Abstract
Virus onslaughts continue to spread fear and cause rampage across the world every now and then. The twenty first century is yet again witnessing a gross global pandemic, Coronavirus Disease 2019 (COVID-19) caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Globally no vaccines or drug specific to COVID-19 is available. Corona viruses have been in mutual relationship with humans and other hosts over many decades though aggressive zoonotic strains have caused havoc. Zoonotic emergent corona viruses prior to SARS-COV-2 included severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), with the former leading to aggressive infectious spread and the later with high mortality rate. Although they emerged in the early period of the twenty first century, resilient biomedical and expertise in pharmaceutical domain could not appropriate any proprietary therapeutics. Studies envisaged towards curtailing their spread employed different stages of the virus life cycle with all zoonotic coronaviruses (CoVs) sharing genomic and structural similarities. Hence the strategies against SARS-CoV and MERS-CoV could prove effective against the recent outbreak of SAR-CoV-2. The review unravels key events involved in the lifecycle of SARS-CoV-2 while highlighting the possible avenues of therapy. The review also holds the scope in better understanding a broad-spectrum antivirals, monoclonal antibodies and small molecule inhibitors against viral glycoproteins, host cell receptor, viral mRNA synthesis, RNA-dependent RNA polymerase (RdRp) and viral proteases in order to design and develop antiviral drugs for SARS-CoV-2.
Keywords: SARS-CoV-2, COVID-19, Therapeutics, Antivirals, Antiviral targets
Graphical abstract
1. Introduction
Since the beginning of the twenty-first century, corona viruses have succeeded in their adaptive potential by traversing through host barrier to cause deadly diseases in humans. So far, they have evolved into three emerging viruses of zoonotic origin, severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). SARS-CoV, which was identified as the cause of severe acute respiratory syndrome (SARS) outbreak, was reported in the year 2002 in China, and within a short time it infected 8089 people from five continents to claim 774 lives (de Wit et al., 2016). Effective control measures facilitated in containing SARS-CoV by 2004 with no new reported cases till date. Saudi Arabia witnessed the emergence of Middle East respiratory syndrome (MERS), a viral respiratory syndrome caused by MERS-CoV, in 2012 spreading across 27 countries and killing 858 out of 2500 affected persons (de Wit et al., 2016). Though both SARS-CoV and MERS-CoV caused severe respiratory disease in humans with similar disease progression, the mortality rate of MERS-CoV was 35%, which was much greater than a 10% mortality rate with SARS-CoV (Petrosillo et al., 2020).
The emergence of a novel corona virus which was later named SARS-CoV-2 was first reported in the city of Wuhan, China during December 2019. SARS-CoV-2 infection causes coronavirus disease 2019 (COVID-19) which is predominantly characterized by respiratory distress of varying severity with a fatality rate of about 3%. By January 2020, the World Health Organization (WHO) declared that it is a Public Health Emergency of International Concern. Remarkably, SARS-CoV-2 and SARS-CoV is associated with milder infection and rapid transmission in the community, compared to MERS-CoV (Munster et al., 2020).
Precise vaccination or treatment is currently unavailable for CoVs though several therapeutics have been identified successfully in cell lines and animal models. Moreover, symptomatic treatments and control measures alone cannot dampen the severity of diseases or future virus spill overs. The lessons learnt from previous SARS-CoV and MERS-CoV outbreaks reveal a realm of research emphasizing the requirement to develop effective therapeutic measures against SARS-CoV-2. In this review, we describe various treatment modalities for SARS-CoV and MERS-CoV and the scientific avenues that could be employed for the development of drugs towards prevention and control of SARS-CoV-2 infection and COVID-19. This review also draws attention to prospects and challenges likely to be confronted by the scientific and biomedical community during the development of therapeutics for SARS-CoV-2.
2. Origin of coronaviruses
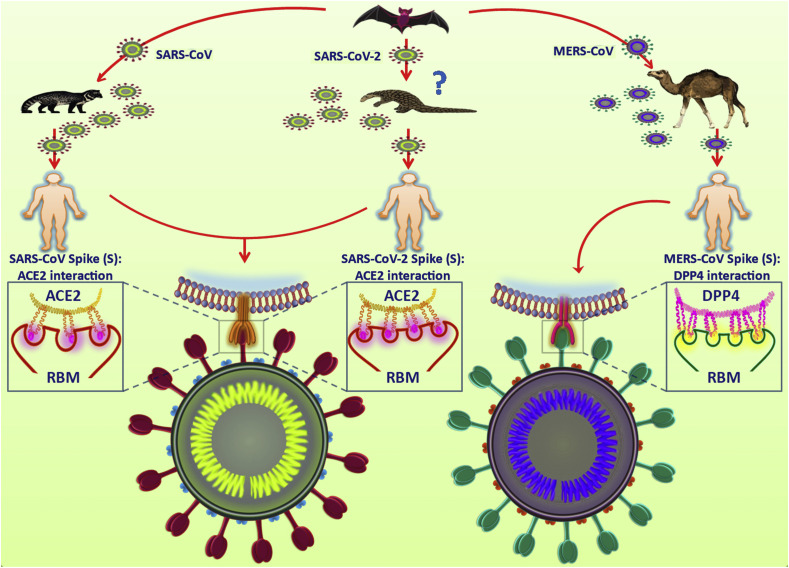
Bats are often considered to be the primary reservoir of all zoonotic CoVs (Cui et al., 2019; Lau et al., 2005; Li et al., 2005b). Civets and camels serve as intermediate hosts for SARS-CoV and MERS-CoV respectively (Gong and Bao, 2018). SARS-CoV-2's similarity to SARS-like bat CoVs potentiates a possible transmission route of SARS-CoV-2 from bats (Zhou et al., 2020). As SARS-like bat CoVs cannot directly infect humans, mutational changes in an intermediate host is inevitable. Reports on the similarity between S1 protein of Pangolin-CoV and SARS-CoV-2 has suggested a possibility of pangolins as intermediate host for SARS-CoV-2, though further evidences are required to prove this concept (Zhang et al., 2020) (Fig. 1 ).
Fig. 1.
