Corresponding Author

Key Words: COVID-19 (coronavirus), echocardiography, right ventricle
It could only be the record of what had to be done…by all who, while unable to be saints but refusing to bow down to pestilences, strive their utmost to be healers.
—Albert Camus, The Plague (1)
COVID-19 has sickened 30 million people worldwide and has resulted in the death of 950,000 (2). It was declared a pandemic by the World Health Organization on March 11, 2020 and a national emergency in the United States 2 days later. Within 2 weeks, New York City, the United States’ most densely populated city and host to many international travelers, became an epicenter, the crowded COVID-19 units of the city hospitals starkly contrasting to the then deserted city streets. In these hospitals, medical staff demonstrated their dedication and skill and battled with this deadly disease using all available technology. To understand and treat the cardiac features of this deadly disease, they used echocardiography, a portable and readily available technology with a role in evaluation of virtually all forms of cardiac disease.
The current retrospective study in this issue of the Journal included 510 patients who underwent treatment for COVID-19 at 3 hospitals in the New York Presbyterian Hospital network who had clinically indicated transthoracic echocardiography (3). Indications included dyspnea and/or respiratory decompensation in 88%, hemodynamic instability in 62%, known and/or suspected myocardial infarction in 12%, and arrhythmia in 7%. Because of the high suspicion of adverse right ventricular (RV) remodeling in COVID-19 due to frequent lung involvement, the investigators appropriately targeted RV assessment, comparing it to conventional clinical and biomarker risk stratification of COVID-19.
Echocardiograms were performed at a median time of the sixth hospital day, and 68% of patients were admitted to the intensive care unit. RV dilatation was present in 35% and RV dysfunction in 15%. These observations were concordant with a recent report of echocardiographic features of 100 hospitalized patients with COVID-19 that found that the most prevalent abnormalities were RV dilatation and dysfunction, which was observed in 39% (4). Left-sided cardiac abnormalities, which were not the emphasis of the current report, were also prevalent, with the finding of left ventricular (LV) ejection fractions of <55% in 41% of the overall population, as well as regional wall motion abnormalities in 13%. These observations were aligned with those of a large prospective international registry of 1,216 patients with COVID-19 in whom LV abnormalities were reported in 39% (5). In the current study, RV contractile dysfunction appeared to occur after geometric remodeling, with RV dysfunction present in less than one-quarter of patients with RV dilation. Dilation is a known early response of the thin-walled RV to increased preload and afterload. RV pressure overload secondary to acute pulmonary hypertension in COVID-19 may cause systolic and diastolic interventricular septal flattening with RV dilation and dysfunction compromising LV function via ventricular interdependence. Tricuspid regurgitation and increased central venous pressure exacerbate RV failure (6). Patients with adverse RV remodeling had lower LV ejection fractions and increased left atrial volumes, as well as higher pulmonary artery systolic pressures. Mechanical ventilation compromised accurate assessment of right atrial pressure. Elevations of troponin, D-dimer, and ferritin, and requirement for intensive care unit−level care were more common in patients with adverse RV remodeling.
During a median follow-up of 20 days, death occurred in 32% of patients, and hospital discharge happened in 45%. Age was a predictor of death (hazard ratio [HR] 1.15 per decade), as were peak levels of D-dimer, ferritin, and troponin. Multivariate analyses showed that RV dysfunction, defined by the impairment of both tricuspid annulus peak systolic excursion and peak systolic tissue Doppler velocity of the tricuspid annulus (HR: 2.57) and dilation, defined using a binary cutoff of RV basal end-diastolic diameter of >4.1 cm (HR: 1.43), were independently associated with mortality. Thus, echo-quantified adverse RV remodeling (dilatation or dysfunction) conferred >2-fold increase in risk of death even after controlling for age and biomarker elevations.
