Abstract
The global crisis caused by SARS-CoV-2 (COVID-19) affected economics, social affairs, and the environment, not to mention public health. It is estimated that near 82% of the SARS-CoV-2 genome is similar to the severe acute respiratory syndrome. The purpose of the review is to highlight how the virus is impacted by the environment and how the virus has impacted the environment. This review was based on an electronic search of the literature in the Scopus, Science Direct, and PubMed database published from December 2019 to July 2020 using combinations of the following keywords: SARS-CoV-2 transmission, COVID-19 transmission, coronavirus transmission, waterborne, wastewater, airborne, solid waste, fomites, and fecal-oral transmission. Studies suggest the thermal properties of ambient air, as well as relative humidity, may affect the transmissibility and viability of the virus. Samples taken from the wastewater collection network were detected contaminated with the novel coronavirus; consequently, there is a concern of its transmission via an urban sewer system. There are concerns about the efficacy of the wastewater treatment plant disinfection process as the last chance to inactivate the virus. Handling solid waste also requires an utmost caution as it may contain infectious masks, etc. Following the PRISMA approach, among all reviewed studies, more than 36% of them were directly or indirectly related to the indoor and outdoor environment, 16% to meteorological factors, 11% to wastewater, 14% to fomites, 8% to water, 9% to solid waste, and 6% to the secondary environment. The still growing body of literature on COVID-19 and air, suggests the importance of SARS-CoV-2 transmission via air and indoor air quality, especially during lockdown interventions. Environmental conditions are found to be a factor in transmitting the virus beyond geographical borders. Accordingly, countries need to pay extra attention to sustainable development themes and goals.
Keywords: COVID-19, SARS-CoV-2, Air transmission, Wastewater, Water, Fomites, Solid waste, Air pollution, Air quality
Graphical abstract
1. Introduction
COVID-19 pandemic has had the most extensive impact on human health on a global scale since the first two decades of the 21st century. On January 30, 2020 World Health Organization (WHO) expressed concern about the spread of COVID-19 for public health. Earlier than that, on 3st December 2019, a pneumonia-like syndrome of an unidentified source was reported in Wuhan City, Hubei province in China (Lu et al., 2019). It is estimated that near 82% of the SARS-CoV-2 genome is similar to severe acute respiratory syndrome (SARS-CoV-1) (Chan et al., 2020). To date, within a matter of a few months, according to the latest reports of the WHO, the COVID-19 pandemic has infected more than 50,266,033 and has killed near 1,254,567 cases (WHO, 2020e). This unprecedented disruption of lives since 75 years ago, made the WHO Emergency Committee declare a global health emergency (Peeri et al., 2020). Unfortunately, the healthcare infrastructure of many countries has failed to properly and adequately respond to this crisis.
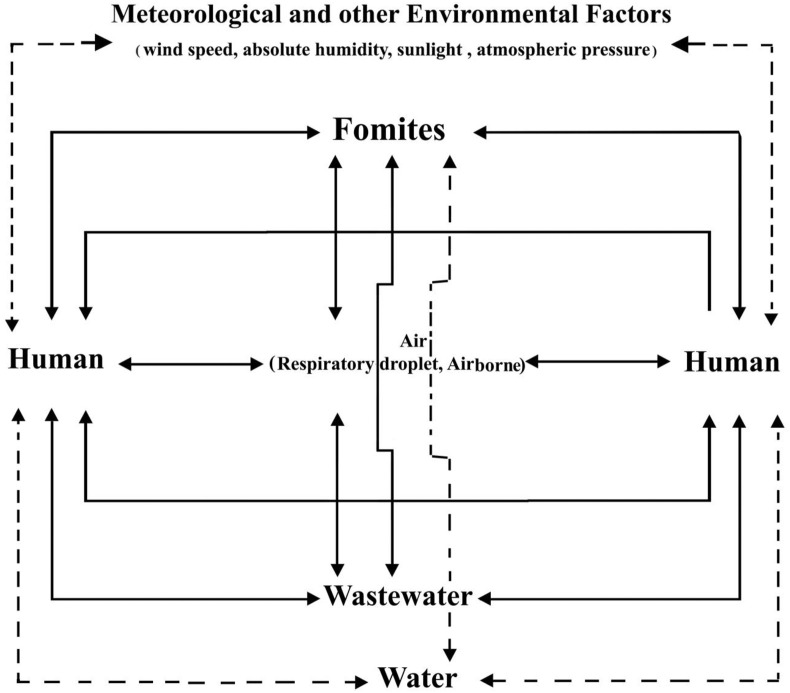
There are two main characteristics of the COVID-19 which have made this virus spread far beyond geographical borders, i.e., clinical and rapid person-to-person transmission. The clinical characteristic suggests the disease is almost unresponsive to conventional treatments; hence, the treatment of those who are in critical conditions is challenging. The rapid transmission or the environment also plays an important role in the global development of the COVID-19. Air, wastewater, water, metrological factors (wind speed, absolute humidity, sunlight, atmospheric pressure, etc.) and fomites are the commonly-known building blocks of the environmental aspect of COVID-19 (see Fig. 1 ). Wang et al. (2020c) suggest an infected hospital environment can transmit the virus to uninfected individuals up to 41%.
Fig. 1.
Person-to-person transmission cycle of COVID-19 by the environment.
The current discussion on the causes of pathogens and microbial resistance is majorly focused on the modernization of urban environments, which may have led to an increased tolerance in microorganisms. Consequently, the current pandemic has shown us the pathogens causing infectious diseases are not limited to the less developed countries with poor environmental and clinical facilities. Hence, it is essential to investigate the environments amplifying the transmissibility of the SARS-CoV-2 virus and to signify the impact of the virus on the environment. In this vein, this review will focus on recent data on the main routes of transmission, the preventative measures, related to the main environmental matrices, to contain the SARS-CoV-2 virus outbreak and secondary effects on the environment are discussed.
2. Methods
This brief review was performed according to the PRISMA approach (Moher et al., 2015). It was based on an electronic search of the literature in the Scopus, Science Direct, and PubMed database published from late December 2019 to November 2020. Keywords were selected and combined (Boolean operators) according to search strategy: SARS-COV-2 transmission, COVID-19 transmission, coronavirus transmission, waterborne, wastewater, airborne, fomites, solid waste, and fecal-oral transmission. The references included in previously published review articles were scanned, and any relevant papers were included. Two investigators independently reviewed each publication to be included in the review, applying the study selection criteria and then reported it. Collectively, we reviewed near 1392 published studies. Fig. 2 shows the proposed approach for reviewing the articles. Interested readers can refer to the appendix section to see the quality assessment checklist (see Table 3), quality assessment of the main studies on which we had discussion (see Table 4), the applied preprocessing criteria before reviewing the studies (see Table 5) and the summary of the main studies on which we had discussion (see Table 6).
Fig. 2.
The flowchart of the proposed systematic review.
3. Results
3.1. Outdoor and indoor air
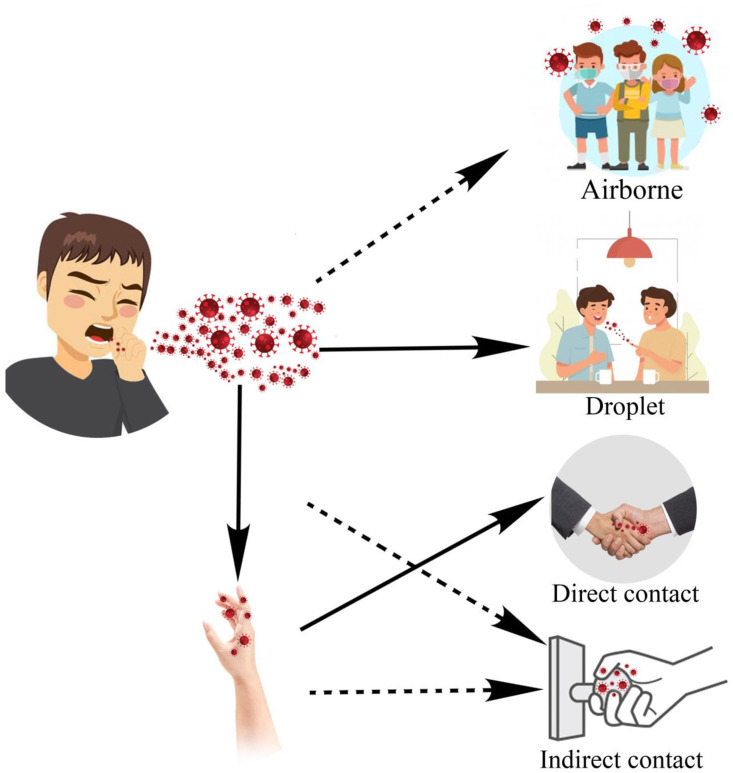
The main route for COVID-19 transmission is the respiratory system (Rothan and Byrareddy, 2020). The virus can either directly (droplet and person-to-person) or indirectly (contaminated objects and airborne transmission) infects the respiratory system (see Fig. 3 ). Droplets and bioaerosols containing the virus, in case of entering into the human body, can cause illness. Any unprotected contact with a contaminated object can potentially increase the transmissibility of the virus. After respiratory infection, in severe cases, the virus can cause acute respiratory distress syndrome and loss of life is not far-fetched without receiving enough treatment (WHO, 2020a). The research carried out in 2005, studying the SARS-CoV-1 epidemic, suggested airborne transmission of SARS-CoV-1 by analyzing air samples taken from infected patients' rooms by PCR test (Booth et al., 2005). In what follows, the studies that address the airborne transmission of the SARS-CoV-2 are reviewed to provide the readers with comprehensive and up-to-date knowledge of the topic.
Fig. 3.
The main routes of SARS-CoV-2 transmission.
According to World Health Organization (WHO) and Center for Disease Control (CDC), the term “airborne transmission” is a characteristics of pathogens that are capable of being transported through small particles less than in diameter and can be suspended into air for hours and long distances (CDC, 2020b; WHO, 2020c). Guo et al. (2020) studied the distribution of SARS-CoV-2 in hospital wards via aerosol transmission in different locations including general COVID-19 wards and ICU wards. In 35% of the samples collected from the air in ICU wards, positive infection with SARS-CoV-2 was reported, while in 12.5% of the samples collected from the general COVID-19 wards reported positive SARS-CoV-2 infection. Higher positivity rates is reported in air outlet samples, near the patients bed, with 66.7% of samples tested positive. Moreover, the maximum aerosol transmission is reported to be about 4 m. It is noteworthy to mention that the samples were tested by quantitative real-time PCR.
To address whether the virus RNA can be transmitted in small size particles, Liu et al. (2020) conducted a study by analyzing aerosols samples by droplet-digital-PCR-based detection method that were in range of sub- and supermicrometer range, i.e., and , respectively. Reportedly, the SARS-CoV-2 RNA was found in both super- and submicrometer samples, indicating that the airborne transmission of the virus RNA is possible. Lednicky et al. (2020a) argues the viable SARS-CoV-2 is detected by RT-qPCR in air samples collected from two COVID-19 patients in 2–4.8m away from the patients. However, as Lewis (2020) and WHO (2020c) suggest, the PCR-based tests are not indicative of viable virus that could be transmitted through airborne pathway, due to out-competition by other respiratory viruses (Lednicky et al., 2020b). To address this, Lednicky et al. (2020a) sought to resolve this conundrum by using air sampling device that does not does not inactivate the virus by adopting water vapor mechanism; hence, conserving their viability. Also, the sampling was taken place in a room dedicated to COVID-19 patients, which is believed to reduce the possibility of the penetration of other respiratory viruses into the sampler. On the other hand, CDC (2020b) suggests the main transmission route for SARS-CoV-2 is via droplets, particularly in close contact distances, although the airborne route for the virus is possible under a specific conditions, including enclosed spaces, long exposure time, and inefficient ventilation.
Santarpia et al. (2020) performed in a hospital in Nebraska, the USA, where air and surface samples were collected in eleven isolation rooms. The results of this study suggest the airborne transmissibility of SARS-CoV-2, both directly and indirectly. The counterexample of these findings is reported in the study which carried out in the biggest hospital of Iran treating COVID-19 patients, indicated that none of the indoor air samples taken from the wards of intensive care unit-Thorax, Internal, ICU-General, and ICU-Heart surgery were infected with SARS-CoV-2. The experimental setups were installed in the mentioned wards at the height of 1.5 m and 2–5 m away from patients' beds where the windows were closed (Faridi et al., 2020a). In this study, the sampling was performed using standard midget impingers; hence, the quality of the samples may have been affected. In another study which carried out in a hospital in Singapore from 24th January to 4th February 2020 supports the findings of Faridi et al. (2020a) by collecting 26 air samples from the COVID-19 patients and suggesting no detection of the virus in any of these samples were observed (Jiang et al., 2020). Low range of limit of detection, experimental setup for sampling SARS-CoV-2, or uncertainties in measurement tools could be the potential reasons for not detecting the virus in Faridi et al. (2020a) and Jiang et al. (2020).