Zoonosis of corona viruses. Bats are the primary reservoirs for the three zoonotic corona viruses, SARS-CoV, SARS-CoV-2 and MERS-CoV. Human transmissions often occur through intermediate hosts. Civets and dromedary camels serve as intermediate hosts for SARS-CoV and MERS-CoV, respectively while intermediate host for SARS-CoV-2 is yet to be confirmed but are anticipated to be pangolins. For cellular entry, the receptor binding domain (RBM) of SARS-CoV S protein interacts with the host ACE2 receptor. SARS-CoV-2 also utilizes the same RBM-ACE2 receptor interaction but possesses a greater binding affinity compared to SARS-CoV. The entry of MERS-CoV is mediated by the interaction of RBM with the host receptor DPP4.
From a phylogenetic point of view, SARS-CoV-2 shares 96.2% genomic similarity with SARS-like bat CoV while SARS-CoV and MERS-CoV are 79% and 50% identical to SARS-CoV-2, respectively (Ren et al., 2020). This suggests an unequivocal origin of SARS-CoV-2 from bats, as in the outbreaks of SARS-CoV and MERS-CoV. However, similar to SARS-CoV, the possibility of human transmission in SARS-CoV-2 is assumed to be from an animal market in China, though the probability of an intermediate host remain unsubstantiated (Walls et al., 2020b). Though studies report similar genomic structures among these CoVs, they differ in exploiting receptors to enter host cell. MERS-CoV uses dipeptidyl peptidase 4 (DPP4) while SARS-CoV and SARS-CoV-2 shares the same receptor angiotensin-converting enzyme 2 (ACE2) for cellular entry with latter having higher affinity to receptor (Wan et al., 2020).
3. SARS-CoV-2
3.1. Structure and replication
SARS-CoV-2 is a spherical virus possessing a positive sense RNA genome of 29.9 kb in size (Wu et al., 2020c). CoVs possess 15 non-structural proteins which are essential for viral replication and four structural proteins, spike (S) glycoprotein, small envelope (E) protein, matrix (M) protein and nucleocapsid (N) proteins (Cui et al., 2019). The RNA genome along with N proteins form the nucleocapsid which is surrounded by an envelope. The M proteins provide shape to the virus whereas the heavily glycosylated S proteins used for cellular entry are embedded on the surface of the virus like a crown, hence the name coronavirus (Siu et al., 2008).
Receptor recognition plays a significant role in cell tropism and forms the primary step of any viral infection. During entry of the virus to human cells, the S glycoprotein of SARS-CoV-2 attaches to the cellular receptor human angiotensin-converting enzyme 2 (ACE2), which is a single pass transmembrane protein found to be expressed in lung, heart, kidney, intestine and testis (Tipnis et al., 2000). The S protein comprises of 2 functional domains, S1 and S2, responsible for receptor binding and membrane fusion respectively (Zhang et al., 2014). Receptor binding domain (RBD) on the S1 domain binds to ACE2 receptor, a major factor influencing the tropism of virus (Kuo et al., 2000). Studies have reported six RBD amino acids to be critical for binding in SARS-CoV, of which none has been reported to be substituted in SARS-CoV-2, however few other amino acids in the RBD have been modified in SARS-CoV-2 (Wu et al., 2020a). Structural analysis confirmed the binding of SARS-CoV-2 with ACE2 at 10 folds greater affinity compared to SARS-CoV which clarifies the efficient transmission of SARS-CoV-2 in humans (Wrapp et al., 2020). Immediately after binding, a conformational change is triggered in S2 domain exposing the fusion peptide mediating the virus-cell membrane fusion eventually delivering the capsid into the cytoplasm (Masters, 2006) (Fig. 2 ).
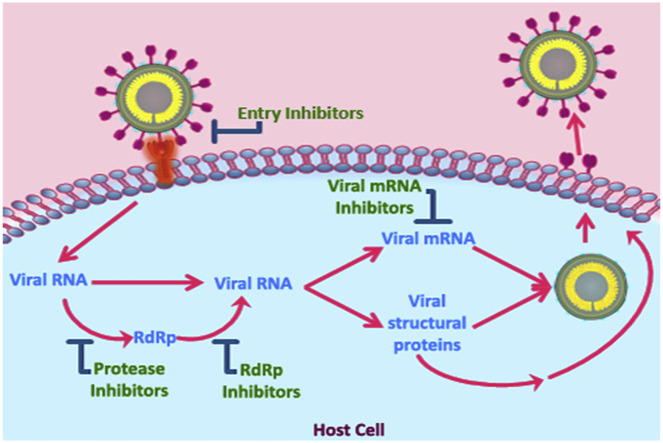
Fig. 2.
Schematic diagram of SARS-CoV-2 life cycle and potential antiviral drug targets based on life cycle. SARS-CoV-2 life cycle comprises several stages. 1) Attachment of the viral S glycoprotein RBM with cellular receptor ACE2 to facilitate entry into the host cell. 2) Disassembly of SARS-CoV-2 to release the RNA into the cytoplasm of the host cell. 3) The viral RNA is translated into replicase polyproteins, which are further cleaved by the viral proteases, papain like protease (PLpro) and 3C like protease (3CLpro) to produce non-structural proteins (nsps). 4) The viral RNA also acts as a template for synthesis of negative sense RNA which subsequently converts to positive sense genomic RNAs. 5) RdRp, one of the nsps, get involved in non-contiguous transcription to produce subgenomic RNAs which are consequently translated into the viral structural proteins. 6) The RNA genome and structural proteins assemble to form new virions. 7) Subsequently mature virions are released from the host cell. Bar-headed lines and red fonts indicate potential antiviral drug targets-viral spike protein/host cellular receptors, protease inhibitors, RdRp inhibitors, and mRNA synthesis inhibitors-that can block different stages of viral life cycle.
Once inside the cytoplasm, the viral RNA uncoats and translates into polyproteins pp1a and pp1ab that encodes non-structural proteins (Fang et al., 2008). Subsequently, polyproteins pp1a is cleaved into 11 non-structural proteins (nsp1-nsp11) while 15 non-structural proteins (nsp1-nsp10 and nsp12-nsp16) are produced due to the cleavage of pp1ab. nsp3 and nsp5 regulate the autoproteolytic cleavage while RNA-dependent RNA polymerase (RdRp) is positioned within nsp12. Mutations in nsp2 and nsp3 may affect the infectious ability and differentiation mechanism of SARS-CoV-2 (Fung and Liu, 2014). In addition to translating polyproteins, the viral RNA also acts as a template to synthesize negative sense genomic RNA which is further used as template to produce positive sense RNA genomes. On the other hand, subgenomic RNAs are produced as a result of non-contiguous transcription of the genome which are later translated into structural proteins (Sawicki et al., 2007). During non-contiguous transcription, RdRp is expected to jump from one template to another resulting in CoV recombination which can play a significant role in virus evolution (Wu and Brian, 2010). The viral assembly occurs in the endoplasmic reticulum-Golgi compartment of the host cell, and the virions are transported to the plasma membrane via vesicles to ultimately release the mature viruses (Masters, 2006; Siu et al., 2008).
