The coronavirus disease 2019 (Covid-19) is an ongoing global pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The SARS-CoV-2 primarily infects the lung epithelial cells via angiotensin-converting enzyme-2 (ACE-2) receptor [1], thereby causing respiratory signs and symptoms. Meanwhile, since ACE-2 is highly expressed in the cardiac myocytes, it is observed that the SARS-CoV-2 can also cause cardiac injury [2,3], thereby leading poor outcomes in Covid-19 cases. However, there is a very limited data about whether admission cardiac troponin levels are associated with a poor survival in Covid-19 cases without cardiovascular risk factors. Therefore, the main objective of the current study was to determine the prognostic significance of admission cardiac troponin level in Covid-19 patients without known such risk factors.
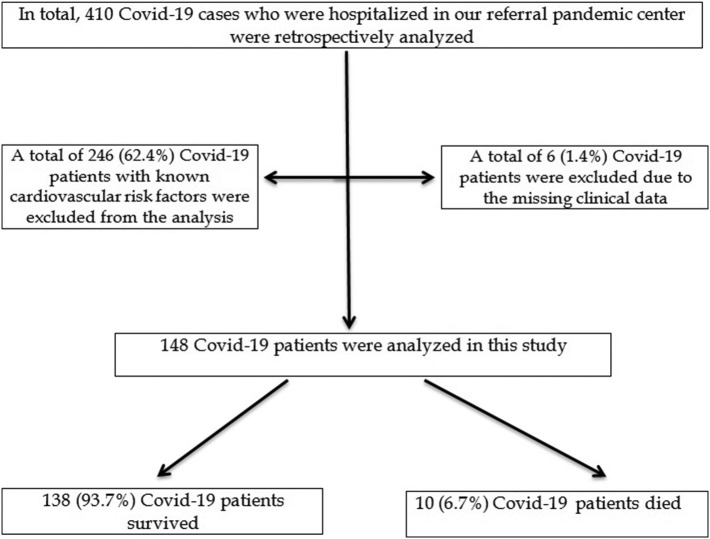
In this retrospective, observational study, the clinical data of consecutive Covid-19 patients who were hospitalized in our tertiary center were collected. In total, 148 Covid-19 patients were analyzed in this study (Fig. 1 ). In all of the cases, Covid-19 infection was diagnosed based on the specific signs and symptoms or imaging findings in computerized thoracic tomography (CT) and confirmed by the RT-PCR test. The present study was registered into the Ministry of Health Scientific Research Covid-19 Committee. Then, the study was approved by the Local Ethics Committee (approval number: 2020/KK/133–2286). The assessment of cardiac troponin I was based on a latex-enhanced immunoturbidimetric assay. In our institution, the normal reference range of cardiac troponin I level was 0–30 ng/L. The primary outcome was the in-hospital all-cause mortality.
Fig. 1.
Flow chart of the study participants.
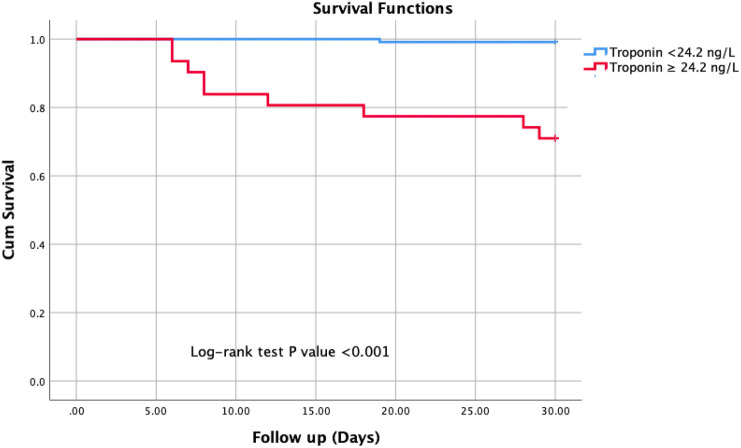
During the in-hospital follow-up, 10 (6.7%) patients died. The sample size was categorized into two groups; survivors and nonsurvivors. Table 1 presents the clinical characteristics, admission symptoms, and laboratory findings of all Covid-19 patients. The frequency of male gender as well as presenting symptoms, including fever, cough, dyspnea, and diarrhea, were not different between the groups. Regarding to laboratory results, nonsurvivors had significantly elevated values of white blood cells (WBC) count, neutrophils, D-dimer, lactate dehydrogenase (LDH), C-reactive protein (CRP), and troponin I levels, while their lymphocytes and albumin levels were significantly decreased. The imaging findings were indifferent for each group. The independent prognosticators of in-hospital death were established using univariable and multivariable LR analysis as displayed in Table 2 . In a multivariable analysis; only cardiac troponin I (OR: 1.110, 95%CI: 1.025–1.201, P = 0.010) and albumin were independent predictors of the in-hospital death for Covid-19 cases without cardiovascular risk factors. In a ROC analysis, the optimal value of cardiac troponin I in predicting in-hospital mortality was 24.2 ng/ with sensitivity of 90.0% and specificity of 85.0% (Fig. 2 ). The Kaplan–Meier analysis displayed that Covid-19 cases with cardiac troponin I values of ≥24.2 ng/L had significantly higher rates of deaths compared to those with cardiac troponin I < 24.2 ng/L (Fig. 3 ).
