Abstract
C-reactive protein-to-albumin ratio (CAR) has been used as an indicator of prognosis in various diseases. Here, we intended to assess the CAR’s diagnostic power in early differentiation of hospitalized severe COVID-19 cases. In this retrospectively designed study, we evaluated 197 patients in total. They were divided into two groups based on their severity of COVID-19 as non-severe (n = 113) and severe (n = 84). The comparison of groups’ demographic data, comorbidities, clinical symptoms, and laboratory test results were done. Laboratory data of the patients within the first 24 h after admission to the hospital were evaluated. The calculation of receiver operating characteristic (ROC) curve was used to determine the diagnostic power of CAR in differentiating severity of COVID-19. Independent risk factors predictive of COVID-19 severity were determined by using logistic regression analysis. Although lymphocyte count levels were lower, severe COVID-19 patients had higher mean age, higher levels of neutrophil count, CRP, aspartate aminotransferase (AST), ferritin, and prothrombin time (P < 0.05). Compared with non-severe patients (median, 0.23 [IQR = 0.07–1.56]), patients with severe COVID-19 had higher CAR levels (median, 1.66 [IQR = 0.50–3.35]; P < 0.001). Age (OR = 1.046, P = 0.003), CAR (OR = 1.264, P = 0.037), and AST (OR = 1.029, P = 0.037) were independent risk factors for severe COVID-19 based on the multivariate logistic regression analysis. ROC curve analysis assigned 0.9 as the cut-off value for CAR for differentiation of severe COVID-19 (area under the curve = 0.718, 69.1% sensitivity, 70.8% specificity, P < 0.001). CAR is a useful marker in early differentiation of severity in patients hospitalized due to COVID-19 that have longer hospital stay and higher mortality.
Keywords: C-reactive protein to albumin ratio, SARS-CoV-2, COVID-19
1. Introduction
In December of 2019, a novel coronavirus disease (COVID-19) started its worldwide spread from Wuhan, China. On March 11, 2020, the World Health Organization (WHO) declared COVID-19 as a pandemic due to its alarming rate of spread and countries' inability to take action [1]. As of August 25, 2020, COVID-19 infected 23,518,843 individuals in virtually all parts of the world and caused 810,492 deaths [2].
COVID-19 is classified into four groups based on the clinical manifestations and disease severity as mild, moderate, severe, and critical [3]. Although most of the patients have mild and moderate cases, early diagnosis of severe and critical cases is important as it prolongs hospital stay and increases mortality rates [4], [5]. Therefore, researchers are putting more effort to detect serious and critical cases at an earlier stage. At this point, the problem faced by researchers is how to monitor this patient group early and actively.
Inflammation triggers the liver to synthesize numerous acute-phase reactants. One of such reactants is C-reactive protein (CRP), which can be used as biomarker in rheumatoid arthritis, cardiovascular disease and in the presence of infection [6], [7]. It has been reported that in severe COVID-19 cases, CRP levels may raise before any findings can be observed on CT and thus CRP can be used to detect severe cases at an early stage [8]. On the other hand, the cytokine storm induced in hospitalized COVID-19 cases may cause critical hypoalbuminemia, increase the risk of death, and low albumin levels at the admission stage can predict the course of the disease independently from other indicators [9], [10].
Prediction of severity of COVID-19 cases by using both the albumin and CRP have been documented as well as benefits of C-reactive protein to albumin ratio (CAR) as inflammation-related indicator of prognosis for diseases such as osteosarcoma [11]. Based on this information, we hypothesized that CAR can be used in early differentiation of the hospitalized severe COVID-19 cases.