3.2. Diagnosis
With the continuous spread of COVID-19 in a tremendous rate, the most important challenge faced by the global health community is rapid and early detection of SARS-CoV-2 positive cases. Various diagnostic methods employed to detect the presence of SARS-CoV-2 are nucleic acid detection tests, immunological tests and imaging techniques. Of which, detection of viral RNA using real time reverse transcriptase PCR (RT-PCR) is considered the most accurate and reliable diagnostic test often conducted using nasopharyngeal or upper respiratory tract swabs (Sethuraman et al., 2020). S, E, N, RdRp/ORF1ab genes of SARS-CoV-2 are the different targets used for conducting real-time PCR (Corman et al., 2020; Tang et al., 2020). For the primary screening of positive cases, WHO recommends to test the target E with subsequent confirmation with RdRp primers as two target assays are most preferred for efficient diagnosis (Corman et al., 2020). Though considered the gold standard in COVID-19 tests, false negatives may occur due to sampling errors or untimely sample collection during a real-time PCR assay (Sethuraman et al., 2020).
Immunological test is an indirect detection method by measuring the antibodies generated due to the host immune response against SARS-CoV-2 infection. Studies have reported the presence of viral specific Immunoglobulin G (IgG) and Immunoglobulin M (IgM) in high levels during the second and third week after infection (Sethuraman et al., 2020). Furthermore, serological diagnosis based on IgG and IgM enzyme-linked immunosorbent assay (ELISA) are reported to have high specificity for detection of COVID-19 (Xiang et al., 2020). However these test are qualitative and can only show the presence or absence of virus specific antibodies but is often considered an important tool to study the prevalence of COVID-19 and spread rate in a community. Even though real-time PCR is the most reliable method of detection, radiological evaluation like the chest computed tomography (CT) scan is also considered a standard diagnosis method of COVID-19 for pneumonia detection (Li and Xia, 2020). However, CT scan is limited to detect any specific viral disease but has proved necessary to ensure early detection and control of transmission during a pandemic situation.
3.3. Current treatment modalities for SARS-CoV-2
No specific vaccines or anti-viral drugs are discovered for the control of SARS-CoV-2 as yet. Therefore, symptomatic treatment is the mainstay of clinical handling at present. Though a number of anti-viral drugs such as remdesivir, arbidol, lopinavir/ritonavir and many more are available for the treatment of SARS-CoV-2, none of them are considered the most potential therapeutics till date (Jin et al., 2020; Wang et al., 2020a).
Remdesivir, an adenosine analogue, has shown anti-viral activity against many RNA viruses including Ebola, SARS-CoV and MERS-CoV (Lo et al., 2019; Sheahan et al., 2017; Warren et al., 2016). It interferes with the viral RdRp and integrates into the nascent viral RNA resulting in premature termination. In addition, it was reported to effectively restrain SARS-CoV-2 in vitro (Wang et al., 2020a). However, its efficacy and side effects in patients need to be substantiated by clinical trials. Arbidol, an indole derivative broad spectrum anti-viral, affects various stages of viral life cycle, particularity targeting virus associated cellular host molecules or viral proteins (Blaising et al., 2014). Arbidol blocks the viral fusion process in influenza virus whereas it inhibits viral attachment and vesicle trafficking in hepatitis C virus (Blaising et al., 2013; Kadam and Wilson, 2017). Similarly, in vitro studies have also reported arbidol's activity to interfere with attachment and vesicular trafficking in SARS-CoV-2 potentiating its candidature for the treatment of COVID-19 though in vivo studies and clinical trials are yet to be accomplished (Wang et al., 2020b). An additional candidate used for the treatment of COVID-19 is a combination of HIV protease inhibitors, lopinavir and ritonavir. They have reported to bind on the target site of M protease (MPro) to supress its activity in SARS-CoV. Treatment with lopinavir and ritonavir could also improve the condition of marmosets infected with MERS-CoV (Chan et al., 2015; Yao et al., 2020). Moreover, they were found to be effective on COVID-19 patients, validating them as potential drug candidates though their potency need to be validated by clinical trials (Lim et al., 2020). Chloroquine, an anti-malarial drug that increases endosomal pH is used as a treatment option against COVID-19. It is reported to increase the endosomal pH required for virus-cell membrane fusion and also interrupts with the glycosylation of host cell receptors of SARS-CoV (Savarino et al., 2003). Moreover, chloroquine also holds promise as an autophagy inhibitor along with its reported anti-tumor properties (Golden et al., 2015). In Vero-E6 cells, chloroquine functioned at both entry, and at post-entry stages of the SARS-CoV-2 infection categorizing its role as a potent COVID-19 drug (Wang et al., 2020a).