Putting all into perspective, the topic of airborne transmission is likely. Moreover, there is a general agreement among scientists that cl Accordingly, adherence to air-conditioning precautions in isolated potentially contaminated environments is strongly encouraged (Van Doremalen et al., 2020).
Based on the most up-to-date studies, the WHO revised the initial reports, as well as the Centers for Disease Control and Prevention, recommended airborne precautions. Both assumed the transmission of the virus via carrier particles and issued one to the 2-m distance to prevent airborne infection (Bahl et al., 2020). Some studies suggest the virus remained viable for 3 h in bioaerosols (Van Doremalen et al., 2020). In this connection, some promising study results indicate the viral shedding of the virus can be reduced by air filtration (specifically HEPA filters) (Elias and Bar-Yam, 2020). Similarly, a study performed in a hospital in Singapore suggests despite swaps taken from air exhaust outlets tested positive for the virus, air samples collected from indoor patient rooms tested negative (Jiang et al., 2020). Besides, a study of several hospitals in Wuhan, China, found that the airborne transmission of SARS-CoV-2 in Cardiac/Coronary Care Unit (CCU), Intensive Care Unit (ICU) rooms were undetectable or low, because of the high air exchange rate and negative pressure ventilation. Nevertheless, the air samples from the patients' toilets and the crowded places of the hospital were positive. They claim that re-suspension of virus-laden aerosol from surfaces can be one of the causes of airborne spread of SARS-CoV-2 (Liu et al., 2020).
The studies suggest the airborne transmission of the virus by considering droplet size and applying free-fall model simulations. The results indicated that the (breathing to sneezing) from an emitter can be considered safe (Lednicky et al., 2020a). The viral shedding of the SARS-CoV-2 via bioaerosols in specific environments, e.g., isolation rooms in hospitals is not unlikely; hence, in closed infected environments, it is recommended to strengthen protective measures for health-care workers. However, based on the current state of knowledge, the significant infection rate in open environments and high distances is improbable (Contini and Costabile, 2020).
Many experts believe air pollution, particularly, gas and particle-based air pollution can potentially increase the vulnerability urban population to COVID-19 (Contini and Costabile, 2020). In this sense, some studies found a positive correlation between air pollution and the COVID-19 mortality rate (Wang et al., 2020a; Travaglio et al., 2020a; Yongjian et al., 2020). The impact of the air quality on COVID-19 cases can be divided into short-term and long-term effect on air quality. Adhikari and Yin (2020) studied the short-term effect of the concentration of tropospheric ozone, particulate matter, and meteorological variables on COVID-19 cases in Queens County, New York. The results indicated a strong negative association between particulate matter concentration. Conversely, a broader perspective has been adopted by Wu et al. (2020a) who found positive association between COVID-19 cases and particulate matter concentration in 3000 counties in the United States. More specifically, they found an increase of in long-term exposure to particulate matter concentration is responsible for 15% increase in COVID-19 mortality. In an even broader perspective, Giani et al. (2020) studied both long- and short-term effect of COVID-19 lockdowns on particulate matter concentration. The results of short-term effect suggested that the lockdown interventions averted 24200 premature deaths in China, and averted 2190 premature mortalities in Europe. Moreover, the long-term effect results suggest 76400 to 287000 reduction in premature fatality in China, while in Europe prevented 13600 to 29500 reduction in premature fatality due to long-term exposure to particulate matter.
Some studies revealed that the COVID-19 outbreak decreased air pollution majorly due to the decrease in transportation and financial activities (Asna-Ashary et al., 2020; Afshari, 2020; Dutheil et al., 2020). More particularly, Muhammad et al. (2020) found 30% decrease in air pollution in populous cities of Wuhan, Italy, Spain, USA, etc.. Another study carried out in Tehran, Iran, signifies that the pandemic has led to higher outdoor particulate matter air pollution owing to the use of more personal transportation despite having more rainfall compared to the same time last year (Faridi et al., 2020b). Within this controversy lays the fact that some countries may promote the use of private transportation to avoid infection, which potentially can escalate air pollution, while some countries may propose lockdown interventions instead, which according to the Giani et al. (2020) may lead to decrease in air pollution.
In contrast, following the stay-at-home orders is not harmless. The studies carried out by Hosseini et al. (2020) and Afshari (2020) signified the flip side of the current lockdown interventions is that it has caused an increase in indoor cooking activities and consequently, decrease in indoor air quality and consequently, favored sick building syndrome.
People living in polluted cities as well as smokers may be at higher risk because of lung disease or reduced lung capacity, which would greatly increase the risk of serious illness. Moreover, smokers are likely to be more vulnerable to COVID-19 as the act of smoking means that fingers (and possibly contaminated cigarettes) are in contact with lips which increases the possibility of transmission of the virus from hand to mouth (Alqahtani et al., 2020). Therefore, in equal conditions, smokers are more vulnerable than non-smokers. As the statistical population of the study is significant, one may extrapolate the findings to similar cities (Cai, 2020).
The significance of bioaerosols and SARS-CoV-2 transmission in the clinical dental care facilities is not overlooked in the literature, as there is a possibility of viral shedding via droplets and bioaerosols in these environments (Ge et al., 2020). In this regard, the use of air ventilation in a healthcare setting is also a primary concern. WHO suggested a ventilation rate of 288 m3/h per person in hospitals and healthcare facilities. Zhao et al. (2020a) propose the use of air purifiers in the healthcare setting as an alternative measure after all precautionary measures have been taken (Zhao et al., 2020a).
There is no report on airborne infection in supermarkets; however, the possibility of the transmission via respiratory droplets that enter the mouth, nose, or eyes by contaminated hands may not be excluded in these environments (Desai and Aronoff, 2020). It is important to bear in mind that no study was found to indicate infection due to food consumption (Desai and Aronoff, 2020). Precautionary measures may include wearing personal protective equipment, e.g., wearing hand gloves, and practicing social distancing in supermarkets and grocery stores (Desai and Aronoff, 2020).
As stated previously, one of the major routes of COVID-19 transmission is through the respiratory system in infected hospitals. In this vein, WHO and the Center for Disease Control and Prevention (CDC) have issued guidelines to prevent SARS-CoV-2 infection. Based on the latest reports, healthcare exposures are assessed via close contact measures, e.g., intake of respiratory droplets produced when an infected person speaks, coughs, or sneezes. Thereby, healthcare exposure can be classified into low, medium, and high based on the accessibility to personal protective equipment in public environments and contact levels with asymptomatic COVID-19-suspected individuals (CDC, 2020a).
Increased antibiotic resistance is known as a possible side-effect of SARS-CoV-2 viral shedding in healthcare facilities. The main cause of this phenomenon is the high consumption of anti-microbial agents in these facilities to manage bacterial infections (Rawson et al., 2020). The high demand for anti-microbial agents can potentially put the lives of people with a weak immune system at higher risk, especially in developing countries (Fouladi Fard and Aali, 2019; Mirhoseini et al., 2018).
3.2. Metrological factors
A large and growing body of literature has investigated the relationship between meteorological variables such as wind speed, absolute humidity, sunlight or cloud percentages, atmospheric pressure, etc. and COVID-19 outbreak.
3.2.1. Temperature and humidity
Tosepu et al. (2020) who found a negative relationship between temperature and COVID-19 transmissibility in Indonesia; however, they did not report any similar association with relative humidity and precipitation. A broader perspective has been adopted by some studies, including Wang et al. (2020f), Roy and Kar (2020), and Nazari Harmooshi et al. (2020) who argued a decreased transmissibility of COVID-19 in higher outdoor temperatures and humidity. In the same vein, Biktasheva (2020) notes a higher infection rate and respiratory system sensibility in dry air conditions; however, it has been reminded that in extreme humidity conditions, bacterial infection can also happen. Xu et al. (2020b) found meteorological parameters in specific situations may affect the reproduction of SARS-CoV-2; however, they conservatively indicated an insignificant control of meteorological variables on the reproduction rate of the virus in most countries around the world.
Based on Otter et al. (2016), SARS-CoV-1 was known to be affected by ambient temperature and relative humidity. A study on SARS-CoV-2 carried out in 122 cities from China has shown a positive association between temperature and the number of COVID-19 cases (Xie and Zhu, 2020). These results are somewhat counterintuitive. In contrast to Xie and Zhu (2020), Qi et al. (2020a) found a negative association between temperature and COVID-19 cases during a 23-days study carried out in Hubei, China. They found a 36%–57% increase in COVID-19 cases by every 1 °C increase in average temperature when relative humidity is in the range 67%–85.5%. Moreover, a negative association was found between average relative humidity and the count of cases. Particularly, it was found that when the average temperature is in range 5.04 °C–8.2 °C, COVID-19 cases dropped 11%–22% (Qi et al., 2020a). The salient difference between the findings of these studies may stem from the time when the studies carried out. The second study was carried out in the cold season and the beginning of the epidemic when there was a lack of sufficient information on SARS-CoV-2 and the number of total cases did not reach 3000. Therefore, it can be concluded that the findings of Xie and Zhu (2020) are more reliable. Another study carried out in Brazil indicates in temperatures lower than 25.8 °C, every 1 °C can decrease the number of cases by 4.89%; however, to this point, there is no evidence that in temperatures higher than 25.8 °C, increase in temperature can decrease the COVID-19 cases counts (Prata et al., 2020).
The variations in temperature and humidity can impact the mortality rate of SARS-CoV-2 infection. A study conducted on 2299 victims of COVID-19 denotes a positive association between the mortality rate and temperature. Notably, it was found that every 1 °C increase in ambient temperature, is responsible for a 2.92% increase in mortality rate. On the other hand, a negative association between relative humidity and the fatality rate was found (Wang et al., 2020a).
Ignoring public health guidelines, the use of public transportation, as well as many other factors, can be responsible for the high infection rate in many countries where the studies were carried out. These key factors should not be disregarded as many studies including Zhou et al. (2017) found an association between weather elements, specifically temperature, rather than other factors on the use of public transportation. Mackenbach et al. (1997) and Faunt et al. (1995) found an increased rate of hospitalization due to elevated temperature, which, taken together, may increase the exposure risk to COVID-19. To date, based on evidence, the increase in temperature cannot be responsible for a decrease in infection rate while there is a shred of growing evidence that obeying public health guidelines can ease the control on COVID-19 before developing an effective vaccine (Mirzaei et al., 2020).
3.2.2. Wind speed and solar radiation
Wind speed and solar radiation, like other meteorological variables, can potentially be classified as covariates of COVID-19 cases (Briz-Redón and Serrano-Aroca, 2020). Recently, there has been a surge of interest to determine how SARS-CoV-2 is affected if the airborne route is one of the modes of viral transmission. In this context, investigating wind speed, as a carrier of the virus-laden aerosols, is a continuing concern within the viral transmissibility of COVID-19. As study carried out by Sarkodie and Owusu (2020), signified the positive relationship of wind speed and COVID-19 confirmed cases in 20 countries. It was also found the mortality rate of COVID-19 increased with the wind speed. These results are supported by Chen et al. (2020) who studied the relationship of COVID-19 confirmed cases and wind speed for 52 days, observed the peak of SARS-CoV-2 transmissibility under a specific wind velocity.
As denoted by Nakada and Urban (2020), there is evidence that solar radiation is a mitigating factor to COVID-19 transmissibility, most possibly through vitamin D synthesis in human skin (Holick et al., 2007), which itself is thought to be negatively correlated with COVID-19 mortality rate (Ilie et al., 2020). Accordingly, it was found that the ultraviolet radiation caused a decrease in COVID-19 reported cases in São Paulo, Brazil. Among different wavelengths of ultraviolet light, the 200–260 nm range is able to damage pathogens RNA or DNA (Tang et al., 2020). Another study indicates 90% the SARS-CoV-2 load can be inactivated after being exposed to the midday summer sunlight for 34 min (Sagripanti and Lytle, 2020). Many studies support the notion of negative correlation between solar radiation and COVID-19 cases including Sfîcă et al. (2020) and Rosario et al. (2020). However, there is a little consensus about whether meteorological variables act in harmony with each other to regulate the virus transmission rate or each individual meteorological variable may impact transmission rate; hence, a systematic need for understanding of the topic still remains (Briz-Redón and Serrano-Aroca, 2020).