2. Materials and methods
2.1. Subjects
This retrospectively designed study included COVID-19 patients hospitalized in a tertiary hospital between 18 March 2020 and 30 May 2020. During hospitalization, the cases were diagnosed as mild, moderate, severe, and critical based on WHO’s interim guidance [3]. We defined the mild and moderate subgroups as non-severe, severe and critical subgroups as severe. The non-severe group included symptomatic patients that had no indication of hypoxia or pneumonia as well as patients that had symptoms of moderate pneumonia such as dyspnea, cough and fever and oxygen saturation (SpO2) ≥ 90% on room air. Meanwhile, the severe group included patients that had at least one of the following: respiratory rate > 30/min, severe respiratory distress, SpO2 < 90% on room air, and arterial partial pressure of oxygen/fraction inspiratory O2 ≤ 300 mmHg in addition to symptoms of pneumonia such as dyspnea, cough and fever as well as patients that required mechanical ventilation due to respiratory failure. Only patients above the age of 18 years were included in the study. Although the data from 310 patients were available, 113 patients were excluded due to incomplete records, leaving a total of 197 patients.
The ethics committee of our institution approved our research (Resolution Number 2020/8–19, dated July 08, 2020). Guidelines of the declaration of Helsinki were followed throughout the study.
2.2. Laboratory assays and data collection
Patients’ demographic data, signs and symptoms, comorbidities, and laboratory data were reviewed and collected from the hospital’s electronic information system. Laboratory data of the patients within the first 24 h after admission to the hospital were evaluated.
Complete blood count analyses were performed in UniCel DxH 800 hematology analyzer (Beckman Coulter, Miami, FL, USA), while C-reactive protein (CRP), glucose, creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), direct bilirubin (DBil), total bilirubin (TBil), and ferritin analyses were performed in AU 5800 chemistry analyzer (Beckman Coulter, High Wycombe, UK). ADVIA Centaur XP immunoassay analyzer (Siemens Healthineers, Erlangen, Germany) was used for high sensitive troponin I (hs-TNI) analyses. CS 2500 automated coagulation analyzer (Sysmex Corporation, Kobe, Japan) was used for prothrombin time (PT) and activated partial prothrombin time (APTT) determination. COVID-19 diagnosis was done by real-time polymerase chain reaction (RT-PCR) (Bio-Speedy COVID-19 RT-qPCR kit, Bioeksen R&D Technologies Ltd, Istanbul, Turkey) of viral nucleic acids form throat swab samples.
2.3. Statistical analysis
Dividing the neutrophil count (NEU) by the lymphocyte count (LYM) provided the neutrophil-to-lymphocyte ratio (NLR), while dividing the LYM by the monocyte count gave the lymphocyte-to-monocyte ratio (LMR). Moreover, dividing the values for CRP by those of albumin gave the CAR.
Frequency (%) was used to express categorical variables. mean ± standard deviation was used to express normally distributed continuous variables, while median (interquartile range) was used to present non-normally distributed continuous variables. The comparison of differences between the groups was done by chi-square test (categorical variables), independent samples t-test (normally distributed continuous variables), and Mann-Whitney U test (non-normally distributed continuous variables). The associations between non-normally distributed variables, the correlation coefficients and their significance were calculated using Spearman test. During the multivariate analysis, the possible factors identified with univariate analysis (age, coronary artery disease, diabetes mellitus, NLR, CAR, glucose, AST, ferritin, and PT) were further entered into the logistic regression analysis to determine independent predictors of COVID-19 severity. While differentiating the severity of COVID-19, the determination of area under the curve (AUC) and cut-off values was done by using receiver operating characteristic (ROC) curve analysis. MedCalc 15.0 (MedCalc, Ostend, Belgium) and SPSS 25.0 (IBM Corp., NY, USA) software packages were used to perform all statistical analyzes. P value of less than 0.05 was considered statistically significant.
3. Results
This retrospectively designed study included 197 cases with 113 of them classified as having non-severe (mild, moderate) COVID-19 while 84 were classified as severe (severe, critical) based on the diagnosis during admission.