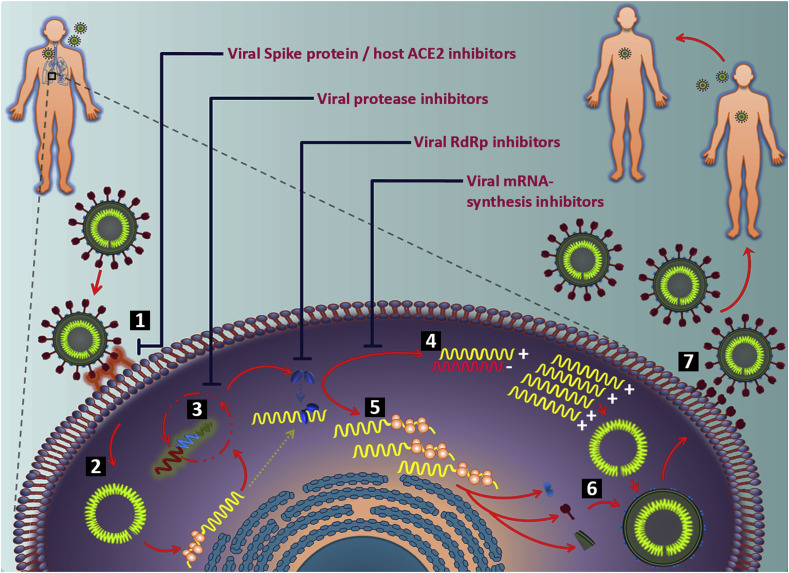
4. Research scope
In an era of emerging novel viruses, the process of developing antiviral drugs is complex yet is of paramount importance for sustenance of mankind. Adversely, the complexity worsens as viruses with lower mortality or comorbidities evolve and re-emerge to elude current therapeutic strategies as observed in the case of SARS-COV-2. Since the discovery of first antiviral drug, a few novel drugs were established to be therapeutically effective and safe but none reckoned for the treatment of CoVs (De Clercq and Li, 2016). Developing antiviral drugs include strategies like screening of existing therapeutic molecule databases, prevailing broad-spectrum antivirals or even de novo synthesis of drugs by harnessing the viral genomic characteristics (Zumla et al., 2016). Systematic analysis have identified significant and potential antiviral targets against SARS-COV-2 like viral spike protein (S), host cellular ACE2 receptor, viral genomic RNA, moieties included in viral mRNA synthesis like the RdRp, replication complex and viral proteases (Wu et al., 2020). Furthermore, many antiviral drugs and small molecules have been proven to block SARS-CoV and MERS-CoV in preclinical studies, while their treatment potency are argued due to meagre results from human clinical trials. Considering the structural and genomic similarity of SARS-CoV-2 to SARS-CoV and MERS-CoV, repurposing the existing drugs may imply a practical solution to ramp down the recent pandemic outbreak. Numerous novel possibilities can be envisaged to prevent and treat COVID-19, such as viral glycoprotein and viral receptor targeted drugs and antibodies, small molecule inhibitors, siRNAs, viral mRNA and replicase targeted drugs, viral protease targeted drugs, and vaccines. However, novel therapeutic interventions entail a prolonged period of time for screening and development.
4.1. Anti-viral drugs targeting viral glycoproteins and viral receptors
A promising target for the treatment of COVID-19 is the viral S glycoprotein. The most variable part of S protein is the RBD domain which is essential for interaction with ACE2 (Wu et al., 2020c; Zhou et al., 2020). Peptide drugs targeting SARS-CoV-2 specific RBD domains could possibly block the RBD-ACE2 interaction during SARS-CoV-2 infection. Consistently, a polypeptide containing two RBD-binding motifs of ACE2 displayed strong inhibitory effects on SARS pseudo virus entry into HeLa cells expressing ACE2 with an IC50 of ~100 μM (Han et al., 2006).
The polybasic cleavage site at the junction of S1 and S2 domains of S protein constitutes another notable target for anti-viral agents as effective cleavage at this site determines the infectivity of SARS-CoV-2 (Walls et al., 2020a). Reports on synthetic peptides derived from cleavage site sequences exhibited inhibitory action against the GZ50 strain of SARS-CoV infection in fetal rhesus kidney cells (Zheng et al., 2005). Therefore, synthesis of such peptides specific to the SARS-CoV-2 polybasic cleavage site can impede the production of functional S1 and S2 domain eventually blocking the fusion of virus-host membranes.
The heptad repeat (HR) domains, HR1 and HR2 in the S2 domain form a six helix bundle fusion core which supports membrane fusion by bringing the cellular and viral membranes into close vicinity. (Gao et al., 2013). During the fusion process, three conformational stages are believed to occur in the CoV HR motifs: pre-fusion native state, a pre-hairpin intermediate state, and a stable post-fusion hairpin state (Eckert and Kim, 2001). The formation of six helix bundle fusion core represents the post-fusion hairpin state. However, prior to this state, both HR1 and HR2 should probably be exposed in an intermediate state where they could function as target for anti-viral therapeutics (Eckert and Kim, 2001). Based on this theory, a number of inhibitory peptides have been designed to stop viral infections of HIV, Ebola and SARS-CoV (Bosch et al., 2004; Shi et al., 2016; Watanabe et al., 2000). One of such peptides that overlapped HR2 could bind and interact with HR1 to form a stable six helix fusion core thereby inhibiting SARS-CoV infection in Vero E6 cells (Liu et al., 2004). A peptide derived from HR2 domain of human coronavirus OC43 (HCoV-OC43) demonstrated fusion inhibition property against many HCoVs (Xia et al., 2019). Nevertheless, these peptides showed poor anti-viral activity compared to the anti-HIV peptides, the reason was attributed to the capability of SARS-CoV S protein HR2 region to form the trimeric coiled-coil which was absent in other class I viral fusion proteins (Du et al., 2009). However, increasing the binding affinity of HR2 peptide with HR1 to form six helix bundle fusion core while reducing the formation of trimer could escalate the antiviral efficiency. Additionally, SARS-CoV-2 exhibits much higher capacity of membrane fusion than SARS-CoV, suggesting the fusion machinery of SARS-CoV-2 as potential target (Xia et al., 2020). In a recent study, a lipopeptide EK1C4, derived from EK1 targeting the HR1 domain was reported to be highly effective against SARS-CoV-2 membrane fusion and pseudovirus infection (Xia et al., 2020). Another group of carbohydrate binding drugs that inhibits SARS-CoV in vitro and in vivo by binding to the viral surface glycoprotein are Griffithsin isolated from the red alga Griffithsia (Barton et al., 2014; O'Keefe et al., 2010). Soluble forms of ACE2 were also demonstrated to block the infection of SARS-CoV with comparable affinities to monoclonal antibodies (Li et al., 2003; Sui et al., 2004). However, to increase the lifespan of such circulating molecule, it is recommended to attach an immunoglobulin Fc domain which alters soluble ACE2 into an immunoadhesin format (Kruse, 2020). Though all the above mentioned peptide drugs are effective in in vitro, the in vivo anti-viral efficacy should be estimated in animal models for further therapeutic development. In addition to the efficacy, the optimum delivery methods and safety profiles of the above mentioned drugs should also be evaluated.
