted to hospital with other symptoms, 1 with subacute bowel obstruction secondary to intra-abdominal adhesions from previous surgery and the other with osteomyelitis of the toe requiring amputation. In both of these patients SARS-CoV-2 testing was performed in line with the hospital’s admitting protocol investigations in order to facilitate admission to appropriate wards. Neither patient developed symptoms consistent with COVID-19 during the course of their inpatient stay or subsequent to discharge. Seven patients are suspected to have suffered nosocomial infection with SARS-CoV-2. Four were already in hospital for at least 1 week for management of non-COVID-19 illness, and 3 were electively admitted for renal transplant graft biopsy because of ongoing dysfunction and suspicion of rejection. These 3 subsequently developed symptoms of COVID-19 and tested positive for SARS-CoV-2, and 1 of these remains an inpatient in hospital at the time of writing.
Postdiagnosis Clinical Characteristics
All patients underwent routine blood tests on the day of diagnosis of SARS-CoV-2. Depending on the clinical picture, a chest radiograph was also undertaken. Patients who were treated on an outpatient basis received welfare phone calls from a member of the transplant nursing staff and subsequently returned for follow-up blood tests. Based on these results, virtual or face-to-face consultation was held by a clinician.
Blood markers on the day of SARS-CoV-2 diagnosis are summarized in Table 3 , including measurements of peak ferritin and D-dimer levels for those managed as inpatients, as well as peak procalcitonin and troponin levels for those patients admitted to ICU. The stage of acute kidney injury was calculated for all patients using Kidney Disease Improving Global Outcomes criteria [6].
Table 3.
Laboratory Values and Stage of AKI on the Day of SARS-CoV-2 Diagnosis
| All (n = 66) | Group 1 (n = 13) | Group 2 (n = 39) | Group 3 (n = 14) | |
|---|---|---|---|---|
| Blood counts, median (IQR) | ||||
| Hb (g/dL) | 113 (98-127) | 124 (101-146) | 110 (97-129) | 111 (99-118) |
| WCC (×109) | 6.5 (4.9-8.5) | 6.1 (5.0-6.6) | 6.9 (5.1-9) | 5.8 (5-8.6) |
| Lymphocytes (×109) | 0.7 (0.5-0.9) | 0.8 (0.7-1.1) | 0.7 (0.5-0.9) | 0.5 (0.48-0.7) |
| Lymphocyte nadir (×109) | 0.6 (0.4-0.8) | 0.77 (0.56-1.1) | 0.6 (0.4-0.8) | 0.4 (0.2-0.5) |
| Graft function, median (IQR) | ||||
| Peak creatinine (μmol/L) | 200 (138-277) | 143 (109-199) | 207 (149-253) | 380 (199-456) |
| eGFR nadir (mL/min) | 26.5 (17.8-45.3) | 41.5 (28.3-52) | 25 (19.5-40) | 14 (9-27.3) |
| Specific analysis, median (IQR) | ||||
| Peak CRP (mg/L) | 160 (59-279) | 31 (8-53) | 159 (77-216) | 362 (304-408) |
| Peak ferritin (μg/L) | 825 (400-1307) | 2130 (1352-3365) | ||
| Peak D-dimer (mg/L FEU) | 2.29 (1.1-6.23) | 9 (3-18.3) | ||
| Peak troponin (ng/L) | 95 (57-227) | |||
| Peak procalcitonin (μg/L) | 4.9 (2.3-8.6) | |||
| Stage of AKI (%) | ||||
| 0 | 26 (39) | 10 (77) | 12 (31) | 4 (29) |
| 1 | 28 (42) | 3 (23) | 21 (54) | 4 (29) |
| 2 | 4 (6) | 0 | 2 (5) | 2 (14) |
| 3 | 3 (5) | 0 | 0 | 3 (21) |
| Insufficient data | 5 (8) | 0 | 4 (10.2) | 1 (7) |
Abbreviations: AKI, acute kidney injury; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; FEU, fibrinogen equivalent units; Hb, hemoglobin; IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WCC, white cell count.