The median age of the non-severe COVID-19 group was significantly lower compared to the severe group (P < 0.001), but there was no significant difference in terms of gender distribution (P = 0.761) (Table 1 ). We also observed that incidence of comorbidities such as hypertension, coronary artery disease, and diabetes mellitus (P = 0.045, 0.002, and 0.001, respectively) was significantly higher in severe COVID-19 group. These three comorbidities were also the most frequent comorbidity parameters in total (44.2%, 16.2%, and 24.4%, respectively). At the time of admission, the most frequently observed clinical symptoms among all patients were cough, fever, and dyspnea (47.2%, 35.5%, and 28.4%, respectively). However, frequency of dyspnea, headache, and pharyngalgia were significantly different between the groups (P = 0.015, 0.037, 0.038, respectively). Severe COVID-19 group also had longer hospital stay and higher mortality as expected (P = 0.001 for both variables).
Table 1.
Baseline characteristics of COVID-19 patients.
| Variables | Total |
Non-Severe |
Severe |
P |
|---|---|---|---|---|
| n = 197 | n = 113 | n = 84 | ||
| Age, years (mean ± SD) | 54.0 ± 18.0 | 48.0 ± 16.6 | 62.1 ± 16.8 | <0.001 |
| Gender Female, n(%) | 89 (45.2) | 50 (44.2) | 39 (46.4) | 0.761 |
| Comorbidities | ||||
| Hypertension, n(%) | 87 (44.2) | 43 (38.1) | 44 (52.4) | 0.045 |
| Coronary artery disease, n(%) | 32 (16.2) | 10 (8.8) | 22 (26.2) | 0.002 |
| Diabetes mellitus, n(%) | 48 (24.4) | 17 (15.0) | 31 (36.9) | 0.001 |
| Hypothyroidism, n(%) | 11 (5.6) | 7 (6.2) | 4 (4.8) | 0.761 |
| Chronic renal failure, n(%) | 12 (6.1) | 7 (6.2) | 5 (6.0) | 1.000 |
| Malignancy, n(%) | 14 (7.1) | 5 (4.4) | 9 (10.7) | 0.156 |
| Chronic obstructive pulmonary disease, n(%) | 17 (8.6) | 6 (5.3) | 11 (13.1) | 0.095 |
| Asthma, n(%) | 10 (5.1) | 6 (5.3) | 4 (4.8) | 1.000 |
| Hepatitis B, n(%) | 3 (1.5) | 2 (1.8) | 1 (1.2) | 1.000 |
| Cirrhosis, n(%) | 3 (1.5) | 2 (1.8) | 1 (1.2) | 1.000 |
| Cerebrovascular disease, n(%) | 5 (2.5) | 2 (1.8) | 3 (3.6) | 0.653 |
| Clinical symptoms | ||||
| Cough, n(%) | 93 (47.2) | 50 (44.2) | 43 (48.8) | 0.334 |
| Fever, n(%) | 70 (35.5) | 42 (37.2) | 28 (33.3) | 0.578 |
| Dyspnea, n(%) | 56 (28.4) | 24 (21.2) | 32 (38.1) | 0.015 |
| Headache, n(%) | 18 (9.1) | 15 (13.3) | 3 (3.6) | 0.037 |
| Pharyngalgia, n(%) | 21 (10.7) | 17 (15.0) | 4 (4.8) | 0.038 |
| Fatigue, n(%) | 43 (21.8) | 29 (25.7) | 14 (16.7) | 0.181 |
| Anosmia/Hyposmia, n(%) | 4 (2.0) | 4 (3.5) | 0 (0) | 0.137 |
| Dysgeusia, n(%) | 4 (2.0) | 3 (2.7) | 1 (1.2) | 0.638 |
| Length of hospital stay, day (median, IQR) | 8 (5–11) | 6 (5–8) | 10 (8–15) | <0.001 |
| Death, n(%) | 8 (4.1) | 0 (0) | 8 (9.5) | <0.001 |
Abbr: n, number; IQR, interquartile range; SD, standard deviation.
Statistically significant P values are shown in bold.