Another potential approach to curtail COVID-19 would be to target the host receptor, ACE2. A few added advantages of targeting ACE2 are that the host protein cannot alter thereby chances of escaping from therapeutic agent are abated. Furthermore, the capability of SARS-CoV-2 to mutate and bind to a different host receptor during an ongoing outbreak is beyond scope. Peptides drugs corresponding to SARS-CoV-2 RBD domain that can successfully bind to ACE2 is an important therapeutic realm to be studied. It has been shown that SARS-CoV infection in Vero cells were inhibited by an RBD overlapping peptide sequence by hindering RBD-ACE2 interaction at an IC50 of ~40 μM (Hu et al., 2005). Similarly a 193 residue fragment of S protein was reported to block the S protein mediated interaction of SARS-CoV by binding on to ACE2 receptor in cell culture (Wong et al., 2004). Enhanced tissue penetration and effective receptor binding could be accomplished by the production of nanobodies as a therapeutic agent (Arbabi-Ghahroudi, 2017). An rRBD protein attached to Fc fragment was demonstrated to effectively block MERS-CoV infection in MERS-CoV specific receptor expressing cells (Du et al., 2013).
4.2. Antibodies targeting viral glycoproteins and viral receptors
Convalescent plasma (CP) therapy is an adaptive immunotherapy that has been used to prevent and treat many contagious diseases. CP therapy is considered to be a promising treatment strategy for COVOD-19 due to the resemblance of viral and clinical characteristics among SARS-CoV, MERS-CoV, and SARS-CoV-2. The donor source of CP therapy are those patients who have recovered from COVID-19 with high neutralizing antibody titers. CP therapy involves transfer of sera containing anti-SARS-CoV-2 antibodies that can block the virus infection and will aid in viral clearance (Rojas et al., 2020). A study of CP therapy in few severe COVID-19 patients in China, showed a decrease in viremia within seven days of treatment with no adverse effects (Duan et al., 2020). Though the therapy was successful and well tolerated by patients, further investigations regarding optimum dose and time points need to be conducted. Contrastingly, some in vitro and in vivo studies have demonstrated disease worsening due to treatment conducted with low titre blood products (Weingartl et al., 2004; Yang et al., 2005b).
To tackle these issues, neutralizing antibodies like monoclonal antibodies (mAbs), their functional antigen-binding fragment (Fab), the single-chain variable region fragment (scFv), or single-domain antibodies (nanobodies), targeting various regions of CoVs have been produced by cloning scFv or Fab from convalescent patients, immortalization of B cells from convalescent patients or immunizing human immunoglobulin into transgenic mice (Coughlin and Prabhakar, 2012; Traggiai et al., 2004). mAbs are a dominant approach of therapeutic intervention which is predominantly significant for viruses in which the neutralizing antibody reaction is vital for protection (Berry, 2005). Majority of these mAbs target the S1 subunit to block the RBD-ACE2 binding while some inhibit the membrane fusion by binding to the S2 subunits. Apart from the common method of using mice and other animals for selecting lead molecules, the faster phage or yeast display methods could be employed in the current pandemic situation (Keck et al., 2019; Shin et al., 2019). However, laborious in vitro and in vivo methods should be performed to confirm the therapeutic efficiency.
Spike protein specific neutralizing human mAbs, 80R and CR3014, inhibited S protein-ACE2 interaction thereby neutralizing the infection by SARS-CoV strains Tor2 and HKU39849 (Sui et al., 2005). Similarly, infection caused by human SARS-CoV strains Urbani, Tor2 and GD03 could also be neutralized by mAbs, m396 and S230.15 (Zhu et al., 2007). Interestingly, CR3014 and m396 were unsuccessful in neutralizing SARS-CoV-2 infections as they failed to bind to the S protein indicating that the cross reactivity seen in mAbs could be due to the difference in the RBD domains of SARS-CoV and SARS-CoV-2 (Tian et al., 2020). Therefore development of mAbs specific for SARS-CoV-2 is crucial in controlling the disease. Moreover, CR3022, a SARS-CoV specific mAb isolated from blood of a convalescent SARS patient was found to interact with the RBD domain of SARS-CoV-2 (Tian et al., 2020). Mouse mAbs are also used for initial and urgent treatment of CoV infections. However repeated use of mouse mAbs can cause human-anti mouse antibody response which subsequently clears the mAb from blood to lessen the therapeutic effect. Mouse mAbs generated using rRBD and inactivated SARS-CoV could inhibit RBD binding and also neutralized pseudoviruses of human SARS-CoV strains Tor2 and GD03T13 (He et al., 2005, 2006). Several MERS-CoV neutralizing antibodies like MERS-27, MERS-GD27, hMS-1, Mersmab1 were reported to recognize RBD epitopes to block infection while SAB-301, isolated from transchromosomic cattle has been evaluated in Phase I clinical trials (Zhou et al., 2019). Apart from isolating mAbs from mice few researchers have used large animals like rhesus macaques, llama and camel for isolating antibodies. Rhesus macaques immunized with combined DNA and protein vaccines isolated a group of mAbs with inhibitory effects that targeted both MERS-CoV RBD and non-RBD S1 region of the S glycoprotein (Wang et al., 2018). Nanobodies targeting RBD isolated from camels immunized with MERS-CoV S protein had potent neutralization capabilities (Stalin Raj et al., 2018). Till now, no SARS-CoV-2 specific antibodies have been reported, though once produced they will have to undergo in vitro evaluation for neutralizing capabilities and in vivo testing for efficacy and safety prior to the approval for clinical trials.
Another approach to prevent COVID-19 would be to deliver antibodies against ACE2 receptors. ACE2 could be effectively blocked by scFv or nanobodies, however, without Fc domain these molecules show shorter half-life. Furthermore, ACE2 binding antibody would eliminate the concern of viral escape which is an advantage over S protein specific antibodies (Kruse, 2020).
4.3. Small molecule inhibitors and siRNAs targeting viral glycoproteins
Studies on several small molecules targeting S protein have been reported, emphasizing the fact that small molecules and other compounds can be effective in inhibiting SARS-CoV-2. Previously, two small molecules tetra-o-galloyl-beta-d-glucose (TGG) and luteolin were identified to block the entry of SARS-CoV into Vero cells while 18 small molecules were also reported to target the S protein- ACE2 mediated viral entry (Kao et al., 2004; Yi et al., 2004). A strong repressive activity of VE607 was observed on SARS-CoV pseudovirus entry into ACE2-expressing 293T cells (Kao et al., 2004). Even though these small molecule inhibitors are effective in vitro, further in vivo studies on efficacy and optimal concentration for drug delivery should be evaluated. Spike specific small interfering RNA (siRNA) repressed SARS-CoV replication in virus-infected Vero E6 cells by RNA interference, enlightens with another potent target for the development of SARS-CoV-2 drugs (Wu et al., 2005).