3.3. Wastewater
Wastewater is known for its role in transmitting a wide variety of pathogens via sewer network. Wastewater provides food, growth, and shelter sources for microorganisms (Simmons and Xagoraraki, 2011). Previous literature supported and confirmed the detection of coronaviruses and coronaviridae family in urban sewer networks (Shahbaz et al., 2016). However, the important note is that some asymptomatic and symptomatic patients discharged the viruses before and after showing any indication of viral infection (Shahbaz et al., 2016). The later studies including Lodder and de Roda Husman (2020) introduced SARS-CoV-2 in the wastewater collection network as a potential health risk. Research has demonstrated that SARS-CoV-1 and SARS-CoV-2 are 80% genetically similar. Wang et al. (2005b) detected the nucleic acid of SARS-CoV-1 in untreated hospital wastewater while the RNA of this virus could be occasionally found after wastewater disinfection (Jia and Zhang, 2020). It seems possible that these results do not necessarily represent the infection localization as one may bring infection to wastewater by washing infected hands. This general lack of methodological rigor may put in question the results as the samples were not taken directly from individuals stool. Some studies are still undergoing to discover the detectability of SARS-CoV-2. This view has been supported in some studies that reported the possibility of the oral-fecal route of SARS-CoV-2 virus, despite the main infection route is supposed to be through respiratory system (Lodder and de Roda Husman, 2020; Mao et al., 2020; Naddeo and Liu, 2020). In these studies, the quantity of samples is appropriate which may reflect the credibility of these studies.
From the clinical point of view, 2 to 10 percent of patients have shown gastrointestinal symptoms such as diarrhea, vomiting, or belly pain (Wang et al., 2020d). The possibility of SARS-CoV-2 can be spread through wastewater may not be totally excluded; bearing in mind that SARS and Middle East Respiratory Syndrome (MERS) were reported to be transmissible through wastewater (Yeo et al., 2020; Wang et al., 2005b). Up to now, some studies confirmed the detection of two main genomes of SARS-CoV-2 in samples taken from wastewater (Medema et al., 2020). Depending on the accuracy of the samples taken from the sewers of seven cities, the statistical population of these studies seems appropriate.
The initial evidence from Holshue et al. (2020) implies the detection of SARS-CoV-2 RNA from the samples of the first case of the COVID-19 patient in the USA. Other studies carried out in a hospital in China reported the detection of the novel coronavirus genomes in wastewater before but not after the disinfection process (Wang et al., 2020d). Limited sample size, i.e., three samples, and sampling location, i.e., before disinfection, fails to properly identify the quantity of the virus genome before the disinfection. Taken together, according to Zhang et al. (2020a), there is a chance of detecting the virus in viable form even after the disinfection of medical wastewater. The virus may embed itself in stool and shield itself from being disinfected.
More strikingly, Wu et al. (2020b) carried out a study on 98 patients and found the virus viable in gastrointestinal tract 11.2 days after the viral clearance of the respiratory tracts. The evidence presented thus far support the idea that viable SARS-CoV-2 can be detectable in raw wastewater. The importance of this notion lies in the fact that each catchment of the modern wastewater network can be a representative of a study zone. This may help the researchers to estimate the prevalence of infections among the population via wastewater-based epidemiology. Ahmed et al. (2020) reported two out of nine cases of SARS-CoV-2 detection in raw wastewater using reverse transcriptase quantitative polymerase chain reaction. The Monte Carlo simulation provided an estimate the median range of 171–1090 cases out of 600 thousand population of a wastewater catchment in Australia could be infected. Considering the sample size and the proposed methodological rigorousness, the findings of the study can effectively contribute to the current understanding of virus localization in sewer networks. This estimate is in agreement with clinical observations of Xing et al. (2020) who studied the dynamic changes of SARS-CoV-2 RNA in the fecal specimens of the children suffering from the novel coronavirus. In a comprehensive review of COVID-19 test results collected from the Shandong Province, China revealed that the respiratory tract of the cases was cleared from SARS-CoV-2 within two weeks after the abatement of fever. However, the virus could be detected in the fecal specimen for longer than four weeks. This may be a clear indication that the virus detectability in the pediatric patients' gastrointestinal tract is significantly longer than the respiratory tract. As both direct and indirect contact is more prevalent among children, the viral shedding of SARS-CoV-2 in feces of children should be taken much more seriously especially in kindergartens and schools. The study holds a valuable contribution from two perspectives. First, the samples were taken from children's stools rather than the sewer network. Second, the virus remained viable two weeks after children's respiratory tract clearance which is more than what was previously mentioned by Wu et al. (2020b). A study on the wastewater of an airport in Amsterdam confirmed the detection of SARS-CoV-2 acid nucleic. Therefore, the fecal-oral transmission route can be taken into consideration (Lodder and de Roda Husman, 2020). Similar to Wang et al. (2020d) considering only the infection rout of wastewater may not yield conclusive results as infection may penetrate into sewer networks by washing infected hands or objects. In this sense, one can consider the existence of SARS-CoV-2 in various treatment processes of a wastewater treatment plant, even in the effluent and the downstream receiving environments such as farmlands and water supplies. Hence, the workers in wastewater treatment plants and farmers are encouraged to take precautionary measures including wearing personal protective equipment (WHO, 2020d). A study carried out in 2003 suggested that aerosolized SARS-CoV-1 was transmitted through faulty wastewater drainage of a high-rise apartment building in Hong Kong, came down with 342 cases of the disease, and killed 42 people (Hung, 2003). A similar case can happen for SARS-CoV-2 in a wastewater treatment plant, particularly in the aeration process, the proximity of manholes, and screw pumps.
Although containing and inactivating the novel coronavirus in wastewater may not be practical due to variations in temperature, pH, etc. (Gundy et al., 2009), the exposure risk can be reduced by taking proper preventive and protective measures. It is suggested to use higher than normal doses of disinfectants in wastewater treatment plants. Previous studies suggest SARS-CoV-1 can be inactivated by 20 mg/L of chlorine in a contact time of 20 min, while E. coli can be 99% inactivated by 5 min in the same experimental setup. Similarly, 40 mg/L of chlorine dioxide in a contact time of 5 min can inactivate all SARS-CoV-1 and 99.99% of E. coli. Taking into account that by increasing the concentration of disinfectant, contact time decreases, and vice versa (Wang et al., 2005a). A more efficient disinfection method may involve the use of two-stage disinfection processes including chemical (e.g., ozone and chlorine) and physical (e.g., ultraviolet) disinfection (WHO, 2020d).
Healthcare facilities wastewater are commonly-known as environments with significant viral load. Unfortunately in many countries, due to the lack of separate wastewater treatment plants, the viral load of hospitals is imposed on urban wastewater treatment plants, which are often ill-equipped to handle. In this manner, WHO (2020d) suggests a wastewater stabilization pond (e.g., an oxidation pond or lagoon) as a practical pathogen destroyer due to the long retention time (20 days or longer), sunlight and high pH levels.
All things considered, there are still many unanswered questions about the interactions of SARS-CoV-2 and wastewater in sewer networks. To exemplify (Kitajima et al., 2020):
-
•
Is there any possibility of mutation of SARS-CoV-2 under varying physicochemical conditions in sewer systems?
-
•
How much temperature variations can affect the stability of SARS-CoV-2 in sewer drainage networks?
-
•
Can aerosolized SARS-CoV-2 emitted from wastewater treatment plants expose nearby workers?
-
•
Does the pathogenicity of the novel coronavirus vary over time?
-
•
How is the stability of this virus in solid waste digestion processes?
-
•
Can SARS-CoV-2 enter into the downstream receiving environments?
3.4. Water
Previous studies suggest surrogate coronaviruses can potentially be viable in liquid water depending on the physicochemical and biological properties of it. Gundy et al. (2009) found coronaviruses can survive at 23 °C water for 10 days, while some of them remained viable after 100 days in 4 °C water. However, the same rule may not apply in urban water distribution networks majorly due to rapid pressure and temperature difference, etc. (Casanova et al., 2009). It is noteworthy to mention that, to date, none of WHO reports, owing to the lack of research, indicates the transmission of SARS-CoV-2 via the water distribution network. Based on WHO reports, the viral envelope of SARS-CoV-2 is a lipid bilayer; hence, lower resistance to commonly-used detergents and ultraviolet light is expected (WHO, 2020d). Thereby, the infection risk of SARS-CoV-2 from treated water resources might be considered low. It is noteworthy to mention that to date, there is no indication of the SARS-CoV-2 infection through water; however, the infection risk through water, polluted by infected wastewater is still being investigated. However, tighter measures including wastewater disinfection should be evaluated based on the current settings in wastewater treatment plants.
3.5. Fomites
The studies on fomites are qualified as comprehensive ones with relatively low sampling limitations as samples can be taken from every surface exposed to every individual. Fomites have a significant role in the viral shedding of infectious viruses including SARS-CoV-2. According to the latest reports of WHO, frequent surface hygiene using appropriate techniques and regular disinfection practices are introduced as protective measures for SARS-CoV-2 infection, especially in healthcare facilities dealing with COVID-19 patients (WHO, 2020c). However, a debate has long prevailed as to whether the persistence of the human and veterinary surrogate coronaviruses on various surfaces is significant or not. Owing to this, Kampf et al. (2020) reviewed the literature to collect the persistence of SARS, MERS coronavirus, or endemic human coronaviruses (HCoV) on inanimate surfaces.
Based on Table 1 , the persistence of surrogate coronaviruses depending on temperature and surface size vary from 2 h to 9 days. Therefore, in order to inactivate the virus based on the finding of 22 studies, it is suggested to apply commonly-used disinfectants, including 72-62% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite for at least 1 min. Other disinfectants including 0.05–0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate were found to be less successful for inactivating the above-mentioned viruses. The recent literature review indicated that relative humidity may also be a factor for coronavirus's persistence on surfaces (Kampf et al., 2020; WHO, 2020b). Jiang et al. (2020) study results in the First Hospital of Jilin University indicate SARS-CoV-2 in a specimen taken from the surfaces of the nurse station in the isolation area were detected by Reverse Transcription-Polymerase Chain Reaction (Jiang et al., 2020; Van Doremalen et al., 2020).
Table 1.
Persistence of various surface types.
| Surface Type | Virus | Temperature | Persistence | |
|---|---|---|---|---|
| Steel | MERS | 20 °C | 48 h | Van Doremalen et al. (2013) |
| Steel | HCOV | 21 °C | 5 days | Warnes et al. (2015) |
| Aluminum | HCOV | 21 °C | 2–8 h | Sizun et al. (2000) |
| Metals | SARS-CoV-1 | 20 °C | 5 days | Dong (2003) |
| Wood | SARS-CoV-1 | Room temperature | 4 days | Dong (2003) |
| Paper | SARS-CoV-1 | Room temperature | 4–5 days | Dong (2003) |
| Glass | SARS-CoV-1 | Room temperature | 4 days | Dong (2003) |
| Plastic | SARS-CoV-1 | Room temperature | 6–9 days | Rabenau et al. (2005) |
| Ceramic | HCOV | 21 °C | 5 days | Warnes et al. (2015) |
| Teflon | HCOV | 21 °C | 5 days | Warnes et al. (2015) |
People may follow social distancing rules; however, the possibility of habitual indirect contact might still be notoriously significant. The importance of this cannot be ignored as it can fuel the life cycle of the novel coronavirus (Yen et al., 2020). SARS-CoV-2 can stay viable up to 9 days on unsterilized public surfaces (Kampf et al., 2020) in a city including cell phones, doors, toilet bowl, sink, public transportation handles or straps, and doorknobs (Hossain et al., 2019; Jiang et al., 2020; Lai et al., 2020). The spike glycoprotein in coronaviruses and SARS-CoV-2 helps the virus hook on surfaces as well as target cells (RAJ et al., 2013; Tamal and Bhaskar, 2020).
Ding et al. (2020) detected SARS-CoV-2 on 60% of hospital bathroom doorknobs and 80% of inanimate surfaces in China. Similarly, Hirotsu et al. (2020) detected SARS-CoV-2 on 50% of nurse calls attached to the COVID-19 infected beds in Japan.