Table 2 depicts the laboratory results from both groups. The severe COVID-19 group had significantly higher NEU, NLR, CRP, CAR, glucose, AST, ferritin, and PT values (P = 0.038, 0.003, <0.001, <0.001, 0.014, 0.047, 0.003, and 0.024, respectively), while its LYM values were significantly lower compared to the non-severe group (P = 0.012).
Table 2.
Laboratory results of the COVID-19 patients.
| Parameter | Total |
Non-Severe |
Severe |
P |
|---|---|---|---|---|
| n = 197 | n = 113 | n = 84 | ||
| WBC (x 109/L) | 7.40 (5.40–9.85) | 7.20 (5.30–8.90) | 7.50 (5.45–10.35) | 0.266 |
| NEU (x 109/L) | 4.60 (3.30–7.00) | 4.40 (3.10–6.40) | 5.10 (3.80–8.20) | 0.038 |
| LYM (x 109/L) | 1.50 (1.00–2.00) | 1.60 (1.10–2.10) | 1.20 (0.80–1.80) | 0.012 |
| MON (x 109/L) | 0.60 (0.40–0.90) | 0.60 (0.40–0.90) | 0.60 (0.50–0.88) | 0.556 |
| PLT ((x 109/L) | 216.0 (172.0–299.0) | 216.0 (175.0–296.0) | 219.0 (165.0–334.0) | 0.691 |
| NLR | 3.13 (1.92–6.62) | 2.53 (1.75–4.38) | 4.31 (2.13–8.34) | 0.003 |
| LMR | 2.40 (1.58–3.50) | 2.53 (1.70–3.67) | 2.23 (1.43–3.00) | 0.052 |
| CRP (mg/L) | 19.6 (5.78–89.53) | 9.5 (2.75–63.0) | 58.3 (16.3–99.7) | <0.001 |
| CAR | 0.53 (0.14–2.56) | 0.23 (0.07–1.56) | 1.66 (0.50–3.35) | <0.001 |
| Glucose (mmol/L) | 6.4 (5.6–8.4) | 6.2 (5.2–7.7) | 6.7 (6.1–8.6) | 0.014 |
| Creatinine (µmol/L) | 79.56 (70.72–106.08) | 79.56 (70.72–97.24) | 88.40 (70.72–114.92) | 0.093 |
| AST (U/L) | 26.0 (19.0–37.0) | 25.0 (18.0–35.0) | 28.0 (21.0–44.0) | 0.047 |
| ALT (U/L) | 21.0 (13.0–34.0) | 22.0 (12.0–36.0) | 20.0 (14.0–33.0) | 0.787 |
| TBil (µmol/L) | 9.23 (7.39–13.17) | 9.41 (7.52–13.42) | 9.06 (7.35–12.65) | 0.672 |
| DBil (µmol/L) | 1.80 (1.37–2.52) | 1.71 (1.20–2.27) | 1.88 (1.37–2.78) | 0.336 |
| Ferritin (µg/L) | 142.0 (54.0–294.7) | 84.7 (38.0–255.0) | 217.7 (101.1–433.5) | 0.003 |
| hs-TNI (ng/L) | 2.50 (0.02–8.34) | 2.50 (0.02–7.94) | 2.50 (0.02–9.82) | 0.849 |
| PT, second | 12.6 (11.9–13.7) | 12.4 (11.7–13.4) | 13.0 (12.1–13.8) | 0.024 |
| APTT, % | 25.1 (23.5–27.5) | 24.6 (23.6–26.7) | 26.2 (23.3–28.8) | 0.336 |
Abbr: WBC, white blood cell count; NEU, neutrophil count; LYM, lymphocyte count; MON, monocyte count; PLT, platelet count; NLR, neutrophil-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; CRP, C-reactive protein; CAR, C-reactive protein to albumin ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase; TBil, total bilirubin; DBil, direct bilirubin; hs-TNI, high sensitive troponin I; PT, prothrombin time; APTT, activated partial prothrombin time; n, number.
