Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-cov-2), first described in December 2019, has now infected more than 28 million cases with almost one million deaths. Reinfection is not definitely established however disease recurrence is increasingly reported.
Cases presentation
Four patients presented with a second episode of coronavirus disease 2019 (COVID-19) occurring 27–85 following their first illness. The initial episode was mild or asymptomatic while the second attack was severe requiring hospital admission. All four patients had a SARS-CoV-2 PCR test positive in the second episode. The chest-X-ray and/or computerized tomography (CT) scan showed bilateral alveolar shadows. Furthermore, the inflammatory markers were raised in the four patients. Three patients recovered following treatment with favipravir in addition tocilizumab and/or dexamethasone.
Conclusion
Covid19 reinfection Recurrent COVID-19 is increasingly reported. However; other etiologies including superadded infection or pulmonary embolism should be ruled out, particularly if recurrence occurs less than 3 weeks.
Keywords: SARS-cov-2, COVID-19, Reinfection, Recurrence, Episode
Introduction
The exact reason why certain COVID-19 patients have a recurrence of their illness is not clear. An incomplete eradication of the virus from the tissues was previously suggested [1]. Lack of protective immunity due to insufficient development of antibodies or a rapid decay of these antibodies might be a driving factor. Specifically, humoral immunity in SARS-COV-2 is of short duration in persons with mild illness [2,3]. Conversely patients with clinically severe disease have a propensity to produce high antibody titers. Nevertheless, detection of IgG antibodies does not equate with immunity. Remarkably, robust SARS-CoV-2-specific T cells were demonstrable in antibody-seronegative individuals with a history of asymptomatic or mild COVID-19 [4]. The interplay of both humoral and cellular immunity requires further longitudinal studies to help guide therapeutic and preventive interventions in SARS-COV-2 infection [5].
In this article we present four patients with a possible recurrence/reinfection of COVID-19. Their initial illness was mild or asymptomatic while the second episode was severe but with a favorable outcome in three of them.
Case 1
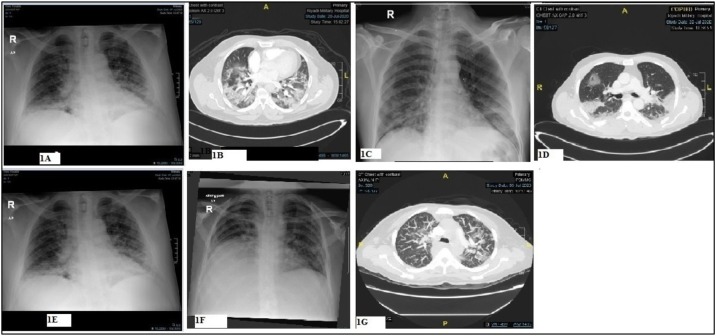
A previously healthy 51-year-old man tested SARS-COV-2 positive on 20/05/2020 (D0). He remained asymptomatic till July 17, 2020 (D58) when he presented with a 3 days history of fever, cough, generalized weakness, and shortness of breath (SOB). There was no recent contact with a COVID-19 patient. His pulse rate (PR) was 99 beats/min, BP 110/68 mmHg, respiratory rate (RR) 28/min, temperature 39 °C, and oxygen saturation (SPO2) 88% on room air. A chest X-ray showed bilateral diffuse patchy airspace disease while a CT scan revealed bilateral patchy central and peripheral ground glass opacities most likely related to COVID-19 (Fig. 1 A & B). There was no evidence of pulmonary emboli. SARS-COV-2 reverse transcription–polymerase chain reaction (RT-PCR) assay (nasopharynx and oropharynx swab) was positive with a cycle threshold value (CT-V) of 21.06. Laboratory investigations showed lymphopenia, and high inflammatory markers including IL-6. Serum SARS-COV-2 IgG antibodies (Abbot) was 7.04 index positive. He received favipravir and two doses of tocilizumab (400 mg each) in addition to supportive care (Table 1 ). The CRP dropped from 164 to 5 and lymphocytes increased from 1.1 to 3.41 with normalization of inflammatory markers. He was discharged home on D67.
Fig. 1.
Chest X-ray and CT scan of patient 1 (A & B), patient 2 (C & D), patient 3 (E) and patient 4 (F & G).
Table 1.