3.6. Solid waste
The COVID-19 pandemic has caused major problems including municipal solid waste (MSW) management and hazardous waste management. The Solid Waste Association of North America reported on the possible shift in the volume and the source of MSW due to the COVID-19 restrictions. In China, according to the March 11 press release, a 30% decrease in the amount of MSW was observed while in Hubei Province, the amount of hospital solid waste strikingly increased by 370% (Kulkarni and Anantharama, 2020). In addition, the patients being treated at home produce infectious solid waste which is disposed of as MSW posing danger to MSW management workers (Mol and Caldas, 2020).
The elevated use of personal protective equipment (PPE) for healthcare workers including hand gloves, facemasks, and surgical gowns and wearing facemasks in public has caused a dramatic increase in the appearance of these kinds of wastes in hospital solid waste and MSW (Nzediegwu and Chang, 2020).
MSW management workers are considered to be more exposed to the contaminated PPE in developing countries including Nigeria than the developed ones. Although other virus containment measures are taken seriously, the management of contaminated MSW along with adopting a coherent MSW strategy has been overlooked in some developing countries (Nzeadibe and Ejike-Alieji, 2020). The impugned legitimacy of using single-use plastic is now restoring due to the pandemic and high demand for PPE. Consequently, it is not far-fetched to see the severe impact of the elevated use of single-use plastics on the environment (Kalina and Tilley, 2020).
A study carried out in February 2020 by Yu et al. (2020) in China, concluded solid waste incineration as a temporary solution for decontamination and disposal of solid medical waste in a proper location.
The use of special trashcans or waste containers for collecting PPE for residential, governmental, and hospital buildings could be a safe approach to prevent hazardous exposure to the virus. Such measures require trained personnel to empty the containers on a daily basis. The disposed of plastic bottles could also be decontaminated by 70% ethanol solution before being reused by local packaging companies (Nzediegwu and Chang, 2020).
3.7. COVID-19 and secondary effects on environment
COVID-19 pandemic impacted the sustainable development themes in many cities (Lau et al., 2020). Owing to the current pandemic and increase in poverty and unemployment rate have classified sustainable development in low priority (Khan et al., 2020; Chowdhury et al., 2020). Besides, the high mortality rate in many countries has led to a substantial decrease in promoting the concept of protecting the environment through sustainable development, especially in developing countries. As a side-effect, economic crunch due to the closure of industries can prevail hunger and poverty despite governmental relief packages (Javed, 2020). After managing the crisis, to put sustainable development back in track, it is required for impacted countries to reach an agreement to revive United Nations Environment Programme.
Cadotte (2020) reported improved air quality in five out of six cities in February 2020 compared to the same month in 2019. Higher air quality is majorly due to government restrictions in response to the COVID-19 pandemic. Although the effects of these restrictions are supposed to be short-term, the results of Cadotte (2020) confirm that governmental policies potentially have the capacity to improve air quality by introducing new air quality control policies.
The use of disinfectants in response to COVID-19 pandemic has distressingly increased in both open and closed spaces including streets, houses, governmental departments, etc.. Zhang et al. (2020b) stated only in Wuhan, China, at least two thousand tons of disinfectants were dispensed. There is growing concern that discharging high amounts of disinfectants in sewer systems may pollute water resources or aquatic environments. The environmental threat posed by chlorine-based sanitizers can be classified into three cases. First, chlorine is known to be a powerful oxidizer, and owing to this feature it can destroy the living cell membrane. Second, the bonding of chlorine to dissolved organic matter can produce hazardous byproducts including, trihalomethanes or haloacetic acids. Besides, the carcinogenic formation as a result of the chemical reaction of chlorine with nitrogen is inherently dangerous (Zhang et al., 2020b; Sedlak and Von Gunten, 2011; Liu and Zhang, 2014; Bei et al., 2016). All things together, it is strongly suggested that countries conduct an environmental impact assessment to study how much the environment is affected by COVID-19 pandemic. This approach can benefit biological diversity and help to mitigate any negative impacts of future crises (Karr, 1993). Besides, excessive use of disinfectants can lead to an increase in antibiotic resistance by developing the ability to endure being destroyed. In this regard, studies have shown that antibiotic resistance, including multiple antibiotic resistance, in bacteria living in the disinfection process of wastewater treatment plants has been substantially increased (Aali et al., 2014, 2019; Yeganeh et al., 2018). Table 2 provides a summary of COVID-19 prevalence on the environmental factors.
Table 2.
The summarized effects of COVID-19 prevalence on the environmental factors.
| Risk factors | Risk measure | Effects of COVID-19 outbreak | methodological basis | orientation and effort of the study | References | |
|---|---|---|---|---|---|---|
| Atmosphere | PM10, PM2.5, NO2, CO | 10–43% | Decreased air pollution | Data obtained from a network of air quality monitoring stations across 22 different cities in India for the past four years (2017–2020) for the time period of March 16th to April 14th. | Determined the effect of restricted emissions during COVID-19 on air quality in India | Sharma et al. (2020) |
| CO | 97.3–207.0% | Increased air pollution | The hourly concentrations from 35 monitoring stations were obtained from China's National Environmental Monitoring Centre and meteorological data were collected from the Meteorological Information Comprehensive Analysis and Process System (MICAPS) of the Chinese Meteorological Administration so modeling was carry out by HYSPLIT, PSCF and CWT. | modeling the effect of COVID-19 on air quality in 3 regions of China | Zhao et al. (2020b) | |
| 50–58% | Decreased air pollution | Data from the eight air quality stations located in various points in the city of Naples was collected | Analysis of Air Quality during the COVID-19 Pandemic Lockdown in Naples | (Sannino et al.) | ||
| 20% | Decreased air pollution | For the CO data Atmospheric Infrared Sounder (AIRS) introduces the grating spectrometer aboard AQUA satellite, launched on May 4, 2002 was used. | Compare the concentrations of atmospheric pollutants in the period before the lockdown and during the implementation of preventive control measures COVID-19. By focusing on East China | (Filonchyk et al.) | ||
| 36.2% | Decreased air pollution | Data were obtained from the platform: http://www.aqistudy.cn/. | Air Quality Index, Indicatory Air Pollutants and Impact of COVID-19 Event on the Air Quality near Central China | Xu et al. (2020a) | ||
| NO2 | 4.5–89.5% | Increased air pollution | The hourly concentrations from 35 monitoring stations were obtained from China's National Environmental Monitoring Centre and meteorological data were collected from the Meteorological Information Comprehensive Analysis and Process System (MICAPS) of the Chinese Meteorological Administration so modeling was carry out by HYSPLIT, PSCF and CWT. | The hourly concentrations from 35 monitoring stations were obtained from China's National Environmental Monitoring Centre and meteorological data were collected from the Meteorological Information Comprehensive Analysis and Process System (MICAPS) of the Chinese Meteorological Administration so modeling was carry out by HYSPLIT, PSCF and CWT. | (Zhao et al., 2020b) | |
| Nearly 50% | Decreased air pollution | The data was collected in two phases, 15 days before the lockdown (i.e., March 10th–March 24th) and 15 days after the lockdown (25th March–April 8th, 2020) Implementation from these targeted cities. The daily air quality data was obtained from the data repository maintained by Central Pollution Control Board (CPCB) under the Ministry of Environment, Forest and Climate Change, India (https://app.cpcbccr.com/AQI_India/). | Assess the changes in air quality parameters during the implementation of the lockdown measures in the four major metropolitan cities of India, viz., Delhi, Mumbai, Kolkata and Chennai for a one-month period (15 days before lockdown and 15 days after the implementation of lockdown). | (Bedi et al., 2020) | ||
| 20–40% | Decreased air pollution | The space-based air quality measurements from the National Aeronautics and Space Administration (NASA) Ozone Monitoring Instrument (OMI) onboard the Aura satellite and the TROPOspheric Monitoring Instrument (TROPOMI) onboard the European Space Agency's (ESA) Sentinel-5 Precursor (Sentinel-5P) satellite were used for assessing the spatial and temporal evolution of tropospheric NO2 throughout California during the pre- and post-initiation of COVID-19 containment measures in the state. | Investigate the impact of COVID-19 Containment Measures on Air Pollution in California | Naeger and Murphy (2020) | ||
| 30% | Decreased air pollution | Nitrogen dioxide (NO2) emission data obtained from Ozone Monitoring Instrument (OMI) on board the AURA satellite launched in 2004 as part of the NASA EOS (Earth Observation System) was used | Compare the concentrations of atmospheric pollutants in the period before the lockdown and during the implementation of preventive control measures COVID-19. By focusing on East China | (Filonchyk et al.) | ||
| SO2 | Negligible | Increased air pollution | Data obtained from a network of air quality monitoring stations across 22 different cities in India for the past four years (2017–2020) for the time period of March 16th to April 14th. | Determined the effect of restricted emissions during COVID-19 on air quality in India | Sharma et al. (2020) | |
| 16.9–33.9% | Increased air pollution | The hourly concentrations from 35 monitoring stations were obtained from China's National Environmental Monitoring Centre and meteorological data were collected from the Meteorological Information Comprehensive Analysis and Process System (MICAPS) of the Chinese Meteorological Administration so modeling was carry out by HYSPLIT, PSCF and CWT. | The hourly concentrations from 35 monitoring stations were obtained from China's National Environmental Monitoring Centre and meteorological data were collected from the Meteorological Information Comprehensive Analysis and Process System (MICAPS) of the Chinese Meteorological Administration so modeling was carry out by HYSPLIT, PSCF and CWT. | Zhao et al. (2020b) | ||
| 70% | Decreased air pollution | Data from the eight air quality stations located in various points in the city of Naples was collected | Analysis of Air Quality during the COVID-19 Pandemic Lockdown in Naples | (Sannino et al.) | ||
| 52.5% | Decreased air pollution | Data were obtained from the platform: http://www.aqistudy.cn/. | Air Quality Index, Indicatory Air Pollutants and Impact of COVID-19 Event on the Air Quality near Central China | Xu et al. (2020a) | ||
| O3 | 17% | Increased air pollution | Data obtained from a network of air quality monitoring stations across 22 different cities in India for the past four years (2017–2020) for the time period of March 16th to April 14th. | Determined the effect of restricted emissions during COVID-19 on air quality in India | Sharma et al. (2020) | |
| 16.4–33.9% | Decreased air pollution | The hourly concentrations from 35 monitoring stations were obtained from China's National Environmental Monitoring Centre and meteorological data were collected from the Meteorological Information Comprehensive Analysis and Process System (MICAPS) of the Chinese Meteorological Administration so modeling was carry out by HYSPLIT, PSCF and CWT. | The hourly concentrations from 35 monitoring stations were obtained from China's National Environmental Monitoring Centre and meteorological data were collected from the Meteorological Information Comprehensive Analysis and Process System (MICAPS) of the Chinese Meteorological Administration so modeling was carry out by HYSPLIT, PSCF and CWT. | Zhao et al. (2020b) | ||
| PM2.5 | 20.5% | Increase air pollution | Tehran Air Quality Control Company data in two years | Compare the concentrations of ambient air PM10 and PM2.5 in Tehran during the SARS-CoV-2 outbreak and over the same period of last year. | (Faridi et al.) | |
| Nearly 50% | Decreased air pollution | The data was collected in two phases, 15 days before the lockdown (i.e., March 10th–March 24th) and 15 days after the lockdown (25th March–April 8th, 2020) Implementation from these targeted cities. The daily air quality data was obtained from the data repository maintained by Central Pollution Control Board (CPCB) under the Ministry of Environment, Forest and Climate Change, India (https://app.cpcbccr.com/AQI_India/). | Assess the changes in air quality parameters during the implementation of the lockdown measures in the four major metropolitan cities of India, viz., Delhi, Mumbai, Kolkata and Chennai for a one-month period (15 days before lockdown and 15 days after the implementation of lockdown). | Bedi et al. (2020) | ||
| 46.5% | Decreased air pollution | Data were obtained from the platform: http://www.aqistudy.cn/. | Air Quality Index, Indicatory Air Pollutants and Impact of COVID-19 Event on the Air Quality near Central China | Xu et al. (2020a) | ||