Chest radiograph and computed tomography (CT) findings are summarized in Table 4 . CT imaging of the chest was limited as far as possible because of the critical state of these patients, as well as to reduce spread of infection within the hospital. Generally chest CT imaging was only undertaken if CT of the head or abdomen was already planned or if there was a high index of suspicion for pulmonary embolism. Interestingly, only 1 patient within the cohort was found to have small subsegmental pulmonary emboli on CT imaging.
Table 4.
Chest Imaging Findings at the Time of SARS-CoV-2 Diagnosis and During the Course of Inpatient Stay
| All (n = 66) | Group 1 (n = 13) | Group 2 (n = 39) | Group 3 (n = 14) | |
|---|---|---|---|---|
| CXR findings | ||||
| Normal | 10 (15) | 2 (15) | 8 (21) | 0 |
| Consolidation | 39 (59) | 0 | 26 (67) | 12 (86) |
| No CXR | 10 (15) | 9 (69) | 1 (3) | 0 |
| Insufficient data | 7 (11) | 2 (15) | 3 (8) | 2 (14) |
| CT findings | ||||
| Consolidation | 11 (17) | 7 (18) | 4 (29) | |
| PE | 1 (2) | 0 | 1 (7) | |
| No CT | 41 (62) | 25 (64) | 5 (36) | |
| Insufficient data | 14 (21) | 7 (18) | 5 (36) |
Abbreviations: CT, computed tomography; CXR, chest radiograph; PE, pulmonary emboli; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Comparison of Disease Severity
There was no statistically significant difference in the age, body mass index, time since transplantation, and baseline graft function between patients who suffered a milder illness and those with life-threatening severity. Lymphocyte count at the time of SARS-CoV-2 diagnosis was significantly lower in patients who ended up with severe COVID-19 than in those who were managed as outpatients or discharged after ward-based care. Patients with more severe COVID-19 illness also had significantly lower nadir estimated glomerular filtration rate (and consequently higher peak creatinine) and higher peak C-reactive protein (CRP) levels, as well as a lower lymphocyte nadir during the course of COVID-19 illness. Patients who either died or were admitted to ICU had a significantly lower baseline hemoglobin level and a lower hemoglobin level at the time of diagnosis with SARS-CoV-2, though the latter did not reach statistical significance. These comparisons are shown in Tables 5 and 6 .
Table 5.
Comparison of Baseline Characteristics by Disease Severity
| Baseline Characteristic, Median (IQR) | Mild/Moderate Illness (n = 45) | Severe Illness (n = 21) | P Value |
|---|---|---|---|
| Age | 57 (48-63) | 60 (49-64) | .432 |
| Months since transplant | 65 (17-116) | 72 (17-110) | .874 |
| BMI (kg/m2) | 27 (24-30) | 29 (25-32) | .521 |
| Baseline creatinine (μmol/L) | 134 (106-184) | 178 (138-242) | .072 |
| eGFR (mL/min) | 41 (32-54) | 30 (21-46) | .056 |
| Hb (g/dL) | 126 (107-136) | 111 (98-121) | .050 |
| WCC (×109) | 6.5 (5-8.8) | 6.3 (4.8-7.4) | .398 |
| Lymphocytes (×109) | 1.2 (0.9-1.7) | 1.2 (0.7-1.6) | .458 |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; IQR, interquartile range; WCC, white cell count.
Table 6.
Comparison of Graft Function and Laboratory Values by Disease Severity
| Postdiagnosis Characteristic, Median (IQR) | Mild/Moderate Illness (n = 45) | Severe Illness (n = 21) | P Value |
|---|---|---|---|
| Hb (g/dL) | 121 (98-134) | 108 (90-117) | .069 |
| WCC (×109) | 6.6 (5.2-8.3) | 5.7 (4.2-9.2) | .572 |
| Lymphocytes (×109) | 0.7 (0.5-1.2) | 0.5 (0.4-0.6) | .025 |
| Lymphocyte nadir (×109) | 0.7 (0.5-0.9) | 0.4 (0.2-0.4) | .000 |
| eGFR nadir (mL/min) | 30 (22-47) | 16 (10-27) | .003 |
| Peak creatinine (μmol/L) | 178 (124-246) | 300 (203-455) | .003 |
| Peak CRP (mg/L) | 105 (42-169) | 311 (202-485) | .000 |
Abbreviations: CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; IQR, interquartile range; WCC, white cell count.