Investigations, radiological changes, and treatment of the patients SOB = shortness of breath; CTV = cycle threshold value; DVT = deep vein thrombosis; CRP = C-reactive protein; LDH = lactate dehydrogenase; PCT = procalcitonin; PT = prothrombin time, PE = pulmonary embolism, *>2 ng/ml indicate high risk of infection.
| Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Normal range |
|---|---|---|---|---|---|
| Age(years)/Sex | 51M | 55M | 60M | 48F | |
| First episode symptoms(D0) | Asymptomatic | Runny nose | Asymptomatic | SOB, fatigue | |
| Day of second episode | D58 | D31 | D 27 | D85 | |
| Second episode symptoms | Fever-cough–SOB- | Fever-Dry cough- sore throat | Cough- SOB | SOB-Fever-vomiting | |
| Co-morbidity | None | Relapsed NHL | IHD, HTN, DM | Metastatic breast cancer | |
| O2 saturation (RA) on second admission | 88% | 93% | 83% | 89% | |
| CXR on a second admission | Bilateral patchy airspace disease | Bilateral patchy airspace disease | Bilateral patchy airspace disease | Bilateral airspace disease | |
| CT-Scan on second admission | Bilateral patchy central and peripheral ground glass opacities (GGO). No PE | Bilateral patchy GGO with thickened interlobular septa and peripheral consolidation. No PE | Not done | Bilateral patchy GGO. No PE | |
| Laboratory investigations during second admission | |||||
| WBC 10^9/l | 5.0 | 4.2 | 3.9 | 3.4 | 4.0 - 11.0 |
| Lymphocytes 10^9/l | 1.1 | 0.4 | 1.5 | .24 | 1.5 - 4.0 |
| Troponin ng/ml | 0.009 | Not done | 0.019 | .304 | 0.1 |
| D-Dimer ug/ml | 0.72 | .58 | 0.760 | 16.11 | .000 - .500 |
| LDH U/l | 349 | 304 | 263 | 514 | 135 - 225 |
| CRP mg/l | 164 | 115 | 19 | 60 | 0 - 6 |
| Ferritin ng/ml | 746 | 3453 | 444 | 4885 | 30 - 400 |
| Fibrinogen g/l | 6.6 | 4.7 | 6.7 | 3.7 | 2.0 - 5.0 |
| PCT ng/ml | 0.39 | 0.17 | 0.08 | 0.70 | * |
| PT sec | 14.0 | 13.1 | 12.5 | 21.9 | 11.5 - 14.5 |
| creatinine umol/l | 120 | 132 | 67 | 67 | 59- 104 |
| Interleukin 6 pg/ml | 9.8 | 43.7 | 11 | Not done | up to 0.7 |
| SARS-COV-2 IgG (Abbot) | 7.04 index positive | 0.01 index negative | Not done | Not done | |
| SARS-CoV-2 PCR (date &CTV) | D0 CTV 27.1, D58 CTV22.2, D62 CTV 21.06 | D0+v, D10-ve, D32 CT 6.41 D42 CTV 10.7 | D0+VE, D27+ve CTV-Not available | D0+ve, D7-ve, D9-ve, D85+ve CTV not available | |
| Treatment | |||||
| Favipravir* | Yes | Yes | Yes | yes | |
| Antibiotic therapy | Yes | Yes | Yes | yes | |
| Dexamethasone | No | yes | yes | ||
| Tocilizumab (400 mg per dose) | Yes (two doses) | No | No | Yes(two doses) | |
| DVT prophylaxis | Yes | Yes | Yes | yes | |
Case 2
A 55-years-old man with a relapsed follicular non-Hodgkin’s lymphoma was diagnosed as mild COVID-19 on 12/06/2020 (D0). A repeat nasopharyngeal aspirate for SARS-CoV-2 PCR was negative on D10. He remained stable till D30 when he presented with a high grade fever, dry cough, and sore throat. Vital signs were normal apart from tachycardia and (SPO2) 93% on room air. SARS-CoV-2 PCR on D30 was positive with a CT-V of 6.41. Admission chest X-ray showed bilateral lower lung linear air space opacity. A CT scan of the chest was negative for PE but showed bilateral patchy ground glass opacities (GGO) with thickening interlobular septa and peripheral consolidation (Fig. 1C & D). Laboratory investigations disclosed marked lymphopenia, hypogammaglobulinemia (low IgG, IgM, and IgA), high inflammatory markers and normal procalcitonin level. Serum SARS-CoV-2 IgG antibodies (Abbot) was 0.01 index negative. Both sputum and blood culture were negative for bacteria (Table 1). He recovered following treatment with standard supportive care, favipravir and dexamethasone.