| PM10 | 15.7% | Increase air pollution | Compared Tehran Air Quality Control Company data | Compare the concentrations of ambient air PM10 and PM2.5 in Tehran during the SARS-CoV-2 outbreak and over the same period of last year. | (Faridi et al.) | |
| Nearly 50% | Decreased air pollution | The data was collected in two phases, 15 days before the lockdown (i.e., March 10th–March 24th) and 15 days after the lockdown (25th March–April 8th, 2020) Implementation from these targeted cities. The daily air quality data was obtained from the data repository maintained by Central Pollution Control Board (CPCB) under the Ministry of Environment, Forest and Climate Change, India (https://app.cpcbccr.com/AQI_India/). | Assess the changes in air quality parameters during the implementation of the lockdown measures in the four major metropolitan cities of India, viz., Delhi, Mumbai, Kolkata and Chennai for a one-month period (15 days before lockdown and 15 days after the implementation of lockdown). | Bedi et al. (2020) | ||
| 48.9% | Decreased air pollution | Data were obtained from the platform: http://www.aqistudy.cn/. | Air Quality Index, Indicatory Air Pollutants and Impact of COVID-19 Event on the Air Quality near Central China | Xu et al. (2020a) | ||
| Water | Water quality | decreased 15.9% of suspended particulate matter | Improved water quality | The suspended particulate matter was measured by the red band (655 nm) | Investigate the effect of COVID-19 lockdown on surface water quality | Yunus et al. (2020) |
| The BOD and COD values reduced by 42.83% and 39.25%, respectively, Faecal Coliform declined by over 40%. | Improved water quality | Water quality data was obtained from the Delhi Pollution Control Committee (DPCC) for nine monitoring stations. The time period primarily examined is from January to April of 2020. The data obtained for 6 January, 13 February and 13 March 2020, was taken to represent the pre-lockdown state while that of 6 April and 14 April 2020, as indicative of the water quality status during the lockdown phase. | Examining the Yamuna's water quality at Delhi during the COVID-19 lockdown period | Patel et al. (2020) | ||
| Clean beaches | The lack of tourists | Decreasing beaches solids and improved seaside water quality | Positive and negative indirect effects of COVID-19 on the environment are presented and compared with the period of time which COVID-19 is not exist. | Presentation Indirect effects of COVID-19 on the environment | Zambrano-Monserrate et al. (2020) | |
| Wastewater | Disinfectants | To prevent the new coronavirus from spreading | The excess of chlorine in the water is harmful for human | Positive and negative indirect effects of COVID-19 on the environment are presented and compared with the period of time which COVID-19 is not exist. | Presentation Indirect effects of COVID-19 on the environment | Zambrano-Monserrate et al. (2020) |
| To prevent the new coronavirus from spreading | Increased disinfectants using | Authors comparison the usage of disinfectants by economic and feasible factors | Investigate the amount and type of disinfection technology of hospital wastes and wastewater | Wang et al. (2020e) | ||
| Wastewater disinfection process | Amount of liquid chlorine, sodium hypochlorite, chlorine dioxide that discharge to sewage | Killing viruses and viruses gens so effluent can discharge for agricultural | Authors comparison the usage of disinfectants by economic and feasible factors | Investigate the amount and type of disinfection technology of hospital wastes and wastewater | Wang et al. (2020e) | |
| Solid waste | Medical waste | – | Medical waste increased | effects of COVID-19 on the hospital waste production was presented by available data | determined the present and future plastic waste, energy and environmental footprints related to COVID-19 | Klemeš et al. (2020) |
| MSW management | – | Decreased MSW management | effects of COVID-19 on the municipal waste production Was investigated using existing data and information from the past | Determine the COVID-19 pandemic on municipal solid waste management | Kulkarni and Anantharama (2020) |
4. Limitations
The survey was hindered by a lack of accurate resources and language. Particularly, the authors did not have access to specific accurate information on the reviewed papers, e.g., sampling height and ventilation rate. Hence, to be more conclusive, one may need this information to compare each based on the physicochemical properties of the study area. Moreover, the authors were limited to review literature which was in English as a minor portion of the literature was published in non-English resources.
5. Summary of conclusions
After a long time of studying non-communicable diseases, apparently, it is time for the world to exert and extra effort to study contagious diseases. The current crisis has shown us COVID-19 and other transmissible diseases can potentially impact the whole world as well as the environment. Based on the current scientific understanding of COVID-19, airborne SARS-CoV-2 can be transmissible in 4 m in closed spaces; hence, following airborne precautions is required. Also been proven that SARS-CoV-2 can be transmitted by holding on aerosols. In this case, it is highly recommended to use air conditioning systems with extra persuasions, especially when the air is possibly infected. As pointed out by Sfîcă et al. (2020) and Briz-Redón and Serrano-Aroca (2020), although meteorological variables are believed to be a contributing factor to COVID-19 cases, social practices and local regulation, including lockdown interventions are believed to be the principal components of the virus spread.
There is evidence that virus-containing aerosols are re-suspended from contaminated surfaces, which should be cleaned and disinfected, especially in the toilets. Wearing masks can lower the chance of infection through the air, particularly in closed-space populated locations. Clearly, obeying the stay-at-home orders and traffic restrictions can hinder the further spread of this disease (Wang et al., 2020b). MSW management is also affected by the pandemic. The MWS management workers are recommended to handle MWS with extra care as they may be infected by the virus.
The current understanding of the SARS-CoV-2 is constantly changing. Fecal-oral transmission route control can significantly be advantageous to contain the virus. As the virus has been detected in wastewater and stool samples, considering this can help us originate the virus through the sewer system and assess the efficiency of wastewater treatment processes (Núñez-Delgado, 2020; Pan et al., 2020). These findings are reliable as these two studies samples were taken directly from patients' stool and prove the virus can spread from patient feces to sewer networks.
Accordingly, epidemiological approaches in wastewater can aid us screen and classify suspicious areas from clean areas (Núñez-Delgado, 2020). Besides, the need for studying several wastewater treatment techniques to contain the virus, not to disinfect it, including bio sorbents is more being stressed (Núñez-Delgado, 2020). In the case of disinfection of the virus in the wastewater disinfection process, applying the recommended dose of disinfectant based on state-of-art studies is vital.
A while ago, common knowledge about the persistence of the virus was limited to a few days (Kampf et al., 2020). Now, knowing that the virus can persist up to nine days, the possibilities of the virus spread from place to place, person to person, reproduction, and mutation are now serious (Núñez-Delgado, 2020). As the persistence time increases, the chance of the virus being transmitted via vaporized droplets increases; hence, transmission over the distance of 10 m is not far-fetched (Morawska et al., 2009). Although there is no indication of airborne transmitted SARS-CoV-2 in indoor environments, it is essential that governments increase public awareness of the droplet or particle transmission (Morawska and Cao, 2020). The novel coronavirus can be transmitted both directly and indirectly. In this regard, doorknobs, cash, nurse calls, hospital beds, and street fences are some of the most infected objects. Accordingly, the disinfection of these high-contact objects can decrease the chance of SARS-CoV-2 spread while increasing the possibility of containing it. It is noteworthy to mention that disinfection using truck-size white-fog-spraying machines may pose several chronic side-effects on humans and the environment. Promising studies suggest the application of technology for tracking infected individuals including Min-Allah and Alrashed (2020) who found the application of smart campus and internet of things to track infected persons.
COVID-19 may become a seasonal disease; hence, measuring its persistence in various environments including water, wastewater and airborne aerosols based on different factors including temperature, humidity, etc. is crucial. The bottom line for counties in the world is to adhere to sustainable development themes and goals and support those countries with poor infrastructures; as the whole international community is integrated and an epidemic in one country can rapidly turn into a pandemic.
6. Conclusion of systematic review
Following the PRISMA approach, 49 studies were comprehensively reviewed and near 100 studies were cited to explore and provide the readers with the most up-to-date knowledge on the bidirectional relationship between COVID-19 and the environment. The study sets out a systematic review on broad topics of COVID-19 and the way it is being impacted by the environment and vice versa.
The mean score of the reviewed studies is ~49, which indicates all of the explored studies are of high quality according to Table 3 criteria. Among these studies, 33% in China, 25% in the United States, 13% in Europe, 4% in Australia 12% were carried out in Iran, 2% in Africa, and 11% in the rest of the world. Therefore, the spatial distribution of the studies is not uniform.
Among all reviewed studies, more than 36% of them were directly or indirectly related to the indoor and outdoor environment, similarly, 16% of them were related to meteorological factors, 11% were related to wastewater, 14% were related to fomites, 8% were related to water, 9% to solid waste, and 6% to the secondary environment. The growing body of literature on airborne transmissibility of SARS-CoV-2 suggests the new ways to detect the viable virus in air samples are being developed, which is the introduction for manufacturing portable devices that does so, perhaps more quickly and effortlessly than the current PCR-based tests. As the virus is capable of being transmitted via droplets and airborne routes, the fact that social practices of COVID-19 prevention are of great importance, should not be ignored; more than the effect of temperature or solar radiation on viral transmission.
Accordingly, based on the share of those mentioned topics, airborne and droplet transmission, and their association with air pollution has drawn more attention. This may be due to humans living in polluted and concentrated urban environments, which has favored both exposures to the virus-laden particles and air pollution. The COVID-19 pandemic effect on the environment has been explored more than the effect of the environment on COVID-19. This has to be due to still unknown nature of the virus and the fact that we are still learning its behavior.
Collectively, at least, the current pandemic has thought us how to contain future epidemics and hopefully, avoid another pandemic. The post-pandemic era can be a place where social hygiene is more practiced, where crisis management may be handled more efficiently, and where technology plays a more salient role in human life.
Author statement
Nayereh Rezaie Rahimi: Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization. Reza Fouladi-Fard: Conceptualization, Methodology, Validation, Investigation, Resources, Writing – original draft, Writing – review & editing, Supervision, Project administration. Rahim Aali: Investigation, Writing – original draft, Writing – review & editing. Ali Shahryari: Investigation, Writing – original draft, Writing – review & editing. Mostafa Rezaali: Validation, Investigation, Writing – original draft, Writing – review & editing. Yadollah Ghafouri: Writing – original draft, Writing – review & editing. Mohammad Rezvani Ghalhari: Investigation, Writing – original draft, Writing – review & editing. Mahdi Asadi Ghalhari: Investigation, Writing – review & editing. Babak Farzinnia: Investigation, Writing – review & editing. Maria Fiore: Formal analysis, Investigation, Supervision. Oliveri Conti Gea: Formal analysis, Investigation, Supervision, Writing – original draft,
Funding
None of the authors received any funding or financial support for this study.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The current review study has been approved by the Qom University of Medical Sciences (approval ID: IR.MUQ.REC.1399.224). The authors would like to appreciate all healthcare workers involved in treating COVID-19 patients around the world.
Appendix.
Table 3.
Quality assessment checklist of quality assessment results.
| Questions | YES | NO |
|---|---|---|
| 1. Is the study population clearly described? | ||
| 2. Are competing alternatives clearly described? | ||
| 3. Is a well-defined research question? | ||
| 4. Is the study design appropriate to the stated objective? | ||
| 5. Is the time chosen appropriate? | ||
| 6. Is the actual perspective chosen appropriate? | ||
| 7. Are all important variables, whose are uncertain, appropriately subjected to sensitivity analysis? | ||
| 8. Do the conclusions follow from the data reported? | ||
| 9. Does the study discuss the generalizability of the results to other settings and patient groups? | ||
| 10. Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | ||
| 11. Are ethical and distributional issues discussed appropriately? |
Table 4.