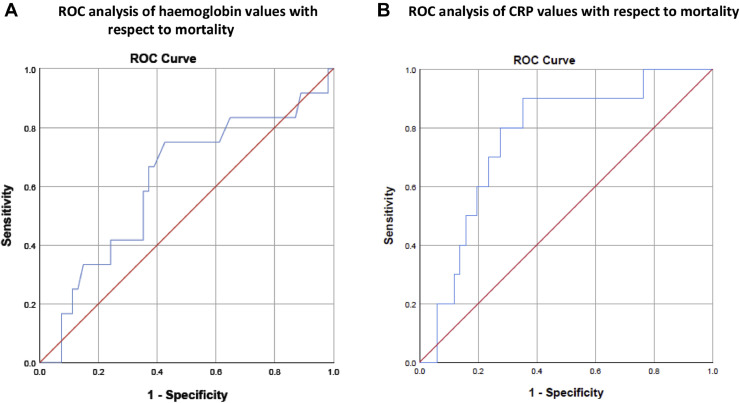
There was no association between disease severity and sex, ethnicity, donor type (living donor, donation after brainstem death, or donation after circulatory death), type of transplant (kidney only vs simultaneous pancreas and kidney), or the cause of end-stage kidney disease. Similarly, no association was found between disease severity and a history of cancer, diabetes, hypertension, or chronic lung disease (see Table 7 ). Of note, peak CRP and baseline hemoglobin were independently associated with severe COVID-19 in multivariable binary logistic regression analysis (OR = 1.019; 95% CI, 1.008-1.031; P = .001, and OR = 0.931; 95% CI, 0.874-0.992; P = .028, respectively; see Table 8 ). We performed a receiver operating characteristic analysis of hemoglobin values with respect to mortality. The area under the curve was 0.616 (P = .212), and the cutoff value was 117.5 with a positive predictive value of 0.28 and negative predictive value of 0.91 (Fig 1A). We also performed receiver operating characteristic analysis of CRP values with respect to mortality. The area under the curve was 0.765 (P = .009), and the cutoff value was 212.5 with a positive predictive value of 0.32 and negative predictive value of 0.92 (Fig 1B).
Table 7.
Associations Between Disease Severity and Patient and Transplant Characteristics
| Mild/Moderate Illness n (%) | Severe Illness n (%) | P Value | ||
|---|---|---|---|---|
| Gender | Male | 29 (67) | 14 (33) | .860 |
| Female | 16 (70) | 7 (30) | ||
| Ethnicity | White | 18 (67) | 9 (33) | .826 |
| Non-white | 27 (69) | 12 (31) | ||
| Type of transplant | Kidney only | 38 (66) | 20 (34) | .419 |
| SPK | 7 (88) | 1 (12) | ||
| Donor type | Live donor | 12 (75) | 4 (25) | .501 |
| Deceased donor | 33 (66) | 17 (34) | ||
| Cause of ESKD | Diabetes mellitus | 11 (69) | 5 (31) | .772 |
| Hypertension | 6 (60) | 4 (40) | ||
| Autoimmune | 11 (79) | 3 (21) | ||
| Other | 17 (65) | 9 (35) | ||
| Diabetes mellitus | Yes | 17 (61) | 11 (39) | .264 |
| No | 28 (74) | 10 (26) | ||
| Cancer | Yes | 6 (60) | 4 (40) | .714 |
| No | 39 (70) | 17 (30) | ||
| Hypertension | Yes | 37 (69) | 17 (31) | 1.000 |
| No | 8 (67) | 4 (33) | ||
| Chronic lung disease | Yes | 4 (80) | 1 (20) | 1.000 |
| No | 41 (67) | 20 (33) |
Abbreviations: ESKD, end-stage kidney disease; SPK, simultaneous pancreas and kidney.
Table 8.