Case 3
An asymptomatic 60-year-old man with DM, HTN and IHD, tested SARS-COV-2positive on June 11, 2020(D0). He was then admitted on D27 with a 5 days history of cough and SOB. The PR was 100 beats/min, BP 120/68 mmHg, RR 32/min, temperature 37.0 °C and (SPO2) 83–88% on room air. SARS-CoV-2 PCR D30 was positive. A chest X-ray showed bilateral airspace disease (Fig. 1E). Investigations are shown in Table 1. He received standard supportive care and favipravir. His condition improved and was discharged home on D30.
Case 4
A 48-years-old lady with metastatic breast cancer on Palbociclib (a cyclin- dependent kinases inhibitor), was initially diagnosed as covid19 pneumonia on April 14, 2020. She received hydroxychloroquine and azithromycin and then discharged home upon two negative nasopharyngeal aspirate SARS-Cov-2 PCR tests. She was readmitted on July 8, 2020(D85) with a one week history of fever and SOB. On admission the PR was 110 beats/min, BP 114/66 mmHg, RR 32/min, temperature 37.0 °C and (SPO2) 89% on room air. Investigations are shown in (Table 1). A chest X-ray showed bilateral airspace disease while a CT scan of the chest was negative for PE but showed bilateral patchy ground glass opacities (GGO) (Fig. 1 F&G). She received favipravir, dexamethasone and tocilizumab but continued to deteriorate requiring mechanical ventilation and died two weeks following admission.
Discussion
We present here four patients aged 48–60 years who presented with symptoms suggestive of a second episode of COVID-19. Their illness followed a symptom-free period of 58, 32, 27 and 85 days for patient 1, 2,3, and 4 respectively. Two of the patients had a negative SARS-CoV-2 PCR following their initial infection. All the patients had a severe second episode as evidenced by the presence of tachypnoea, radiological signs and hypoxia. In addition to supportive therapy, they received O2 supplementation, antivirals and dexamethasone and/or an IL-6 inhibitor, tocilizumab.
Reinfection with SARS-CoV-2 is a potential etiology; however, it is difficult to confirm. Up till recently, there was no concrete evidence to support Sars-cov-2 re-infection. However, a 33 years-old- immunocompetent man from Hong Kong was reported as the first confirmed case of covid re-infection in August 2020. The patient’s two episodes were related to two different clades with clearly different genome sequences. Viral genomic studies showed the first episode virus particles belongs to GISAID clade V, Nextstrain 19A, and Pangolin Lineage B.2 with a remarkably close probability of 0.99. On the other hand, the second viral genome fit in the GISAID clade V, Nextstrain 19A, and Pangolin Lineage B.1.79 with a probability of 0.70. The second attack occurred 142 days following recovery from a mild infection in March 2020 [6]. His early serology for SARS-COV-2 -specific immunoglobulin G was negative. These findings could indicate that immunity following a natural infection may be absent or short lived. In contrast, there is evidence that the virus does not replicate in primates previously infected with SARS-COV-2. It is postulated that the primary infection of SARS-COV-2 had induced a protective immunity against subsequent infections in these primates [7,8]. However; it is not yet clear if humans recovering from an initial infection will mount an adequate immune response and for how long they will remain sufficiently protected against a future infection [9]. In one study, nearly one third of recovered COVID-19 individuals tested negative for neutralizing antibodies within an average of 39 days from their onset of symptoms [10]. Remarkably; some data suggest that the disease severity may influence the magnitude of antibody response [11,12]. Noteworthy, three of our patients’ initial presentation was asymptomatic or mild and may not have formed sufficient neutralizing antibodies. On the other hand, our first patient developed a severe disease despite a positive serology for anti-SARS-CoV-2 antibodies. It is possible those antibodies lacked the neutralizing activity needed to control the infection. Prolonged detection of SARS-COV-2 RNA was shown in one obstetric patient despite adequate antibody seroconversion [13]. Another possibility is that the antibodies were formed in response to the second episode as the test was done late in his current illness.