Statistically significant P values are shown in bold.
Correlation analysis in all subjects showed that age was negatively correlated with albumin (r = -0.419, P < 0.001). However, there was no correlation between age and both CRP and CAR (r = 0.096, P = 0.179 and r = 0.132, P = 0.064; respectively). Multivariate logistic regression analysis identified that age, CAR, and AST are independent risk factors that can forecast how severe the patients COVID-19 disease will progress (Table 3 ).
Table 3.
Regression analysis of risk factors for severe COVID-19.
| Parameter | B | SE | Wald | P | OR (95% CI) |
|---|---|---|---|---|---|
| Age | 0.045 | 0.015 | 8.939 | 0.003 | 1.046 (1.016–1.077) |
| CAR | 0.235 | 0.113 | 4.330 | 0.037 | 1.264 (1.014–1.577) |
| AST | 0.028 | 0.014 | 4.334 | 0.037 | 1.029 (1.002–1.057) |
Abbr: CAR, C-reactive protein to albumin ratio; AST, aspartate aminotransferase; OR, Odds ratio; CI, confidence interval.
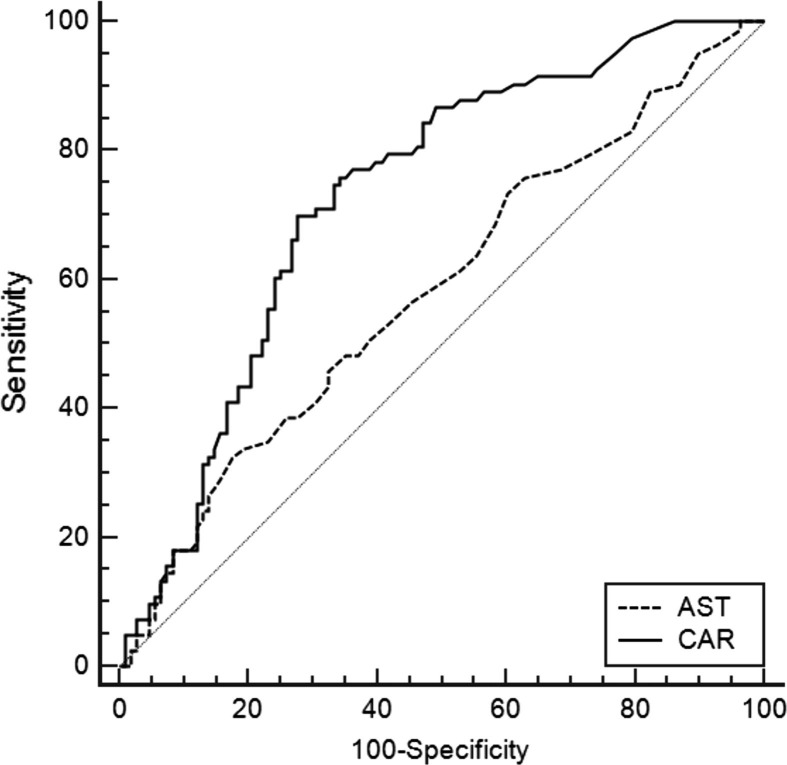
ROC curve analysis is shown in Fig. 1 . Based on the interpretation of AUC proposed by Hosmer and Lemeshow [12], AUC values indicated poor discrimination between our groups by AST (AUC = 0.584, 95% confidence interval: 0.511-0.655, 32.5% sensitivity, 82.4% specificity, P = 0.044) and acceptable discrimination by CAR. ROC curve analysis assigned 0.9 as the cut-off value for CAR for discrimination severe COVID-19 with an AUC of 0.718 (95% confidence interval: 0.649–0.779, 69.1% sensitivity, 70.8% specificity, P < 0.001). Comparison of efficacy of CAR and CRP in predicting severity of COVID-19 by ROC analysis revealed that CRP’s cut-off value was 19.9 and AUC value was 0.697 (95% confidence interval: 0.624–0.771, 73.8% sensitivity, 64.6% specificity, P < 0.001).