The investigators used commercial full-sized echocardiography systems. Although echocardiography can be performed with handheld devices with simplified cleaning and storage, image quality can be more challenging, and disease may be underestimated (7), especially when patients have lung disease (acute respiratory distress syndrome [ARDS] was present in 58% of patients in the current study) or require mechanical ventilation (60% in this study). As recommended by professional societies, echocardiography was performed according to a protocol to minimize acquisition time and diminish sonographer exposure (8). Thus, assessments of RV size and systolic function were assessed from a single apical 4-chamber view.
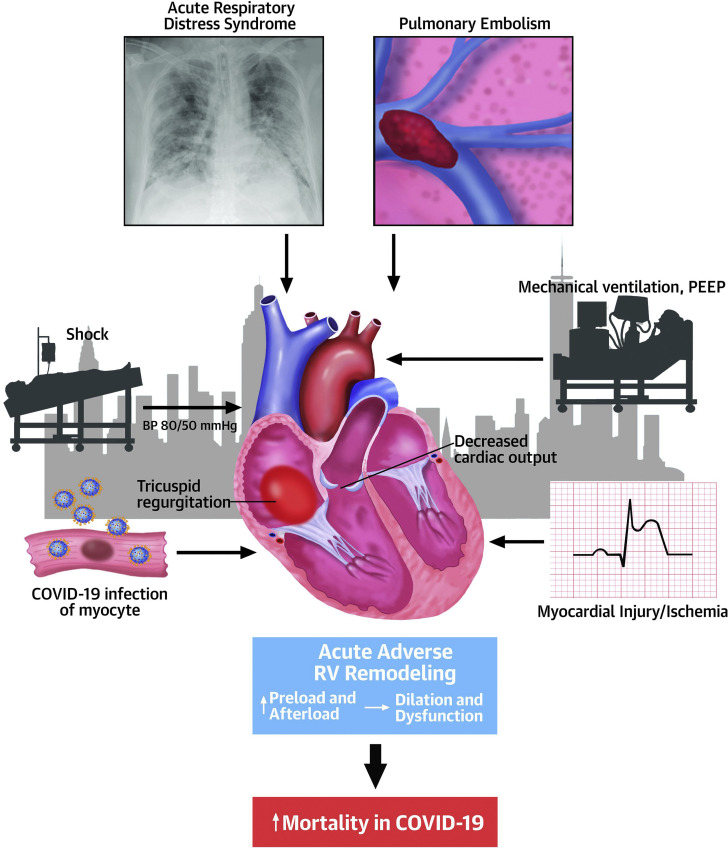
The study had important strengths. Echocardiographic images were reviewed in a core laboratory. Comparisons with previous echocardiography were available in 14% of patients, which allowed documentation that adverse RV remodeling was often new. Limitations were missing clinical and echocardiographic data, including RV systolic function assessment (missing in 47%) and RV systolic pressure (missing in 45%), limited duration of follow-up, and lack of information regarding the total number of patients hospitalized with COVID-19 during the study. Hypoxia, underlying lung disease, ARDS, myocardial ischemia or infarction, or cardiogenic shock coexisted in many of these patients, but the contribution of these, as well as those of pulmonary thromboembolism, disseminated intravascular coagulation, myocardial inflammation, and myocarditis, to RV remodeling and mortality (Figure 1 ) could not be ascertained from the information presented. The hemodynamic impact of vasopressor use and mechanical ventilation on the RV versus other effects of COVID-19 could not be distinguished.
Figure 1.
Adverse RV Remodeling By Echocardiography Was Associated With Doubling of Mortality in Patients With COVID-19
In this setting, acute respiratory distress syndrome, pulmonary embolism, mechanical ventilation, myocardial ischemia and infarction, shock, and coronavirus disease 2019 (COVID-19) related infection and inflammation of the myocyte may contribute to right ventricular (RV) dilation and dysfunction. BP = blood pressure; PEEP = positive end-expiratory pressure.