Quality assessment of articles
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Faridi et al. (2020a) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Jiang et al. (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Santarpia et al. (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Guo et al. (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Morawska and Cao (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Elias and Bar-Yam (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | N | 8 |
| Liu et al. (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | N | Y | 8 |
| Lednicky et al. (2020a) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | N | 8 |
| Wang et al. (2020a) | Y | N | Y | N | Y | Y | Y | Y | N | Y | Y | 8 |
| Travaglio et al. (2020b) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Yongjian et al. (2020) | Y | N | Y | N | Y | Y | Y | Y | N | Y | Y | 8 |
| Asna-Ashary et al. (2020) | Y | N | Y | N | Y | Y | Y | Y | N | Y | N | 7 |
| (Faridi et al.) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | N | 8 |
| Tosepu et al. (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Wang et al. (2020f) | Y | N | Y | N | Y | Y | Y | Y | N | Y | N | 7 |
| Adhikari and Yin (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | N | Y | 8 |
| Wang et al. (2020e) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Giani et al. (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | N | Y | 8 |
| Roy and Kar (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Xu et al. (2020b) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Zhao et al. (2020a) | Y | N | Y | Y | Y | Y | Y | Y | N | N | Y | 8 |
| Lodder and de Roda Husman (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Mao et al. (2020) | Y | N | Y | N | Y | Y | Y | Y | N | Y | Y | 8 |
| Wu et al. (2020b) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Ahmed et al. (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Briz-Redón and Serrano-Aroca (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | N | 8 |
| Sarkodie and Owusu (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Chen et al. (2020) | Y | N | Y | N | Y | Y | Y | Y | N | Y | N | 7 |
| Nakada and Urban (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Tang et al. (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | N | 8 |
| Sfîcă et al. (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | N | 8 |
| Rosario et al. (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Xing et al. (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | N | Y | 8 |
| Cadotte (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Zhu and Xie (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Zhao et al. (2020b) | Y | N | Y | Y | Y | Y | Y | Y | N | N | Y | 8 |
| (Sannino et al.) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| (Filonchyk et al.) | Y | N | Y | Y | Y | Y | Y | Y | N | N | Y | 8 |
| Xu et al. (2020a) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Bedi et al. (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Naeger and Murphy (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | N | Y | 8 |
| Yunus et al. (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Patel et al. (2020) | Y | N | Y | N | Y | Y | Y | Y | N | Y | Y | 8 |
| Zambrano-Monserrate et al. (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 |
| Qi et al. (2020b) | Y | N | Y | Y | N | Y | Y | N | N | Y | Y | 7 |
| Klemeš et al. (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | N | 8 |
| Kulkarni and Anantharama (2020) | Y | N | Y | Y | Y | Y | Y | Y | N | Y | N | 8 |
Table 5.
The inclusion and exclusion criteria used for preprocessing studies
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
| Cohort, case-control, or cross-sectional studies, ecologic studies | Review and meta-analyses articles; proceeding articles; and policy articles, abstract, letters, short communications, and chapter in books |
| case-crossover, or time-series studies, WHO, and World Bank report | |
| examining the impacts of COVID-19 on environment |
|
Humidity and temperature Metrological factors Wastewater Water Fomites Solid Waste | |
| COVID-19 Pandemic and Secondary Effects on Environment | |
| |
| Articles published from the late December 2019 to November 2020 | |
|
Table 6.
The summary of some reviewed studies
| # | Journal | Date | Study Design (RCT,prospective, retrospective, etc.) | Sample Size | Country | Province | Environmental Factor (air, water …) | Main Finding | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Science of the Total Environment | March-2020 | Cross Sectional | 44 | Iran | Tehran | Air | SARS-CoV-2 is not airborne | Faridi et al. (2020a) |
| 2 | Med Rxiv | _ | Cross Sectional | 26 | _ | _ | Air | no detection of the virus in any of air samples were observed | Jiang et al. (2020) |
| 3 | Med Rxiv | _ | Cross Sectional | 163 | USA | Nebraska | Air | SARS-CoV-2 have airborne transmission potential | Santarpia et al. (2020) |
| 4 | Emerging Infectious Diseases | March-2020 | Cross Sectional | 39 | China | Wuhan | Air | Positive for SARS-CoV-2 virus infection at 4 m away from infected patients | Guo et al. (2020) |
| 5 | New England Complex Systems Institute | _ | Cross Sectional | _ | _ | _ | Air | some promising study results indicate the viral shedding of the virus can be reduced by air filtration | Elias and Bar-Yam (2020) |
| 6 | Nature | February-2020 | Cross Sectional | 35 | China | Wuhan | Air | re-suspension of virus-laden aerosol from surfaces can be one of the causes of airborne spread of SARS-CoV-2 | Liu et al. (2020) |
| 7 | medRxiv | July −2020 | Pro-spective | _ | USA | _ | Air | demonstrated of viable virus isolated from air samples collected 2–4.8m away from the patients should be included | Lednicky et al. (2020a) |
| 8 | MedRxiv | January-2020 | Cross Sectional | 50 | China | Shanghai | Air | a positive correlation between air pollution and the COVID-19 mortality rate | Wang et al. (2020a) |
| 9 | MedRxiv | February-2020 | Cross Sectional | 200 | England | _ | Air | positive correlation between air pollution and the COVID-19 mortality rate | Travaglio et al. (2020b) |
| 10 | Science of the Total Environment | January-2020 | Cross Sectional | 120 | China | _ | Air | There is a significant relationship between air pollution and COVID-19 infection | Yongjian et al. (2020) |
| 11 | Econstor | February-2020 | Cross Sectional | 682 | Iran | _ | Air | the COVID-19 outbreak decreased air pollution majorly due to the decrease in transportation and financial activities | Asna-Ashary et al. (2020) |
| 12 | Aerosol and Air Quality Research | February-2020 | Cross Sectional | 22 | Iran | Tehran | Air | signifies that the pandemic has led to higher outdoor particulate matter air pollution owing to the use of more personal transportation despite having more rainfall compared to the same time last year | Faridi et al. (2020b) |
| 13 | Science of the Total Environment | January-2020 | Cross Sectional | _ | Indonesia | Jakarta | Air | a negative relationship between temperature and COVID-19 transmissibility | Tosepu et al. (2020) |
| 14 | Available at SSRN 3551767 | January-2020 | Cross Sectional | _ | China | Wuhan | Temperature and humidity | a decreased transmissibility of COVID-19 in higher outdoor temperatures and humidity | Wang et al. (2020f) |
| 15 | MedRxiv | March-2020 | Cross Sectional | 15 | India | _ | Temperature and humidity | a decreased transmissibility of COVID-19 in higher outdoor temperatures and humidity | Roy and Kar (2020) |
| 16 | SSRN | May-2020 | Pro-spective | 3739 | _ | _ | Temperature and humidity | Warmer temperature and moderate outdoor ultraviolet exposure may offer a modest reduction in transmission | Xu et al. (2020b) |
| 17 | Building and Environment | June-2020 | Pro-spective | _ | China | _ | Air | The indoor air purifiers should be used as a supplementary | Zhao et al. (2020a) |
| 18 | The Lancet Gastroenterology & Hepatology | February-2020 | Cross Sectional | 232 | Netherlands | Amsterdam | Wastewater | introduced SARS-CoV-2 in the wastewater collection network as a potential health risk | Lodder and de Roda Husman (2020) |
| 19 | Environmental science and technology | February-2020 | Pro-spective | 16 | China | Shanxi | Waste water | The paper-based device has the potential to be used as a small, portable device to detect SARS-CoV-2 in wastewater on site and to track virus carriers in the community. | Mao et al. (2020) |
| 20 | The Lancet Gastroenterology & Hepatology | January-2020 | Pro-spective | 98 | China | Zhuhai | Wastewater | The evidence presented thus far support the idea that viable SARS-CoV-2 can be detectable in raw wastewater | Wu et al. (2020b) |
| 21 | Science of the Total Environment | March-2020 | Cross sectional | _ | Australia | A catchment | Wastewater | The presence of SARS-CoV-2 was con-firmed by sequencing. | Ahmed et al. (2020) |
| 22 | Journal of Microbiology, Immunology and Infection | January-2020 | Cross Sectional | 3 | China | Shandong | Wastewater | dynamic changes of SARS-CoV-2 RNA in the fecal specimens of the children suffering from the novel coronavirus | Xing et al. (2020) |
| 23 | EarthArXiv | February-2020 | Pro-spective | _ | Thailand.India.Chinese Special Administrative Region.Indonesia.South Africa.Japan. United Kingdom.USA.Mexico.Italy.Brazil.Bosnia and Herzegovina.South Korea.China.Israel.Canada.China | Bangkok.Delhi.Hong Kong.Jakarta.Johannesburg.Kyoto.London.Los Angeles.Mexico City.Milano.Sao Paulo.Sarajevo.Seoul.Shanghai.Tel Aviv.Toronto.Wuhan | Air | Declines in air pollution in response to activity changes during the quarantine period | Cadotte (2020) |
| 24 | Science of The Total Environment | January-2020 | Pro-spective | 122 | China | _ | Temperature and humidity | When mean temperature was below 3 °C, each 1 °C rise was associated with a 4.861% increase in the daily number of COVID-19 confirmed cases. . | Zhu and Xie (2020) |
| 25 | Science of the Total Environment | December-2019 | Pro-spective | 31 | China | All province | Temperature and humidity | Temperature and humidity showed negative associations with COVID-19. | Qi et al. (2020b) |
| 26 | Renewable and Sustainable Energy Reviews | April-2020 | Pro-spective | _ | _ | _ | Solid waste | Sudden surge in the volume of plastic waste | Klemeš et al. (2020) |
| 27 | Science of The Total Environment | July-2020 | Pro-spective | _ | India | Karnataka | Solid waste | Medical and household waste generated affects municipal waste management | Kulkarni and Anantharama (2020) |
References
- Aali R., Baragh S., Asgari E., Fard R.F., Izanloo H., Hosseinpoor S., Olia J.B.H., Naseri R., Rabori M.M. Tracking of chloramphenicol, erythromycin, and sulfamethoxazole antibiotic-resistant bacteria from untreated wastewater effluents to receiving river. Environmental Health Engineering and Management Journal. 2019;6(2):89–96. [Google Scholar]
- Aali R., Nikaeen M., Khanahmad H., Hejazi Z., Kazemi M., Hassanzadeh A. Occurrence of tetracycline resistant bacteria and resistance gene (fetW) in hospital and municipal wastewaters. Fresenius Environ. Bull. 2014;23:2560–2566. [Google Scholar]
- Adhikari A., Yin J. Short-term effects of ambient ozone, PM2. 5, and meteorological factors on COVID-19 confirmed cases and deaths in Queens, New York. Int. J. Environ. Res. Publ. Health. 2020;17:4047. doi: 10.3390/ijerph17114047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshari R. Indoor air quality and severity of COVID-19: where communicable and non-communicable preventive measures meet. Asia Pacific Journal of Medical Toxicology. 2020;9:1–2. [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J. Science of The Total Environment; 2020. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community; p. 138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqahtani J.S., Oyelade T., Aldhahir A.M., Alghamdi S.M., Almehmadi M., Alqahtani A.S., Quaderi S., Mandal S., Hurst J.R. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PloS One. 2020;15 doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asna-Ashary M., Farzanegan M.R., Feizi M., Sadati S.M.… . Philipps-Universität Marburg, Faculty of Business Administration and; 2020. COVID-19 Outbreak and Air Pollution in Iran: A Panel VAR Analysis. [Google Scholar]
- Bahl P., Doolan C., De Silva C., Chughtai A.A., Bourouiba L., Macintyre C.R. Airborne or droplet precautions for health workers treating COVID-19? J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi J.S., Dhaka P., Vijay D., Aulakh R.S., Gill J.P.S. Assessment of air quality changes in the four metropolitan cities of India during COVID-19 pandemic lockdown. Aerosol and Air Quality Research. 2020;20 [Google Scholar]
- Bei E., Shu Y., Li S., Liao X., Wang J., Zhang X., Chen C., Krasner S. Occurrence of nitrosamines and their precursors in drinking water systems around mainland China. Water Res. 2016;98:168–175. doi: 10.1016/j.watres.2016.04.013. [DOI] [PubMed] [Google Scholar]