Binary Logistic Regression Analysis of COVID-19 Severity
| Variable | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Hb at baseline | 0.941 | 0.892-0.992 | .024 |
| Lymphocytes at time of COVID-19 diagnosis | 0.357 | 0.011-11.247 | .558 |
| eGFR nadir | 1.010 | 0.959-1.063 | .703 |
| Peak CRP | 1.019 | 1.008-1.030 | .001 |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; Hb, hemoglobin.
Fig 1.
(A) Receiver operating characteristic analysis of hemoglobin values with respect to mortality. (B) Receiver operating characteristic analysis of C-reactive protein values with respect to mortality.
Treatment and Outcomes
Transplant patients admitted to hospital had reduction in immunosuppression with the antimetabolite being withheld in the first instance and reduction of calcineurin inhibitor or complete withdrawal in very sick patients, as per guidance published by the British Transplantation Society (BTS) [7]. Patients were treated with antibiotics for pneumonia and sepsis as indicated. The changes in immunosuppression regime following SARS-CoV-2 diagnosis are reflected in Table 9 . Note that 12 patients were not on any maintenance antimetabolite agent, 4 patients were not on calcineurin inhibitor, and 1 patient was commenced on steroids during his inpatient stay. None of our patients received antiviral or immunomodulatory treatment.
Table 9.
Changes in Immunosuppression
| All (n = 66) | Group 1 (n = 13) | Group 2 (n = 39) | Group 3 (n = 14) | |
|---|---|---|---|---|
| Antimetabolite (%) | ||||
| Ceased | 48 (73) | 11 (85) | 26 (67) | 11 (79) |
| Maintained | 3 (5) | 0 | 3 (8) | 0 |
| None at baseline | 12 (18) | 2 (15) | 7 (18) | 3 (21) |
| Insufficient data | 3 (5) | 0 | 3 (8) | 0 |
| CNI (%) | ||||
| Reduced | 22 (33) | 0 | 13 (33) | 9 (64) |
| Ceased | 2 (3) | 0 | 0 | 2 (14) |
| Unchanged | 32 (48) | 12 (92) | 20 (51) | 0 |
| None at baseline | 4 (6) | 1 (8) | 2 (5) | 1 (7) |
| Insufficient data | 6 (9) | 0 | 4 (10) | 2 (14) |
| Steroid (%) | ||||
| Increased | 24 (36) | 1 (8) | 12 (31) | 11 (79) |
| Unchanged | 30 (45) | 9 (69) | 19 (49) | 2 (14) |
| None at baseline | 9 (14) | 3 (23) | 6 (15) | 0 |
| Insufficient data | 3 (5) | 0 | 2 (5) | 1 (7) |
Abbreviation: CNI, calcineurin inhibitor.
The mortality within this cohort of 66 kidney and pancreas transplant recipients with SARS-CoV-2 was 18% (12 patients), of whom 5 patients died in the ICU setting. Seven patients died on a general ward (18%), and this includes patients who were deemed unsuitable for escalation to ICU with a predefined ward-based ceiling of care. The overall mortality rate within our entire cohort of 2848 kidney and pancreas transplant recipients undergoing follow-up at GSTT, KCH, and K&C is 0.4%, which is comparable to that of the UK kidney transplant population [8].
Ten patients died with a functioning graft (15%). Five patients required renal replacement therapy during the course of their COVID-19 illness, of whom 2 have recovered function and been discharged from hospital. One patient died in ICU, and 2 remain in ICU with ongoing renal replacement therapy.
The median LOS for those patients admitted to hospital (groups 2 and 3) was 10 days (IQR = 4-21). Of the patients who died of COVID-19 in hospital, the median length of inpatient stay was 8 days (IQR = 5-10). The median LOS for patients admitted to ICU was 24 days (IQR = 11-44). At the time of writing, 6 patients remain in hospital, of whom 3 are in ICU and 3 are on a general ward, with median LOS of 47 and 40 days, respectively.