Table 1.
Comparison of clinical characteristics and laboratory parameters of patients hospitalized with the diagnosis of Covid-19 pneumonia according to in-hospital mortality
| Survivor, (n = 138) | Nonsurvivor, (n = 10) | P value | |
|---|---|---|---|
| Age, y | 52.4 ± 15.2 | 71.8 ± 10.7 | <0.001 |
| Male gender, n (%) | 81 (58.7) | 9 (90.0) | 0.080 |
| Systolic blood pressure, mmHg | 130.1 ± 15.3 | 128.3 ± 15.3 | 0.713 |
| Diastolic blood pressure, mmHg | 79.9 ± 7.2 | 82.6 ± 7.5 | 0.273 |
| Admission symptoms, n (%) | |||
| Fever | 75 (54.6) | 7 (70.0) | 0.513 |
| Cough | 73 (52.9) | 7 (70.0) | 0.344 |
| Dyspnea | 27 (19.6) | 4 (40.0) | 0.218 |
| Diarrhea | 7 (5.1) | 0 (0.0) | 1.000 |
| Asymptomatic | 10 (7.2) | 0 (0.0) | 1.000 |
| Laboratory values | |||
| White blood cells, cells/μL | 6.1 ± 2.9 | 8.5 ± 4.2 | 0.037 |
| Neutrophils, | 4.3 ± 2.7 | 5.3 ± 1.8 | 0.048 |
| Lymphocytes | 1.4 ± 0.7 | 0.8 ± 0.3 | 0.003 |
| Platelets, cells/μL | 205.8 ± 67.8 | 194.1 ± 86.1 | 0.405 |
| Hemoglobin, g/dL | 13.1 ± 2.0 | 12.8 ± 2.3 | 0.957 |
| Glucose, mg/dL | 111.6 ± 44.2 | 107.1 ± 18.4 | 0.601 |
| Lactate dehydrogenase, U/L | 483.8 ± 204.1 | 1214.4 ± 1523.6 | 0.002 |
| Fibrinogen, mg/L | 487.4 ± 180.0 | 561.6 ± 228.8 | 0.521 |
| D-dimer, μg/mL | 553.7 ± 588.9 | 2593.6 ± 3276.6 | 0.005 |
| C-reactive protein, mg/dL | 59.4 ± 65.9 | 110.8 ± 70.9 | 0.022 |
| Alanine aminotransferase, U/L | 36.0 ± 36.6 | 43.8 ± 38.1 | 0.564 |
| Aspartate aminotransferase, U/L | 31.4 ± 24.0 | 116.0 ± 231.0 | 0.069 |
| Creatinine, mg/dL | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.227 |
| Potassium, mEq/L | 4.2 ± 0.4 | 4.2 ± 0.4 | 0.748 |
| Sodium, mEq/L | 137.3 ± 3.0 | 135.5 ± 3.1 | 0.065 |
| Troponin I, ng/L | 6.2 ± 9.6 | 89.6 ± 66.0 | <0.001 |
| Albumin, g/L | 40.6 ± 5.7 | 26.9 ± 5.0 | <0.001 |
| Hospital stay, day | 8.4 ± 5.9 | 14.6 ± 9.8 | 0.019 |
| Pneumonia region in the lungs, n (%) | |||
| Bilateral | 107 (79.9) | 9 (90.0) | 0.687 |
| Right | 19 (14.4) | 0 (0.0) | 0.358 |
| Left | 12 (9.1) | 1 (10.0) | 1.000 |
Continuous variables are presented as mean ± SD, nominal variables presented as frequency (%).