4.4. Anti-viral drugs targeting viral mRNA synthesis and replicase
The viral RdRp is another promising therapeutic target of SARS-CoV-2 that does not cause any significant side effects in the host. Repurposing the existing therapeutic drugs can also be considered as an accomplishable strategy. Remdesivir, HIV reverse transcriptase inhibitor and one of the approved drugs for treatment during the COVID-19 outbreak was shown effective in animal models against SARS-CoV and MERS-CoV and is also under phase III clinical trials for the treatment of Ebola virus (de Wit et al., 2020; Sheahan et al., 2017). Favipiravir, a broad spectrum anti-viral drug has gained its attention as a potentially promising drug for specifically untreatable RNA viral infections due to its unique profile. This purine nucleotide analogue undergoes intracellular phosphoribosylation to an active favipiravir-ribofuranosyl-5′-triphosphate (RTP) form, thus acting as a substrate and inhibits RdRp activity and is already under clinical trials for COVID-19 (Furuta et al., 2017; Mifsud et al., 2019). Since various types of RNA viruses share similar catalytic domain, favipiravir can be considered as a therapeutic strategy to prevent COVID-19. A fleximer nucleoside analogue designed based on the acyclic sugar scaffold of acyclovir were capable of inhibiting Human coronavirus NL63 (HCoV-NL63) and MERS-CoV replication in cell culture by disrupting viral replication through an imprecise mechanism of action (Peters et al., 2015). The RdRp inhibitor guanosine analogue, ribavirin, is also a potent drug candidate for COVID-19 as ribavirin treatment in combination with interferons has shown to be effective during MERS-CoV and SARS-CoV infections (Omrani et al., 2014; Saijo et al., 2005). However the therapeutic efficiency of all these RdRp inhibitor drugs in COVID-19 patients must be estimated by clinical trials. K22, a compound targeting the CoV replication complex, inhibited a broad range of CoV RNA synthesis in vitro (Lundin et al., 2014). A chimeric RNA-DNA ribozyme targeting the loop region of SARS-CoV could considerably minimize the expression of recombinant SARS-CoV RNA in cell culture. Conversely, delivery of ribozymes in humans has to be optimized for productive clinical procedure as they were rapidly degraded in vivo (Fukushima et al., 2009). In addition to ribozymes, a chimeric protein, dsRNA-activated caspase oligomerizer (DRACO) possessing viral dsRNA binding domain that selectively kill cells harboring viral dsRNA was found to be effective against many RNA viruses (Rider et al., 2011). Such broad spectrum antivirals that potentially target the viral RNA sequences could also be employed as an effective therapeutic strategy against COVID-19. Production of mAbs against RdRp or nsp12, a fundamental component that plays a central role in the replication and transcription of SARS-CoV-2, along with the support of nsp7 and nsp8 has to be studied in detail.
4.5. Anti-viral drugs targeting viral proteases
SARS-CoV-2 genome encodes two types of proteases 3C-like protease (3CLpro) also called main protease (Mpro) and papain-like protease (PLpro) which are necessary for the cleavage of viral polyproteins. They are considered perfect drug targets as they are distinct from host cellular proteases. Protein sequence similarity of 96% has been identified between SARS-CoV Mpro and SARS-CoV-2 Mpro (Chen et al., 2020). HIV protease inhibitors, lopinavir and ritonavir have been reported to effectively inhibit SARS-CoV and MERS-CoV though their efficacy should be evaluated through clinical trials (Chan et al., 2015; Yao et al., 2020). Therefore, substantiated clinical trial studies can establish lopinavir and ritonavir as an effective treatment against COVID-19. Additionally, few peptidomimetic chymotrypsin-like protease inhibitors were found to inhibit SARS-COV at high concentrations (Ghosh et al., 2005). Two PLpro targeting compounds, 6-mercaptopurine and 6-thioguanine, were reported to inhibit SARS-CoV and MERS-CoV while mycophenolic acid effectively blocked replication of MERS-CoV in vitro (Cheng et al., 2015; Hart et al., 2014). Benzodioxole was found to inhibit PLpro, whereas zinc and its conjugates was reported to inhibit the activity of both PLpro and 3CLpro of SARS-CoV (Baez-Santos et al., 2014; Han et al., 2005). Small molecule inhibitors of 3CLpro like ML188, ML300 and N3 have also reported inhibitory effects (Jacobs et al., 2013; Turlington et al., 2013; Yang et al., 2005a). Additionally, mycophenolic acid did not exhibit any effect on marmosets, whereas the efficiency of other molecules are yet to be tested in animal models (Chan et al., 2015) (Table 1 ). However, high levels of functional complexities mark SARS-CoV-2 proteases a significant target for therapeutic interventions.
Table 1.
Potential antivirals against corona viruses and their therapeutic targets and mechanisms of action.