It is conceivable that our patients’ second episode is a delayed presentation of an initial infection, or a post viral inflammatory changes. Three of our patients had a mild illness or were asymptomatic in the first episode. There are scarce reports on the long-term follow-up to evaluate for disease progression in asymptomatic or pre-symptomatic patients. Recent data from Korea, showed that 21 (19.1%) of asymptomatic subjects developed symptoms during isolation. The median (IQR) interval time from diagnosis to symptom onset was 15 (13–20) days [14]. All our patients are outside this this interval with symptoms developing 27–85 days post initial episode.
Recurrence of COVID-19 after apparent recovery is increasingly reported [1]. In a study from Wuhan, China, 9% (5/55 patients) had a recurrence of the disease. Both asymptomatic and minimally symptomatic patients had the potential to reactivate [15]. A Collaborative study COvid RECurrences (COCOREC) proposed a COVID-19 recurrence to be considered if the second episode occurred 21 days following a symptom free period and alternative etiologies are excluded [1].
The persistence of RT-PCR positivity and/or oscillating positive/ negative tests despite clinical and radiological recovery has been previously described [16]. As a result the presence of a positive RT-PCR in the absence of clinical symptoms will suggest more of a prolonged viral shedding and does not automatically imply an acute infection [17]. Nevertheless, We believe our patients had a re-infection or recurrence of the disease rather than a bacterial pneumonia with a persistence of non-viable RNA of SARS-CoV-2 following the first infection. First, the long interval between the initial and second episodes (27–85 days) argues against prolonged shedding. Viral shedding is undetectable in the majority of patients one month following infection. Unusually, in two previously reported patients, viral shedding was detected 55 and 104 days following initial episode [13,18]. Secondly, the normal procalcitonin and negative blood and sputum culture makes bacterial pneumonia unlikely. Thirdly the radiological changes with bilateral, peripheral shadowing and ground glass changes are highly suggestive of active covid19 though not pathognomonic [19]. Furthermore, elevated pro-inflammatory cytokine (IL-6) and inflammatory markers including ferritin, CRP, LDH, D-dimer and lymphopenia support an active SARS-CO-V-2 infection rather than a simple prolonged shedding. Additionally, the low CT value in the first two patients suggests an active SARS-COV-2 infection and a high viral load. Ct value is inversely related to the viral load and can be used as a surrogate for the copy number of viral RNA. The association of SARS-COV-2 low Ct values and increased probability of progression to severe disease and increased mortality was previously described [20].
The second episode in our patients varied between 27 and 85days. It is not clear why and when some patients reactivate their infection. Host factors, immunosuppressive therapy and viral dynamic are suggested as contributory factors. High viral load and viral genome are important consideration. Regrettably three of our patients did not have a baseline CT- value to reflect their viral load. Two of our patients were immunosuppressed; the second patient is immunocompromised with hypogammaglobulinemia following anti-CD20 monoclonal antibody therapy (Obinutuzumab) while the third patient is on Palbociclib for advanced metastatic breast cancer. However, the period of the second episode cannot be clearly attributed to immunosuppression. It has been suggested that the virus emerges from a concealed focus and get activated [1]. Immunosuppressive factors including drugs or pathological conditions could contribute to delayed viral clearance and leads to SARS-CoV-2 reactivation [21].
There are several limitations in this case series. We failed to quantify neutralizing antibodies in the described patients. Similarly, SARS-COV- 2 virus was not isolated on culture and genotyping was not performed. However; the long interval between the first and second episode and the raised inflammatory markers pointed more to an active infection or reactivation than a prolonged viral shedding. The number of cases included in our study is too small to reach a conclusion a large number is needed to study factors and mechanisms underlying reinfection and recurrences of COVID-19.
In conclusion, we suspect this cluster of patients had a recurrence of their disease though re-infection cannot be excluded. Nevertheless, re-infection is extremely rare and should not impede efforts to control this pandemic. Persistence, reactivation and re-infection can have therapeutic and transmission risk impact. Further studies are vital to quantify this risk.
Funding
No funding sources.
Competing interests
None declared.