Table 2.
Univariable analysis and multivariable model for in-hospital mortalitya.
| Univariable analysis | P value | OR (95% CI) | Multivariable analysis | P value | OR (95% CI) |
|---|---|---|---|---|---|
| Age | 0.001 | 1.115 (1.045–1.189) | – | – | – |
| White blood cell count | 0.021 | 1.205 (1.028–1.413) | – | – | – |
| Lymphocyte | 0.006 | 0.081 (0.013–0.490) | – | – | – |
| Lactate dehydrogenase | 0.008 | 1.003 (1.001–1.005) | – | – | – |
| D-dimer | 0.001 | 1.001 (1.000–1.002) | – | – | – |
| C-reactive protein | 0.028 | 1.009 (1.001–1.017) | – | – | – |
| Troponin I | <0.001 | 1.107 (1.053–1.164) | Troponin I | 0.010 | 1.110 (1.025–1.201) |
| Albumin | <0.001 | 0.654 (0.528–0.810) | Albumin | 0.021 | 0.656 (0.458–0.939) |
OR, Odds ratio; CI, confidence interval.
All clinically relevant parameters were included in the model.
Fig. 2.
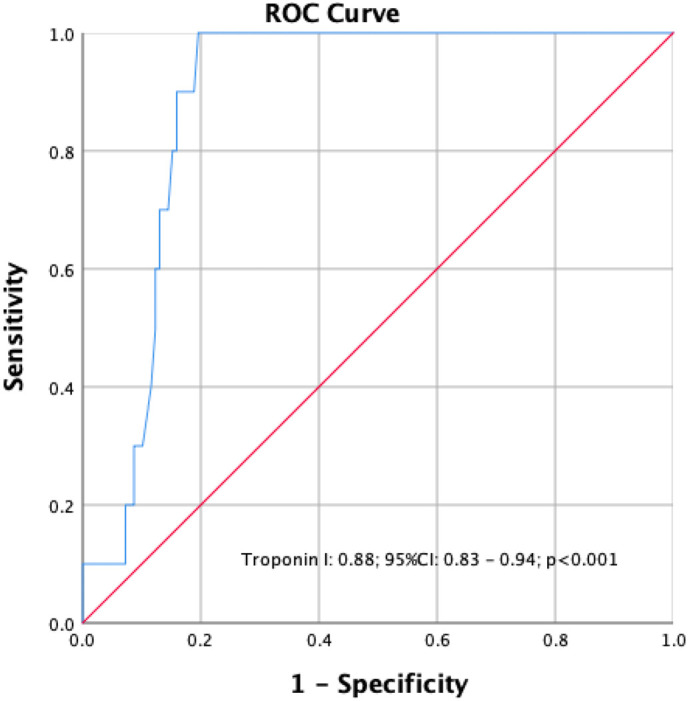
The optimal cut-off value of cardiac troponin I to predict the in-hospital mortality in patients without cardiovascular risk factors.
Fig. 3.
The Kaplan–Meier cumulative survival analysis of the patients based on cardiac troponin I level.
In the current study, we observed that after controlling of some possible confounders, myocardial injury, indicated by the elevation of cardiac troponin I level, is a strong predictor of in-hospital death in Covid-19 cases without cardiovascular risk factors. Based on the study results, we considered that admission cardiac troponin level should be treated as an important prognostic marker, thus patients with higher levels of admission troponin levels should be followed-up closer in order to predetermine Covid-19-associated complications. More aggressive treatment strategies, such as biologic agents or Covid-19 convalescent plasma, may be used earlier in order to decrease mortality due to Covid-19 infection in subjects with higher admission troponin levels.
References
- 1.Li G., He X., Zhang L., Ran Q., Wang J., Xiong A., et al. Assessing ACE2 expression patterns in lung tissues in the pathogenesis of COVID-19. J Autoimmun. 2020;112:102463. doi: 10.1016/j.jaut.2020.102463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen C., Chen C., Yan J.T., Zhou N., Zhao J.P., Wang D.W. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48(7):567–571. doi: 10.3760/cma.j.cn112148-20200225-00123. [DOI] [PubMed] [Google Scholar]