- Biktasheva I.V. Science of the Total Environment; Forthcoming: 2020. Role of a Habitat's Air Humidity in COVID-19 Mortality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth T.F., Kournikakis B., Bastien N., Ho J., Kobasa D., Stadnyk L., Li Y., Spence M., Paton S., Henry B. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J. Infect. Dis. 2005;191:1472–1477. doi: 10.1086/429634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briz-Redón Á., Serrano-Aroca Á. Progress in Physical Geography. Earth and Environment; 2020. The effect of climate on the spread of the COVID-19 pandemic: a review of findings, and statistical and modelling techniques. [Google Scholar]
- Cadotte M. 2020. Early Evidence that COVID-19 Government Policies Reduce Urban Air Pollution. [Google Scholar]
- Cai H. Sex difference and smoking predisposition in patients with COVID-19. The Lancet Respiratory Medicine. 2020;8 doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova L., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43:1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . Center for Disease Control and Prevention; 2020. Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with Coronavirus Disease (COVID-19) [Google Scholar]
- CDC Scientific brief: SARS-CoV-2 and potential airborne transmission. 2020. https://www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-sars-cov-2.html [Online]. Available:
- Chan J.F.-W., Kok K.-H., Zhu Z., Chu H., To K.K.-W., Yuan S., Yuen K.-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microb. Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Liang H., Yuan X., Hu Y., Xu M., Zhao Y., Zhang B., Tian F., Zhu X. MedRxiv; 2020. Roles of meteorological conditions in COVID-19 transmission on a worldwide scale. [Google Scholar]
- Chowdhury A., Kabir K.A., Tanimoto J. 2020. How Quarantine and Social Distancing Policy Can Suppress the Outbreak of Novel Coronavirus in Developing or under Poverty Level Countries: a Mathematical and Statistical Analysis. [Google Scholar]
- Contini D., Costabile F. Multidisciplinary Digital Publishing Institute; 2020. Does Air Pollution Influence COVID-19 Outbreaks? [Google Scholar]
- Desai A.N., Aronoff D.M. Food safety and COVID-19. J. Am. Med. Assoc. 2020;323(19):1982. doi: 10.1001/jama.2020.5877. [DOI] [PubMed] [Google Scholar]
- Ding Z., Qian H., Xu B., Huang Y., Miao T., Yen H.-L., Xiao S., Cui L., Wu X., Shao W. medRxiv; 2020. Toilets Dominate Environmental Detection of SARS-CoV-2 Virus in a Hospital. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J.H.X.-P. 2003. Stability of SARS Coronavirus in Human Specimens and Environment and its Sensitivity to Heating and UV Irradiation. [PubMed] [Google Scholar]
- Dutheil F., Baker J.S., Navel V. Barking; Essex: 2020. COVID-19 as a Factor Influencing Air Pollution? Environmental Pollution. 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias B., Bar-Yam Y. 2020. Could air filtration reduce COVID-19 severity and spread? [Google Scholar]
- Faridi S., Niazi S., Sadeghi K., Naddafi K., Yavarian J., Shamsipour M., Jandaghi N.Z.S., Sadeghniiat K., Nabizadeh R., Yunesian M. Science of the Total Environment; 2020. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran; p. 138401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faridi S., Yousefian F., Niazi S., Ghalhari M.R., Hassanvand M.S., Naddafi K. Vol. 20. Aerosol and Air Quality Research; 2020. Impact of SARS-CoV-2 on ambient air particulate matter in tehran. [Google Scholar]
- Faunt J., Wilkinson T., Aplin P., Henschke P., Webb M., Penhall R. The effete in the heat: heat‐related hospital presentations during a ten day heat wave. Aust. N. Z. J. Med. 1995;25:117–121. doi: 10.1111/j.1445-5994.1995.tb02822.x. [DOI] [PubMed] [Google Scholar]
- Fouladi Fard R., Aali R. Airborne antibiotic resistant bacteria: hospital indoor air pollution and the challenge of nosocomial infection. Journal of Environmental Health and Sustainable Development. 2019;4:859–861. [Google Scholar]
- Ge Z.-Y., Yang L.-M., Xia J.-J., Fu X.-H., Zhang Y.-Z. Journal of Zhejiang University-SCIENCE B; 2020. Possible aerosol transmission of COVID-19 and special precautions in dentistry; pp. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giani P., Castruccio S., Anav A., Howard D., Hu W., Crippa P. Short-term and long-term health impacts of air pollution reductions from COVID-19 lockdowns in China and Europe: a modelling study. The Lancet Planetary Health. 2020;4:e474–e482. doi: 10.1016/S2542-5196(20)30224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food and Environmental Virology. 2009;1:10. [Google Scholar]
- Guo Z.-D., Wang Z.-Y., Zhang S.-F., Li X., Li L., Li C., Cui Y., Fu R.-B., Dong Y.-Z., Chi X.-Y. vol. 26. Emerging infectious diseases; 2020. (Aerosol and Surface Distribution of Severe Acute Respiratory Syndrome Coronavirus 2 in Hospital Wards, Wuhan, China, 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu Y., Maejima M., Nakajima M., Mochizuki H., Omata M. Environmental cleaning is effective for the eradication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in contaminated hospital rooms: a patient from the Diamond Princess cruise ship. Infect. Contr. Hosp. Epidemiol. 2020:1–8. doi: 10.1017/ice.2020.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick M.F., Chen T.C., Lu Z., Sauter E. Vitamin D and skin physiology: AD‐lightful story. J. Bone Miner. Res. 2007;22:V28–V33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- Holshue M.L., Debolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain A., Rana J., Benzadid S., Ahsan B.G.U. 2019. COVID-19 and Bangladesh. [Google Scholar]
- Hosseini M.R., Fouladi-Fard R., Aali R. Indoor and Built Environment; 2020. COVID-19 Pandemic and Sick Building Syndrome. 1420326X20935644. [Google Scholar]
- Hung L.S. The SARS epidemic in Hong Kong: what lessons have we learned? J. R. Soc. Med. 2003;96:374–378. doi: 10.1258/jrsm.96.8.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilie P.C., Stefanescu S., Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020:1–4. doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed A. 2020. Policy Review Economic Impact of Coronavirus and Revival Measures: Way Forward for Pakistan. [Google Scholar]
- Jia S., Zhang X. Elsevier; 2020. Biological HRPs in Wastewater. High-Risk Pollutants In Wastewater. [Google Scholar]
- Jiang Y., Wang H., Chen Y., He J., Chen L., Liu Y., Hu X., Li A., Liu S., Zhang P. medRxiv; 2020. Clinical Data on Hospital Environmental Hygiene Monitoring and Medical Staff Protection during the Coronavirus Disease 2019 Outbreak. [Google Scholar]
- Kalina M., Tilley E. This is our next problem": cleaning up from the COVID-19 response. Waste Manag. 2020;108:202–205. doi: 10.1016/j.wasman.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr J.R. Defining and assessing ecological integrity: beyond water quality. Environ. Toxicol. Chem.: Int. J. 1993;12:1521–1531. [Google Scholar]
- Khan S., Siddique R., Ali A., Xue M., Nabi G. Novel coronavirus, poor quarantine, and the risk of pandemic. J. Hosp. Infect. 2020;104:449–450. doi: 10.1016/j.jhin.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020:139076. doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemeš J.J., Van Fan Y., Tan R.R., Jiang P. Minimising the present and future plastic waste, energy and environmental footprints related to COVID-19. Renew. Sustain. Energy Rev. 2020;127:109883. doi: 10.1016/j.rser.2020.109883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni B.N., Anantharama V. Science of The Total Environment; 2020. Repercussions of COVID-19 pandemic on municipal solid waste management: challenges and opportunities; p. 140693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai T.H., Tang E.W., Fung K.S., Li K.K. Reply to “Does hand hygiene reduce SARS-CoV-2 transmission?”. Graefes Arch. Clin. Exp. Ophthalmol. 2020;1 doi: 10.1007/s00417-020-04653-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau H., Khosrawipour V., Kocbach P., Mikolajczyk A., Ichii H., Schubert J., Bania J., Khosrawipour T. Internationally lost COVID-19 cases. J. Microbiol. Immunol. Infect. 2020;53(3):454–458. doi: 10.1016/j.jmii.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lednicky J.A., Lauzardo M., Fan Z.H., Jutla A., Tilly T.B., Gangwar M., Usmani M., Shankar S.N., Mohamed K., Eiguren-Fernandez A. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int. J. Infect. Dis. 2020;100:476–482. doi: 10.1016/j.ijid.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lednicky J.A., Shankar S.N., Elbadry M.A., Gibson J.C., Alam M.M., Stephenson C.J., Eiguren-Fernandez A., Morris J.G., Mavian C.N., Salemi M. Vol. 20. Aerosol and Air Quality Research; 2020. Collection of SARS-CoV-2 virus from the air of a clinic within a university student health care center and analyses of the viral genomic sequence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. Is the coronavirus airborne? Experts can't agree. Nature. 2020;580:175. doi: 10.1038/d41586-020-00974-w. [DOI] [PubMed] [Google Scholar]
- Liu J., Zhang X. Comparative toxicity of new halophenolic DBPs in chlorinated saline wastewater effluents against a marine alga: halophenolic DBPs are generally more toxic than haloaliphatic ones. Water Res. 2014;65:64–72. doi: 10.1016/j.watres.2014.07.024. [DOI] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Lodder W., de Roda Husman A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. The Lancet Gastroenterology & Hepatology. 2020;5(6):533–534. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Stratton C.W., Tang Y.W. 2019. Outbreak of Pneumonia of Unknown Etiology in Wuhan China: the Mystery and the Miracle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenbach J.P., Borst V., Schols J.A. Heat-related mortality among nursing-home patients. Lancet. 1997;349:1297–1298. doi: 10.1016/S0140-6736(05)62510-X. [DOI] [PubMed] [Google Scholar]
- Mao K., Zhang H., yang Z. ACS Publications; 2020. Can a paper-based device trace COVID-19 sources with wastewater-based epidemiology? [DOI] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. medRxiv; 2020. Presence of SARS-Coronavirus-2 in Sewage. [DOI] [PubMed] [Google Scholar]
- Min-Allah N., Alrashed S. Smart campus—a sketch. Sustainable Cities and Society. 2020;59:102231. doi: 10.1016/j.scs.2020.102231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirhoseini S.H., Nikaeen M., Shamsizadeh Z., Aali R. Prevalence and molecular identification of antibiotic resistant airborne bacteria at intensive care units. Koomesh. 2018;20 [Google Scholar]
- Mirzaei R., Mohammadzadeh R., Mahdavi F., Badrzadeh F., Kazemi S., Ebrahimi M., Soltani F., kazemi S., Jeda A.S., Darvishmotevalli M., Yousefimashouf R., Keyvani H., Karampoor S. Overview of the current promising approaches for the development of an effective severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine. Int. Immunopharm. 2020;88:106928. doi: 10.1016/j.intimp.2020.106928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol M.P.G., Caldas S. Can the human coronavirus epidemic also spread through solid waste? Waste Manag. Res. 2020;38:485–486. doi: 10.1177/0734242X20918312. [DOI] [PubMed] [Google Scholar]
- Morawska L., Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ. Int. 2020;139:105730. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Johnson G., Ristovski Z., Hargreaves M., Mengersen K., Corbett S., Chao C.Y.H., Li Y., Katoshevski D. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J. Aerosol Sci. 2009;40:256–269. doi: 10.1016/j.jaerosci.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad S., Long X., Salman M. COVID-19 pandemic and environmental pollution: a blessing in disguise? Sci. Total Environ. 2020:138820. doi: 10.1016/j.scitotenv.2020.138820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naddeo V., Liu H. Editorial Perspectives: 2019 novel coronavirus (SARS-CoV-2): what is its fate in urban water cycle and how can the water research community respond? Environ. Sci.: Water Research & Technology. 2020;6:1213–1216. doi: 10.1039/D0EW90015J. [DOI] [Google Scholar]
- Naeger A.R., Murphy K. Impact of COVID-19 containment measures on air pollution in California. Aerosol and Air Quality Research. 2020;20 [Google Scholar]
- Nakada L.Y.K., Urban R.C. Environmental Science and Pollution Research; 2020. COVID-19 pandemic: environmental and social factors influencing the spread of SARS-CoV-2 in São Paulo, Brazil. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazari Harmooshi N., Shirbandi K., Rahim F. SSRN; 2020. Environmental concern regarding the effect of humidity and temperature on SARS-COV-2 (COVID-19) survival: fact or fiction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez-Delgado A. Science of The Total Environment; 2020. What do we know about the SARS-CoV-2 coronavirus in the environment? p. 138647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nzeadibe T.C., Ejike-Alieji A.U. Local Environment; 2020. Solid Waste Management during Covid-19 Pandemic: Policy Gaps and Prospects for Inclusive Waste Governance in Nigeria; pp. 1–9. [Google Scholar]