Ethical approval
Ethical approval was obtained from the ethical committee of PSMMC.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jiph.2021.01.002.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Batisse D., Benech N., Botelho-Nevers E., Bouiller K., Collarino R., Conrad A. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect. 2020;81(5):816–846. doi: 10.1016/j.jinf.2020.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ibarrondo F.J., Fulcher J.A., Goodman-Meza D., Elliott J., Hofmann C., Hausner M.A. Rapid decay of anti–SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med. 2020;383(11):1085–1087. doi: 10.1056/NEJMc2025179. NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;29:1–4. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 4.Sekine T., Perez-Potti A., Rivera-Ballesteros O., Straling K., Gorin J.-B., Olsson A. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. BioRxiv. 2020;183(1):158–168. doi: 10.1101/2020.06.29.174888. 2020.06.29.174888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirkcaldy R.D., King B.A., Brooks J.T. COVID-19 and postinfection immunity: limited evidence, many remaining questions. JAMA J Am Med Assoc. 2020 doi: 10.1001/jama.2020.7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.To K.K.-W., Hung I.F.-N., Ip J.D., Chu A.W.-H., Chan W.-M., Tam A.R. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin Infect Dis. 2020;(Aug 25) doi: 10.1093/cid/ciaa1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandrashekar A., Liu J., Martinot A.J., McMahan K., Mercado N.B., Peter L. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science (80-.) 2020 doi: 10.1126/science.abc4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao L., Deng W., Gao H., Xiao C., Liu J., Xue J. Lack of reinfection in rhesus macaques infected with SARS-CoV-2. BioRxiv. 2020 doi: 10.1101/2020.03.13.990226. preprint. [DOI] [Google Scholar]
- 9.Patel R., Babady E., Theel E., Storch G., Pinsky B., George K. Report from the american society for microbiology COVID-19 COVID-19. MBio. 2020;11:1–5. doi: 10.1128/mBio.00722-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020 doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynch K.L., Whitman J.D., Lacanienta N.P. Magnitude and kinetics of anti-SARS-CoV-2 antibody responses and their relationship to disease severity. medRxiv. 2020 doi: 10.1101/2020.06.03.20121525. 2020.06.03.20121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L., To K.K.W., Chan K.H., Wong Y.C., Zhou R., Kwan K.Y. High neutralizing antibody titer in intensive care unit patients with COVID-19. Emerg Microbes Infect. 2020;9(1):1664–1670. doi: 10.1080/22221751.2020.1791738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molina L.P., Chow S.-K., Nickel A., Love J.E. Prolonged detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA in an obstetric patient with antibody seroconversion. Obstet Gynecol. 2020;136(4):838–841. doi: 10.1097/aog.0000000000004086. [DOI] [PubMed] [Google Scholar]
- 14.Park S.Y., Son H., Yu S., Park J.W., Choo E.J., Park S. 2020. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the Republic of Korea; pp. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye G., Pan Z., Pan Y., Deng Q., Chen L., Li J. Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. J Infect. 2020;80:e14–e17. doi: 10.1016/j.jinf.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duggan N.M., Ludy S.M., Shannon B.C., Reisner A.T., Wilcox S.R. A case report of possible novel coronavirus 2019 reinfection. Am J Emerg Med. 2020;(Jul 4) doi: 10.1016/j.ajem.2020.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lan L., Xu D., Ye G., Xia C., Wang S., Li Y. Positive RT-PCR test results in patients recovered from COVID-19. JAMA J Am Med Assoc. 2020;(March 31) doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J., Zhang L., Liu B., Song D. Case report: viral shedding for 60 days in a woman with COVID-19. Am J Trop Med Hyg. 2020;45(1):96–101. doi: 10.4269/ajtmh.20-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosseiny M., Kooraki S., Gholamrezanezhad A., Reddy S., Myers L. Radiology perspective of coronavirus disease 2019 (COVID-19): lessons from severe acute respiratory syndrome and Middle East respiratory syndrome. Am J Roentgenol. 2020;214(5):1078–1082. doi: 10.2214/AJR.20.22969. [DOI] [PubMed] [Google Scholar]
- 20.Rao S.N., Manissero D., Steele V.R., Communications H., Pareja J. A narrative systematic review of the clinical utility of cycle threshold values in the context of COVID-19. Infect Dis Ther. 2020:1–14. doi: 10.1007/s40121-020-00324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ling Y., Xu S.B., Lin Y.X., Tian D., Zhu Z.Q., Dai F.H. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020 doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.