| Anti-virals | Anti-viral targets | Mechanism of action | References | |
|---|---|---|---|---|
| Viral S glycoprotein | P6 peptide | RBD of S protein | Binds to S protein preventing the entry of virus | Han et al. (2006) |
| 20-mer synthetic peptides | Cleavage site of S protein | Block the production of functional S1 and S2 domain | Zheng et al. (2005) | |
| EK1 | HR1 | Inhibits fusion of S protein with host receptor | Xia et al. (2019) | |
| EK1C4 peptide | HR1 | Inhibits fusion of S protein with host receptor | Xia et al. (2020) | |
| CP-1 | HR2 | Interacts with HR1 inhibiting viral infection | Liu et al. (2004) | |
| Griffithsin | S protein | Binds to S protein blocking virus –host interaction | (Barton et al., 2014; O'Keefe et al., 2010) | |
| Soluble ACE2 | S1 subunit of S protein | Blocks S1 subunit of S protein | (Li et al., 2003; Sui et al., 2004) | |
| 80R, CR3014, m396, S230.15, CR3022, MERS-27, MERS-GD27, hMS-1, Mersmab1 | RBD of S protein | Blocks interaction with the host receptor | (Sui et al., 2005; Tian et al., 2020; Zhou et al., 2019; Zhu et al., 2007) | |
| TGG and luteolin | S protein | Binds to S protein and blocks entry into the host cell | Yi et al. (2004) | |
| VE605 | S protein | Blocks entry into the host cell | Kao et al. (2004) | |
| siRNA |
S protein |
Inhibits the expression of S protein |
Wu et al. (2005) |
|
| Host cell receptor | S471-503 peptide | ACE2 | Blocks binding between RBD and host receptor | Hu et al. (2005) |
| 193aa fragment (318–510) | ACE2 | Blocks S protein mediated viral infection | Wong et al. (2004) | |
| Recombinant S377-588-Fc |
DPP4 |
Blocks binding of viral RBD to host receptor |
Du et al. (2013) |
|
| Viral mRNA synthesis and replicase | Remdesivir | RdRp | Inhibits viral RNA synthesis | Sheahan et al. (2017) |
| Favipiravir | RdRp | Inhibits viral RNA synthesis | (Furuta et al., 2017; Mifsud et al., 2019) | |
| Fleximer nucleoside analogue | RdRp | Inhibits viral replication | Peters et al. (2015) | |
| Ribavirin | RdRp | Inhibits viral RNA synthesis | (Omrani et al., 2014; Saijo et al., 2005) | |
| K22 | mRNA | Inhibits viral RNA synthesis | Lundin et al. (2014) | |
| Ribozymes | mRNA | Inhibits viral RNA synthesis | Fukushima et al. (2009) | |
| DRACO |
Viral dsRNA |
Kills cells containing dsRNA |
Rider et al. (2011) |
|
| Viral proteases | Lopinavir and ritonavir | 3CLpro | Inhibits the activity of 3CLpro | (Chan et al., 2015; Yao et al., 2020) |
| 6-mercaptopurine and 6-thioguanine | PLpro | Inhibits the activity of PLpro | Cheng et al. (2015) | |
| Mycophenolic acid | PLpro | Blocks replication of virus | Hart et al. (2014) | |
| Benzodioxole | PLpro | Inhibits the activity of PLpro | Baez-Santos et al. (2014) | |
| ML188, ML300 and N3 | 3CLpro | Inhibits the activity of 3CLpro | (Jacobs et al., 2013; Turlington et al., 2013; Yang et al., 2005a) |
4.6. Bioactive compounds against coronaviruses
Numerous bioactive natural products have also been reported to show anti-viral activities against SARS-CoV, MERS-CoV and SARS-CoV-2. Bioactive compounds ginsenoside-Rb1 isolated from Panax ginseng and aescin isolated from Aesculus hippocastanum were reported to possess anti-SARS-CoV properties (Wu et al., 2004). Similarly, pharmacologically active compounds reserpine, leptodactylone and lycorine shown to be effective against SARS-CoV were extracted from Rauwolfia serpentina, Boenninghausenia sessilicarpa and Lycoris radiate respectively (Li et al., 2005a; Wu et al., 2004; Yang et al., 2007). MERS-CoV infection was inhibited by resveratrol and dihydrotanshinone, a lipophilic compound isolated from Salvia miltiorrhiza (Kim et al., 2018; Lin et al., 2017). Few other natural compounds like celastrol, tingenone, pristimererin, iguesterin isolated from Triterygium regelii inhibited the activity of SARS-CoV 3CLpro while PLpro activity was blocked by tanshinones I–VII and hirsutenone (Park et al., 2012a, 2012b; Ryu et al., 2010). In addition, a bioactive compound emodin extracted from Rheum palmatum blocked the interaction of SARS-CoV S protein with ACE2 receptor (Ho et al., 2007). However, a recent study has reported an ACE2 inhibitory activity of cepharanthine extracted from Stephania japonica on SARS-CoV-2 related pangolin CoV infection (Fan et al., 2020). Furthermore, molecular docking studies have identified stilbene-based natural compounds and a potent Mpro inhibitor, oolonghomobisflavan-A from tea plant as promising candidates against SARS-CoV-2 (Bhardwaj et al., 2020; Wahedi et al., 2020) (Table 2 ).
Table 2.
Potential bioactive compounds against corona viruses and their mechanisms of action.
| Bioactive compound | Plant | Mechanism of action | References |
|---|---|---|---|
| Celastrol | Triterygium regelii | Inhibits 3CLpro | Ryu et al. (2010) |
| Tingenone | Triterygium regelii | Inhibits 3CLpro | Ryu et al. (2010) |
| Pristimererin | Triterygium regelii | Inhibits 3CLpro | Ryu et al. (2010) |
| Iguesterin | Triterygium regelii | Inhibits 3CLpro | Ryu et al. (2010) |
| Tanshinones I–VII | Salvia miltiorrhiz | Inhibits PLpro | Park et al. (2012b) |
| Hirsutenone | Alnus japonica | Inhibits PLpro | (Park et al., 2012a, 2012b) |
| Emodin | Rheum palmatum | Blocks the interaction of S protein and ACE2 | Ho et al. (2007) |
| Ginsenoside-Rb1 | Panax ginseng | Blocks glycoprotein | Wu et al. (2004) |
| Aescin | Aesculus hippocastanum | unknown | Wu et al. (2004) |
| Leptodactylone | Boenninghausenia sessilica | unknown | Yang et al. (2007) |
| Lycorine | Lycoris radiate | unknown | Li et al. (2005a) |
| Reserpine | Rauvolfia serpentina | unknown | Wu et al. (2004) |
| Dihydrotanshinone | Salvia miltiorrhiza | unknown | Kim et al. (2018) |
| Resveratrol | Polygonum cuspidatum | unknown | Lin et al. (2017) |
| Oolonghomobisflavan-A | Tea plant | Inhibits Mpro | Bhardwaj et al. (2020) |
| Cepharanthine | Stephania japonica | Blocks ACE2 | Fan et al. (2020) |
4.7. Drugs in clinical trials
Sudden onset of COVID-19 pandemic along with the challenge of discovering specific anti-viral drug against SARS CoV-2 has lead researchers to repurpose potent antiviral and non-antiviral drugs or their combinations to slow down the spread. Drugs repurposed for COVID-19 are still undergoing intensive preclinical and clinical trials and may prove effective in limiting the infection.
The first and foremost clinical trial study on repurposed drug for COVID-19 was with remdesivir on 61 patients with critical condition on compassionate use, although the study was criticized due to absence of control cases (Grein et al., 2020). This potent nucleotide analogue RdRp enzyme inhibitor prodrug has been found to support severe pulmonary cases with little advantage of shorter viral clearance period over placebo control group in a randomized double blind study on 237 infected patients (Wang et al., 2020c). Although the drug had adverse effects, a bigger cohort study of 1063 advanced stage critical patients, remdesivir aided advantage over placebo while had a marginal advantage on reducing mortality (Beigel et al., 2020). Being a non-specific drug, smaller advantage over other therapies could be accounted as a life saver, better combination of drugs are also suggested.