- Nzediegwu C., Chang S.X. Improper solid waste management increases potential for COVID-19 spread in developing countries. Resour. Conserv. Recycl. 2020;161:104947. doi: 10.1016/j.resconrec.2020.104947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter J., Donskey C., Yezli S., Douthwaite S., Goldenberg S., Weber D. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J. Hosp. Infect. 2016;92:235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Zhang D., Yang P., Poon L.L., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P.P., Mondal S., Ghosh K.G. Some respite for India's dirtiest river? Examining the Yamuna's water quality at Delhi during the COVID-19 lockdown period. Sci. Total Environ. 2020;744:140851. doi: 10.1016/j.scitotenv.2020.140851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeri N.C., Shrestha N., Rahman M.S., Zaki R., Tan Z., Bibi S., Baghbanzadeh M., Aghamohammadi N., Zhang W., Haque U. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the NEWEST and biggest global health threats: what lessons have we learned? Int. J. Epidemiol. 2020;49(3):717–726. doi: 10.1093/ije/dyaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata D.N., Rodrigues W., Bermejo P.H. Temperature significantly changes COVID-19 transmission in (sub)tropical cities of Brazil. Sci. Total Environ. 2020;729:138862. doi: 10.1016/j.scitotenv.2020.138862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H., Xiao S., Shi R., Ward M.P., Chen Y., Tu W., Su Q., Wang W., Wang X., Zhang Z. COVID-19 transmission in Mainland China is associated with temperature and humidity: a time-series analysis. Sci. Total Environ. 2020;728:138778. doi: 10.1016/j.scitotenv.2020.138778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H., Xiao S., Shi R., Ward M.P., Chen Y., Tu W., Su Q., Wang W., Wang X., Zhang Z. COVID-19 transmission in Mainland China is associated with temperature and humidity: a time-series analysis. Sci. Total Environ. 2020:138778. doi: 10.1016/j.scitotenv.2020.138778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenau H., Cinatl J., Morgenstern B., Bauer G., Preiser W., Doerr H. Stability and inactivation of SARS coronavirus. Med. Microbiol. Immunol. 2005;194:1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj V.S., Mou H., Smits S.L., Dekkers D.H., Müller M.A., Dijkman R., Muth D., Demmers J.A., Zaki A., Fouchier R.A. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson T., Moore L., Castro Sanchez E., Charani E., Davies F., Satta G., Ellington M., Holmes A. 2020. COVID-19 and the Potential Long Term Impact on Antimicrobial Resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario D.K.A., Mutz Y.S., Bernardes P.C., Conte-Junior C.A. Relationship between COVID-19 and weather: case study in a tropical country. Int. J. Hyg Environ. Health. 2020;229:113587. doi: 10.1016/j.ijheh.2020.113587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A., Kar S. medRxiv; 2020. Nature of Transmission of Covid19 in India. [Google Scholar]
- Sagripanti J.L., Lytle C.D. Estimated inactivation of coronaviruses by solar radiation with special reference to COVID‐19. Photochem. Photobiol. 2020;96(4):731–737. doi: 10.1111/php.13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia J.L., Rivera D.N., Herrera V., Morwitzer M.J., Creager H., Santarpia G.W., Crown K.K., Brett-Major D., Schnaubelt E., Broadhurst M.J. 2020. Transmission Potential of SARS-CoV-2 in Viral Shedding Observed at the University of Nebraska Medical Center. medRxiv. [Google Scholar]
- Sarkodie S.A., Owusu P.A. Impact of meteorological factors on COVID-19 pandemic: evidence from top 20 countries with confirmed cases. Environ. Res. 2020;191:110101. doi: 10.1016/j.envres.2020.110101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak D.L., Von Gunten U. The chlorine dilemma. Science. 2011;331:42–43. doi: 10.1126/science.1196397. [DOI] [PubMed] [Google Scholar]
- Sfîcă L., Bulai M., Amihăesei V.-A., Ion C., Ștefan M. Weather conditions (with focus on UV radiation) associated with COVID-19 outbreak and worldwide climate-based prediction for future prevention. Aerosol and Air Quality Research. 2020;20:1862–1873. [Google Scholar]
- Shahbaz B., Norouzi M., Tabatabai H. Mechanism of action and application of virocids in health care-associated viral infections. Tehran Univ. Med. J. 2016;73:837–855. [Google Scholar]
- Sharma S., Zhang M., Anshika, Gao J., Zhang H., Kota S.H. Effect of restricted emissions during COVID-19 on air quality in India. Sci. Total Environ. 2020;728:138878. doi: 10.1016/j.scitotenv.2020.138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons F.J., Xagoraraki I. Release of infectious human enteric viruses by full-scale wastewater utilities. Water Res. 2011;45:3590–3598. doi: 10.1016/j.watres.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Sizun J., Yu M., Talbot P. Survival of human coronaviruses 229E and OC43 in suspension and after drying onsurfaces: a possible source ofhospital-acquired infections. J. Hosp. Infect. 2000;46:55–60. doi: 10.1053/jhin.2000.0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamal G., Bhaskar B. 2020. Molecular Docking Study of Receptor Binding Domain of SARS-CoV-2 Spike Glycoprotein with Saikosaponin, a Triterpenoid Natural Product. [Google Scholar]
- Tang L., Liu M., Ren B., Wu Z., Yu X., Peng C., Tian J. Sunlight ultraviolet radiation dose is negatively correlated with the percent positive of SARS-CoV-2 and four other common human coronaviruses in the US. Sci. Total Environ. 2020;751:141816. doi: 10.1016/j.scitotenv.2020.141816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosepu R., Gunawan J., Effendy D.S., Lestari H., Bahar H., Asfian P. Science of The Total Environment; 2020. Correlation between weather and covid-19 pandemic in jakarta, Indonesia; p. 138436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travaglio M., Popovic R., Yu Y., Leal N., Martins L.M. medRxiv; 2020. Links between Air Pollution and COVID-19 in England. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travaglio M., Yu Y., Popovic R., Leal N.S., Martins L.M. medRxiv; 2020. Links between Air Pollution and COVID-19 in England. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doremalen N., Bushmaker T., Munster V. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental Conditions. Euro Surveill. 2013;18:20590. doi: 10.2807/1560-7917.es2013.18.38.20590. [DOI] [PubMed] [Google Scholar]
- Wang B., Liu J., Fu S., Xu X., Li L., Ma Y., Zhou J., Yao J., Liu X., Zhang X. medRxiv; 2020. An Effect Assessment of Airborne Particulate Matter Pollution on COVID-19: A Multi-City Study in China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.J., Ng C.Y., Brook R.H. Response to COVID-19 in Taiwan: big data analytics, new technology, and proactive testing. Jama. 2020;323(14):1341–1342. doi: 10.1001/jama.2020.3151. [DOI] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Feng H., Zhang S., Ni Z., Ni L., Chen Y., Zhuo L., Zhong Z., Qu T. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the Coronavirus Disease 2019 outbreak in a Chinese hospital. Int. J. Infect. Dis. 2020;93:103–106. doi: 10.1016/j.ijid.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Shen J., Ye D., Yan X., Zhang Y., Yang W., Li X., Wang J., Zhang L., Pan L. Disinfection technology of hospital wastes and wastewater: suggestions for disinfection strategy during coronavirus Disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2020;262:114665. doi: 10.1016/j.envpol.2020.114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Tang K., Feng K., Lv W. 2020. High temperature and high humidity reduce the transmission of COVID-19. Available at: SSRN 3551767. [Google Scholar]
- Wang X.-W., Li J.-S., Jin M., Zhen B., Kong Q.-X., Song N., Xiao W.-J., Yin J., Wei W., Wang G.-J. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol Methods. 2005;126:171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Li J., Guo T., Zhen B., Kong Q., Yi B., Li Z., Song N., Jin M., Xiao W. Concentration and detection of SARS coronavirus in sewage from xiao tang Shan hospital and the 309th hospital of the Chinese people's liberation army. Water Sci. Technol. 2005;52:213–221. [PubMed] [Google Scholar]
- Warnes S.L., Little Z.R., Keevil C.W. Human coronavirus 229E remains infectious on common touch surface materials. mBio. 2015;6 doi: 10.1128/mBio.01697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; 2020. Considerations for Quarantine of Individuals in the Context of Containment for Coronavirus Disease (COVID-19): Interim Guidance, 19 March 2020. [Google Scholar]
- WHO . World Health Organization; Geneva: 2020. Global Surveillance for Human Infection with Coronavirus Disease (COVID-19) Available via https://www. who. int/publications-detail/global-surveillance-for-human-infection-with-novel-coronavirus-(2019-ncov). Accessed, 27. [Google Scholar]
- WHO Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. 2020. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations [Online]. Available:
- WHO . World Health Organization; 2020. Water, Sanitation, Hygiene and Waste Management for COVID-19: Technical Brief, 03 March 2020. [Google Scholar]
- WHO WHO coronavirus disease (COVID-19) dashboard. 2020. https://covid19.who.int/ [Online]. Available:
- Wu X., Nethery R.C., Sabath B.M., Braun D., Dominici F. 2020. Exposure to Air Pollution and COVID-19 Mortality in the United States. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. The lancet Gastroenterology & hepatology. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Zhu Y. Association between ambient temperature and COVID-19 infection in 122 cities from China. Sci. Total Environ. 2020;724:138201. doi: 10.1016/j.scitotenv.2020.138201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y.-H., Ni W., Wu Q., Li W.-J., Li G.-J., Wang W.-D., Tong J.-N., Song X.-F., Wong G.W.-K., Xing Q.-S. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J. Microbiol. Immunol. Infect. 2020;53(3):473–480. doi: 10.1016/j.jmii.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Cui K., Young L.-H., Wang Y.-F., Hsieh Y.-K., Wan S., Zhang J. Air quality index, indicatory air pollutants and impact of COVID-19 event on the air quality near Central China. Aerosol and Air Quality Research. 2020;20 [Google Scholar]
- Xu R., Rahmandad H., Gupta M., Digennaro C., Ghaffarzadegan N., Amini H., Jalali M. 2020. Weather conditions and COVID-19 transmission: estimates and projections. Available at: SSRN 3593879. [Google Scholar]
- Yeganeh J., Mehrdadi N., Baghdadi M., Aali R. Effect of zero valent iron nanoparticles on removal of antibiotic resistant genes of heterotrophic bacteria in urban wastewater. Journal of Mazandaran University of Medical Sciences. 2018;28:28–44. [Google Scholar]
- Yen M.-Y., Schwartz J., Chen S.-Y., King C.-C., Yang G.-Y., Hsueh P.-R. Interrupting COVID-19 transmission by implementing enhanced traffic control bundling: implications for global prevention and control efforts. J. Microbiol. Immunol. Infect. 2020;53(3):377–380. doi: 10.1016/j.jmii.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? The Lancet Gastroenterology & Hepatology. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yongjian Z., Jingu X., Fengming H., Liqing C. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. Sci. Total Environ. 2020:138704. doi: 10.1016/j.scitotenv.2020.138704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Sun X., Solvang W.D., Zhao X. Reverse logistics network design for effective management of medical waste in epidemic outbreaks: insights from the coronavirus disease 2019 (COVID-19) outbreak in Wuhan (China) Int. J. Environ. Res. Publ. Health. 2020;17:1770. doi: 10.3390/ijerph17051770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunus A.P., Masago Y., Hijioka Y. COVID-19 and surface water quality: improved lake water quality during the lockdown. Sci. Total Environ. 2020;731:139012. doi: 10.1016/j.scitotenv.2020.139012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano-Monserrate M.A., Ruano M.A., Sanchez-Alcalde L. Indirect effects of COVID-19 on the environment. Sci. Total Environ. 2020;728:138813. doi: 10.1016/j.scitotenv.2020.138813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Ling H., Huang X., Li J., Li W., Yi C., Zhang T., Jiang Y., He Y., Deng S., Zhang X., Wang X., Liu Y., Li G., Qu J. Potential spreading risks and disinfection challenges of medical wastewater by the presence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of Fangcang Hospital. Sci. Total Environ. 2020;741:140445. doi: 10.1016/j.scitotenv.2020.140445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Tang W., Chen Y., Yin W. Disinfection threatens aquatic ecosystems. Science. 2020;368:146–147. doi: 10.1126/science.abb8905. [DOI] [PubMed] [Google Scholar]
- Zhao B., Liu Y., Chen C. Building and Environment; 2020. Air Purifiers: A Supplementary Measure to Remove Airborne SARS-CoV-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N., Wang G., Li G., Lang J., Zhang H. Air pollution episodes during the COVID-19 outbreak in the Beijing–Tianjin–Hebei region of China: an insight into the transport pathways and source distribution. Environ. Pollut. 2020;267:115617. doi: 10.1016/j.envpol.2020.115617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Wang D., Li Q., Yue Y., Tu W., Cao R. Impacts of weather on public transport ridership: results from mining data from different sources. Transport. Res. C Emerg. Technol. 2017;75:17–29. [Google Scholar]
- Zhu Y., Xie J. Science of the Total Environment; 2020. Association between ambient temperature and COVID-19 infection in 122 cities from China; p. 138201. [DOI] [PMC free article] [PubMed] [Google Scholar]