Fig. 1.
Receiver operating characteristic curve of independent risk factors for the severe COVID-19.
4. Discussion
In this study we showed that CAR can be effectively used in early differentiation of the COVID-19 severity in hospitalized patients. We found that older age, elevated levels of inflammation markers, along with increased incidence of diabetes mellitus, hypertension, and coronary artery disease are common in patients that have severe COVID-19 and have high CAR levels. Multivariate logistic regression analysis model reinforced the notion that in severe COVID-19, CAR can be used as an independent risk factor.
Elevated inflammation markers at the time of admission and older age have previously been reported as significantly related to COVID-19′s severe manifestation [13]. The positive correlation between CRP levels on admission and the diameter of the lung lesion was also reported to indicate disease severity [14]. Another indicator of COVID-19 severity is presence of hypertension, coronary heart disease, and diabetes mellitus [15]. Glycolipid metabolic disorders can induce cytokine storm and an imbalance between this cytokine storm and angiotensin converting enzyme 2 may affect the severity of COVID-19 [15]. The results of our study are in agreement with the aforementioned literature.
CRP, has been used as a systemic marker for tissue damage, infection, and inflammation ever since it was first identified as an acute phase protein. When bound to macromolecular ligands, CRP strongly activates the classical complement pathway and can also regulate alternative pathway amplification as well as C5 convertases [16]. The CRP expression level is usually low, but increases rapidly during acute inflammatory responses from plasma levels of 1 µg/mL to 500 µg/mL [7]. The prognostic value of CRP has been demonstrated in nasopharyngeal carcinoma [17], colorectal cancer with liver metastasis [18], and amyotrophic lateral sclerosis [19]. Li et al. stated that CRP can be used as an indicator in the progression of COVID-19 and can allow for an early identification of severe cases therefore contributing to the decrease in mortality [20].
Albumin is the main protein synthesized in the liver and can be notably negatively affected by factors such as inflammation rather than nutritional intake [21]. Inflammation may cause a decrease in serum albumin levels by downregulating albumin synthesis by means of IL-6 and TNF-α or by increasing its catabolism [22], [23]. On the other hand, albumin is also a prognostic factor for poor long-term survival after surgery in colorectal cancer, and its low levels at the time of admission increase short and long-term mortality in hospitalized patients [24], [25]. Low serum albumin levels have also been reported to indicate poor prognosis in COVID-19 patients [9].
We hypothesized that combining CRP and albumin in an index may have a prognostic value in inflammation and can predict COVID-19′s severity in hospitalized patients within the first 24 h after admission. Combining albumin and CRP in an index has previously been proposed in various diseases. Fairclough et al. proposed the concept of CAR in 2009 [26]. Ranzani et al. stated that CAR can predict mortality in intensive care unit [27]. Also, Kinoshita et al. demonstrated CAR’s independent prognostic value in hepatocellular carcinoma and stated that it has a prognostic power comparable to other inflammation-based prognostic scores [28]. There is only one study evaluating the efficacy of CAR in COVID-19 patients in the literature. Wang et al. stated that CAR could be a warning sign for early COVID-19 severity [29]. Similar to our study, Wang et al. reported that in patients with severe COVID-19, CAR was significantly elevated compared to the non-severe group. Their multivariate regression analyses also revealed CAR as an independent risk factor for COVID-19′s severity. Because CAR is derived from only two laboratory data and is easily analyzed in many centers, it can be a simple, useful, and inexpensive prognostic marker to foresee severity of COVID-19 in patients when they first arrive at the hospital. In addition, having a higher AUC in ROC analysis than CRP makes CAR a significant marker in early detection severe COVID-19.