Discussion
Since the initial reports of the COVID-19 pandemic, more than 250,000 of the UK population have been affected by SARS-CoV-2 (March 11, 2020), with a high proportion of cases in London [2]. Public Health England advised on “shielding” for extremely vulnerable patients, including transplant recipients on immunosuppression, because this group of patients was considered to be at high risk of infection and to have a poorer prognosis than the general population if infected with the virus [9]. GSTT is one of the largest transplant centers in the UK, performing around 250 adult kidney and/or pancreas transplants per year. We currently have a population of 2848 transplant patients undergoing follow-up at GSTT and our 2 main referral centers, KCH and K&C. During a 40-day period, 66 transplant patients were found to be positive for SARS-CoV-2. Fifty-three of 66 patients required hospital admission (80%) and, of those, 26% were admitted to ICU. Twelve of the 53 admitted patients died (18%). Additionally, at least 12 of our transplant patients had mild symptoms consistent with COVID-19 and were treated at home with regular phone consultation, without undergoing testing for SARS-CoV-2. Because not all patients with symptoms were tested, it is not possible to know the exact disease prevalence in our transplant population.
We were keen to identify predictive risk factors for disease severity in our transplant population. Interestingly, we found no association between disease severity and characteristics such as age, sex, and ethnicity, as well as preexisting comorbidities including hypertension, diabetes, and obesity. Unexpected, patients with severe disease were noted to have lower hemoglobin at baseline (prior to SARS-CoV-2 diagnosis), the clinical significance of which needs to be explored in larger studies. We found that patients who had more severe disease also had a significantly lower lymphocyte count at the time of diagnosis. This could be considered a predictive marker but could also represent a later stage of disease at the time of presentation in this subgroup. Lower estimated glomerular filtration rate and higher CRP were also associated with more severe disease. These are likely reflective of the systemic effect of the infection and the inflammatory response.
Three of our transplant patients were found to have SARS-CoV-2 infection after a planned admission to hospital for a biopsy to investigate declining graft function. This was despite strict infection control measures and admission of SARS-CoV-2-positive and -negative patients to separate wards. Although it is not entirely clear whether these patients acquired the infection prior to or during admission to hospital, it highlights the importance of reducing hospital visits for this vulnerable group as much as possible.
Banerjee et al [10] were the first group from London to report on COVID-19 in kidney transplant patients from London. Of their 7 patients, 5 required hospital admission for supportive treatment, and at the time of their report 1 patient had died. Two of their cohort are included in our study because they were KCH patients; 1 has subsequently died and the other remains on ICU at the time of this report.
Because no definitive treatment has been established for SARS-CoV-2, management of this disease remains challenging. In many countries, empirical treatments such as hydroxychloroquine with or without azithromycin, tocilizumab, and other anti-inflammatory medications have been used for management of this infection [[11], [12], [13], [14], [15], [16]]. The mortality rate in these studies has been reported as 18% to 26% [12,15].
Geleris et al [17] recently published their findings on the effect of treatment with hydroxychloroquine in patients with COVID-19. Of 1376 patients admitted to hospital, 811 had received hydroxychloroquine. Interesting, they found no significant difference between the 2 groups for the outcomes requiring intubation or death. A trial of lopinavir/ritonavir in adults hospitalized with severe COVID-19 [18] where 99 patients were randomized to receive antiviral treatment and 100 had standard care, no difference in outcomes between the 2 groups was noted. In addition to cost, all treatments are associated with potential side effects that may not be justified. Xia and Wang [19] provided a useful commentary, explaining that the interaction of lopinavir/ritonavir with tacrolimus often results in significantly raised tacrolimus levels, which, in addition to causing damage to the kidney, liver, and central nervous system, augments the individual’s immune suppression, which may be detrimental at the time of viral infection.
Our data have several limitations. Although all efforts have been made to capture the incidence of infection in our transplant population cohort, we have relied on direct reporting from other hospitals, as well as communication from transplant patients and family via a helpline set up during the pandemic. Via this helpline, we are also aware of a small group of patients with mild symptoms consistent with SARS-CoV-2 but who remained well in home isolation and hence did not undergo RT-PCR testing. Similarly, we are aware of patients who underwent repeated testing because ongoing characteristic symptoms but who consistently had negative RT-PCR results.
Contrary to other published cohorts, we did not identify a significant difference in the severity of the disease with increasing age. This could be due to the narrow age range observed in our patient cohort. One possible explanation is that younger transplant patients did not develop severe enough symptoms to warrant attendance to health services. We also did not observe major differences in disease severity between males and females or between patients of white and non-white ethnicities. This could be due to the relatively small number of cases within our cohort. As mentioned previously, other than a single case of small subsegmental pulmonary emboli, there were no further reports of thromboembolic disease in our patient cohort. This could partly be related to the limited use of CT imaging of the chest unless there was a clear clinical indication.