RV geometry is complex and best assessed with 3-dimensional volumetric measurements or multiple views (9). Anatomic landmarks for RV measurement are limited. Linear dimensions in the apical 4-chamber view, as used in the current study to define RV dilation, can be changed substantially by minor changes in transducer position. No correction was made for obesity, which was present in 34% of patients; patient size affects normal values for cardiac dimensions (10). Echo contrast was used in only 15% and might have enhanced the detection of ventricular dysfunction or regional wall motion abnormalities. RV longitudinal strain, a more accurate tool to detect RV dysfunction, and previously shown to be a predictor of higher mortality in patients with COVID-19 (11), was not used.
Cardiac sonographers and other practitioners performing cardiac ultrasound are in close contact with patients with COVID-19 and require appropriate personal protective equipment (8). In the future, robotic imaging may optimize safety for patients and providers. Application of artificial intelligence to interpretation of images will expedite echocardiographic imaging protocols and augment recognition of cardiac disease.
The cardiac sonographers and the investigative team, including medical students and house staff who assisted with data abstraction, are to be congratulated on this impressive and detailed work conducted in a short amount of time. The high mortality in the current study (32%) was similar to that reported in other studies (12). Further work is needed to understand how echocardiographic findings could affect patient management and could be used to improve outcome. The impact of therapies, including anticoagulation, antivirals, corticosteroids, anti-inflammatory agents, and convalescent plasma on cardiac function, morbidity, and mortality remains to be understood. A recent study that used cardiovascular magnetic resonance imaging in patients who recently recovered from COVID-19 (only 33% required hospitalization) showed abnormal findings in 78%, including ongoing myocardial inflammation in 60% (13). The long-term cardiovascular sequelae of COVID-19, including the impacts of clinical and subclinical myocardial injury and inflammation, chronic pulmonary dysfunction, exacerbation of underlying heart disease, and profound deconditioning, will be a subject of continued study. Echocardiography will play a key role in our understanding.
Footnotes
Dr. Pellikka has received support for research on a related topic from the Mayo Clinic Department of Cardiovascular Medicine and Ultromics (money paid to institution). Dr. Naqvi has reported that she has no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACCauthor instructions page.
References
- 1.Camus A., Gilbert S. 1st ed. A. A. Knopf; New York: 1948. The Plague. [Google Scholar]
- 2.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Inf Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim J., Volodarskiy A., Sultana R. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID-19. J Am Coll Cardiol. 2020;76:1965–1977. doi: 10.1016/j.jacc.2020.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szekely Y., Lichter Y., Taieb P. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation. 2020;142:342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dweck M.R., Bularga A., Hahn R.T. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 2020;21:949–958. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konstam M.A., Kiernan M.S., Bernstein D. Evaluation and management of right-sided heart failure: a scientific statement from the American Heart Association. Circulation. 2018;137:e578–e622. doi: 10.1161/CIR.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 7.Cullen M.W., Blauwet L.A., Vatury O.M. Diagnostic capability of comprehensive handheld vs transthoracic echocardiography. Mayo Clin Proc. 2014;89:790–798. doi: 10.1016/j.mayocp.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkpatrick J.N., Mitchell C., Taub C., Kort S., Hung J., Swaminathan M. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak: endorsed by the American College of Cardiology. J Am Coll Cardiol. 2020;75:3078–3084. doi: 10.1016/j.jacc.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 10.Singh M., Sethi A., Mishra A.K., Subrayappa N.K., Stapleton D.D., Pellikka P.A. Echocardiographic imaging challenges in obesity: guideline recommendations and limitations of adjusting to body size. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.014609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y., Li H., Zhu S. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. J Am Coll Cardiol Img. 2020 Apr 28 doi: 10.1016/j.jcmg.2020.04.014. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 13.Puntmann V.O., Carerj M.L., Wieters I. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 July 27 doi: 10.1001/jamacardio.2020.3557. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]