The incidence of Kawasaki disease, a rare hyper inflammatory-syndrome that increases the risk of coronary artery aneurysm in children, has increased during the COVID-19 pandemic [30]. Kawasaki disease and acute respiratory distress syndrome are of concern to clinicians, and inclusion of CAR in the initial evaluation of hospital admissions may make it possible to identify and closely monitor patients in whom these conditions may develop. In addition, although there are currently no standardized criteria for COVID-19 patients to be admitted to intensive care units [31], it may be possible to classify patients requiring strict monitoring and early possible therapeutic intervention by using CAR as a risk factor.
Limitations of our study are small size and retrospective design. Moreover, similar to other retrospective studies, we cannot completely rule out the impact of selection bias. There is a need for further prospective studies to confirm our findings.
In conclusion, CAR is a useful marker in early differentiation of severity in patients hospitalized due to COVID-19 that have longer hospital stay and higher mortality.
Ethical approval
The ethics committee of the Tepecik Training and Research Hospital approved this study (Resolution Number 2020/8–19, dated July 08, 2020).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Inanc Karakoyun: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Ayfer Colak: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing - original draft, Writing - review & editing. Melda Turken: Data curation, Investigation, Methodology, Resources, Validation, Visualization, Writing - review & editing. Zeynep Altın: Data curation, Investigation, Methodology, Resources, Validation, Visualization, Writing - review & editing. Fatma Demet Arslan: Formal analysis, Investigation, Methodology, Software, Validation, Writing - review & editing. Veli Iyilikci: Data curation, Formal analysis, Investigation, Software, Validation, Writing - review & editing. Nisel Yilmaz: Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing - review & editing. Sukran Kose: Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None declared.
References
- 1.WHO, WHO Virtual press conference on COVID-19, March 11, 2020.
- 2.World Health Organization, WHO Coronavirus Disease (COVID-19) Dashboard (Updated 25 August 2020).
- 3.World Health Organization, Clinical management of COVID-19: interim guidance, 27 May 2020.
- 4.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang G., Hub C., Luo L., et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felger J.C., Haroon E., Patel T.A., et al. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol. Psychiatry. 2020;25(6):1301–1311. doi: 10.1038/s41380-018-0096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sproston N.R., Ashworth J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan C., Huang Y., Shi F., et al. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J. Med. Virol. 2020;92(7):856–862. doi: 10.1002/jmv.25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang W., Li C., Wang Z., et al. Decreased serum albumin level indicates poor prognosis of COVID-19 patients: hepatic injury analysis from 2,623 hospitalized cases. Sci. China Life Sci. 2020;63:1–10. doi: 10.1007/s11427-020-1733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang J., Cheng A., Kumar R., et al. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J. Med. Virol. 2020;92:2152–2158. doi: 10.1002/jmv.26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y.J., Yao K., Lu M.X., Zhang W.B., Xiao C., Tu C.Q. Prognostic value of the C-reactive protein to albumin ratio: a novel inflammation-based prognostic indicator in osteosarcoma. Oncol. Targets Ther. 2017;10:5255–5261. doi: 10.2147/OTT.S140560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosmer D.W., Lemeshow S., Sturdivant R.X. 3rd ed. Wiley; Hoboken, NJ: 2013. Applied logistic regression. [Google Scholar]
- 13.Liu F., Li L., Xu M., et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L. C-reactive protein levels in the early stage of COVID-19. Med. Mal. Infect. 2020;50(4):332–334. doi: 10.1016/j.medmal.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y., Gong X., Wang L., Guo J. Effects of hypertension, diabetes and coronary heart disease on COVID-19 diseases severity: a systematic review and meta-analysis. medRxiv. 2020 preprint. [Google Scholar]