After considering the available evidence, the British Transplantation Society (BTS) made recommendations for management of transplanted patients affected by SARS-CoV-2 [7], which initially included reduction in immunosuppression and supportive care. We did not use hydroxychloroquine, azithromycin, or other antiviral treatment, and our inpatient mortality rate of 18% is comparable to that of other published worldwide transplant center outcomes.
Encouraging, the most recent preliminary findings of the RECOVERY trial [20] have shown a reduction in mortality in patients with more severe SARS-CoV-2 infection, with use of dexamethasone and remdesivir being shown to shorten the length of symptomatic disease [21]. These are very important and useful findings in the treatment of patients with COVID-19. BTS has since updated its current guidance based on these results [7].
Acknowledgments
We acknowledge the nursing teams, transplant surgeons, and renal physicians from all 3 centers for their valuable contributions to this study.
References
- 1.World Health Organization Coronavirus disease (COVID-19) situation report--135. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200603-covid-19-sitrep-135.pdf?sfvrsn=39972feb_2 [accessed 20.06.03]
- 2.UK Government Coronavirus (COVID-19) in the UK. https://coronavirus.data.gov.uk/?_ga=2.107739851.893381260.1589709324-1340677483.1577435701 [accessed 20.05.28]
- 3.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Docherty A.B., Harrison E.M., Green C.A., et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 7.British Transplantation Society Guidance on the management of transplant recipients diagnosed with or suspected of having COVID19. https://bts.org.uk/information-resources/covid-19-information/ [accessed 20.05.23]
- 8.NHS Blood and Transplant Weekly report on SARS-CoV-2 positive patients in transplantation. 2020. https://www.odt.nhs.uk/deceased-donation/covid-19-advice-for-clinicians/ [accessed 23.05.20]
- 9.UK Government Guidance on shielding and protecting people who are clinically extremely vulnerable from COVID-19. 2020. https://www.gov.uk/government/publications/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19/ [accessed 20.05.23]
- 10.Banerjee D., Popoola J., Shah S., Ster I.C., Quan V., Phanish M. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;97:1076–1082. doi: 10.1016/j.kint.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akalin E., Azzi Y., Bartash R., et al. COVID-19 and kidney transplantation. N Engl J Med. 2020:2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Columbia University Kidney Transplant Program Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol. 2020;31:1150–1156. doi: 10.1681/ASN.2020030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernández-Ruiz M, Andrés A, Loinaz C, et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain [e-pub ahead of print]. Am J Transplant https://doi.org/10.1111/ajt.15929. [accessed 23.05.20]. [DOI] [PMC free article] [PubMed]
- 14.Zhu L., Gong N., Liu B., et al. Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur Urol. 2020;77:748–754. doi: 10.1016/j.eururo.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter [e-pub ahead of print]. Am J Transplant https://doi.org/10.1111/ajt.15941. [accessed 23.05.20]. [DOI] [PMC free article] [PubMed]
- 16.Alberici F., Delbarba E., Manenti C., et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97:1083–1088. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geleris J., Sun Y., Platt J., et al. Observational study of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao B., Wang Y., Wen D., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787–1789. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia T, Wang Y. Coronavirus disease 2019 and transplantation: the combination of lopinavir/ritonavir and hydroxychloroquine is responsible for excessive tacrolimus trough level and unfavorable outcome [e-pub ahead of print]. Am J Transplant https://doi.org/10.1111/ajt.15992. [accessed 23.05.20]. [DOI] [PMC free article] [PubMed]
- 20.RECOVERY Randomised evaluation of COVID-19 therapy. https://www.recoverytrial.net/ [accessed 23.05.20]
- 21.Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Landary M.J., et al. Effect of dexamethasone in hospitalized patients with COVID-19---preliminary report. https://www.medrxiv.org/content/10.1101/2020.06.22.20137273v1.full.pdf [accessed 20.06.22]