- 16.Pepys M.B., Hirschfield G.M. C-reactive protein: a critical update. J. Clin. Invest. 2003;111(12):1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen R.W., Zhou Y., Yuan Y.J., et al. Effect of CRP and Kinetics of CRP in Prognosis of Nasopharyngeal Carcinoma. Front. Oncol. 2019;9:89. doi: 10.3389/fonc.2019.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Køstner A.H., Kersten C., Löwenmark T., et al. The prognostic role of systemic inflammation in patients undergoing resection of colorectal liver metastases: C-reactive protein (CRP) is a strong negative prognostic biomarker. J. Surg. Oncol. 2016;114(7):895–899. doi: 10.1002/jso.24415. [DOI] [PubMed] [Google Scholar]
- 19.Lunetta C., Lizio A., Maestri E., et al. Serum C-Reactive Protein as a Prognostic Biomarker in Amyotrophic Lateral Sclerosis. JAMA Neurol. 2017;74(6):660–667. doi: 10.1001/jamaneurol.2016.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X., Wang L., Yan S., et al. Clinical characteristics of 25 death cases with COVID-19: A retrospective review of medical records in a single medical center, Wuhan, China. Int. J. Infect. Dis. 2020;94:128–132. doi: 10.1016/j.ijid.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishida S., Hashimoto I., Seike T., Abe Y., Nakaya Y., Nakanishi H. Serum albumin levels correlate with inflammation rather than nutrition supply in burns patients: a retrospective study. J. Med. Invest. 2014;61(3–4):361–368. doi: 10.2152/jmi.61.361. [DOI] [PubMed] [Google Scholar]
- 22.Alves F.C., Sun J., Qureshi A.R., et al. The higher mortality associated with low serum albumin is dependent on systemic inflammation in end-stage kidney disease. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0190410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y., Liu F.-Y., Liu Z.-H., et al. Effect of tacrolimus and cyclosporine A on suppression of albumin secretion induced by inflammatory cytokines in cultured human hepatocytes. Inflamm. Res. 2006;55:216–220. doi: 10.1007/s00011-006-0074-0. [DOI] [PubMed] [Google Scholar]
- 24.Lai C.C., You J.F., Yeh C.Y., et al. Low preoperative serum albumin in colon cancer: a risk factor for poor outcome. Int. J. Colorectal Dis. 2011;26:473–481. doi: 10.1007/s00384-010-1113-4. [DOI] [PubMed] [Google Scholar]
- 25.Akirov A., Iraqi H.M., Atamna A., Shimon I. Low Albumin Levels Are Associated with Mortality Risk in Hospitalized Patients. Am. J. Med. 2017;130(12):1465.e11–1465.e19. doi: 10.1016/j.amjmed.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Fairclough E., Cairns E., Hamilton J., Kelly C. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin. Med. 2009;9(1):30–33. doi: 10.7861/clinmedicine.9-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranzani O.T., Zampieri F.G., Forte D.N., Azevedo L.C.P., Park M. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. PloS one. 2013;8(3) doi: 10.1371/journal.pone.0059321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinoshita A., Onoda H., Imai N., et al. The C-Reactive Protein/Albumin Ratio, a Novel Inflammation-Based Prognostic Score, Predicts Outcomes in Patients with Hepatocellular Carcinoma. Ann. Surg. Oncol. 2015;22:803–810. doi: 10.1245/s10434-014-4048-0. [DOI] [PubMed] [Google Scholar]
- 29.Wang X., Xu Y., Huang H., et al. An increased pretreatment C-reactive protein-to albumin ratio predicts severe novel corona virus infected pneumonia. Research Square. 2020 doi: 10.21203/rs.3.rs-31723/v1. [DOI] [Google Scholar]
- 30.Feketea G.M., Vlacha V. The Diagnostic Significance of Usual Biochemical Parameters in Coronavirus Disease 19 (COVID-19): Albumin to Globulin Ratio and CRP to Albumin Ratio. Front. Med. 2020;7 doi: 10.3389/fmed.2020.566591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bannaga A.S., Tabuso M., Farrugia A., et al. C-reactive protein and albumin association with mortality of hospitalised SARS-CoV-2 patients: A tertiary hospital experience. Clin. Med. 2020;20(5):463–467. doi: 10.7861/clinmed.2020-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]