Favipiravir, another nucleoside analogue, had similar effects to that of remdesivir when administered in higher drug doses and supported with IFN-α. A non-randomized open label clinical trial with 80 patients on favipiravir demonstrated that COVID-19 patients could recover rapidly in contrast to Ritonavir/Lopinavir treated patients. Betterment of lung CT opacity was evidenced on favipiravir treatment (Cai et al., 2020). Similarly, broad spectrum purine analogue drug ribavirin in combination with lopinavir/ritonavir along with IFN-α supportive therapy could efficiently clear SARS-CoV-2 virus in phase 2 clinical trial with 127 patients, but the drug ribavirin alone had adverse effects like hypocalcemia, hemolytic anemia and hypomagnesemia (Hung et al., 2020). Ritonavir/Lopinavir both potent protease inhibitors known in use for HIV treatment were known to reduce SARS-CoV and MERS-CoV infection and thus had been a potent candidate of study on humans. Ritonavir/Lopinavir combination treatment in an open label trial of 199 patients improved recovery of critically infected case while failed in reducing mortality rates (Cao et al., 2020; Young et al., 2020).
Apart from the repurposed anti-viral drugs various monoclonal antibody, steroids and bioactive compound are being administered for immediate recovery of critically ill patients. Similarly, hydroxychloroquine and chloroquine although non-anti-viral drug has been used extensively as a first line treatment modality and various clinical trials are under progress evaluating the efficiency of such compounds and drugs towards effectively reducing the disease severity and recovery.
5. Conclusion
World economy and global industries plunged under the rampant spread of SARS-CoV-2 among populations. Unavailability of COVID-19 specific vaccines or drugs forced the authorities to repurpose drugs, however the current calamitous public health and unstable socio-economy, indispensably demands a scientific investigation for COVID-19 therapeutics. Considering genomic similarity of SARS-CoV-2 with SARS-CoV and MERS-CoV and their structural protein homology holds the avenues of therapeutics. Though clinical evaluation of drug candidates on young and naturally non-susceptible model animals often falsifies the outcome and in availing protection to the elderly population. Hence future testing should be mandated in proper and susceptible animal models thereby reducing escape mutants in mAbs as well as clinical trial failures. Additionally, combinatorial antiviral drugs or novel host acting broad spectrum antivirals could overcome impediments of dosage toxicities and immune evasion. Establishing more biosafety level containment facilities accompanied by meticulous research can benefit development of novel therapeutics and also limit future pandemics to greater extend. Nevertheless, ensuring critical research data exchange with data transparency along with proper preparedness and alertness among the global regulatory authorities and public awareness can effectively contain future viral outbreaks.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author agreement
All authors have read and approved the final version of the manuscript. The authors declare no conflict of interest.
CRediT authorship contribution statement
Gayathri Krishna: Writing - review & editing. Vinod Soman Pillai: Writing - review & editing. Mohanan Valiya Veettil: Conceptualization, Writing - review & editing.
Declaration of competing interest
The authors declare no conflict of interest.
References
- Arbabi-Ghahroudi M. Camelid single-domain antibodies: historical perspective and future outlook. Front. Immunol. 2017;8:1589. doi: 10.3389/fimmu.2017.01589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baez-Santos Y.M., Barraza S.J., Wilson M.W., Agius M.P., Mielech A.M., Davis N.M., Baker S.C., Larsen S.D., Mesecar A.D. X-ray structural and biological evaluation of a series of potent and highly selective inhibitors of human coronavirus papain-like proteases. J. Med. Chem. 2014;57:2393–2412. doi: 10.1021/jm401712t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton C., Kouokam J.C., Lasnik A.B., Foreman O., Cambon A., Brock G., Montefiori D.C., Vojdani F., McCormick A.A., O'Keefe B.R., Palmer K.E. Activity of and effect of subcutaneous treatment with the broad-spectrum antiviral lectin griffithsin in two laboratory rodent models. Antimicrob. Agents Chemother. 2014;58:120–127. doi: 10.1128/AAC.01407-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.D., Ruiz-Palacios G.M., Benfield T., Fatkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C., Members A.-S.G. Remdesivir for the treatment of covid-19 - preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J.D. Rational monoclonal antibody development to emerging pathogens, biothreat agents and agents of foreign animal disease: the antigen scale. Vet. J. 2005;170:193–211. doi: 10.1016/j.tvjl.2004.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj V.K., Singh R., Sharma J., Rajendran V., Purohit R., Kumar S. Identification of bioactive molecules from tea plant as SARS-CoV-2 main protease inhibitors. J. Biomol. Struct. Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1766572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaising J., Levy P.L., Polyak S.J., Stanifer M., Boulant S., Pecheur E.I. Arbidol inhibits viral entry by interfering with clathrin-dependent trafficking. Antiviral Res. 2013;100:215–219. doi: 10.1016/j.antiviral.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Blaising J., Polyak S.J., Pecheur E.I. Arbidol as a broad-spectrum antiviral: an update. Antiviral Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch B.J., Martina B.E., Van Der Zee R., Lepault J., Haijema B.J., Versluis C., Heck A.J., De Groot R., Osterhaus A.D., Rottier P.J. Severe acute respiratory syndrome coronavirus (SARS-CoV) infection inhibition using spike protein heptad repeat-derived peptides. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8455–8460. doi: 10.1073/pnas.0400576101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., Liao X., Gu Y., Cai Q., Yang Y., Shen C., Li X., Peng L., Huang D., Zhang J., Zhang S., Wang F., Liu J., Chen L., Chen S., Wang Z., Zhang Z., Cao R., Zhong W., Liu Y., Liu L. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020 doi: 10.1016/j.eng.2020.03.007. (Beijing) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Yao Y., Yeung M.L., Deng W., Bao L., Jia L., Li F., Xiao C., Gao H., Yu P., Cai J.P., Chu H., Zhou J., Chen H., Qin C., Yuen K.Y. Treatment with lopinavir/ritonavir or interferon-beta1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J. Infect. Dis. 2015;212:1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.W., Yiu C.B., Wong K.Y. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CL (pro)) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Res. 2020;9:129. doi: 10.12688/f1000research.22457.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K.W., Cheng S.C., Chen W.Y., Lin M.H., Chuang S.J., Cheng I.H., Sun C.Y., Chou C.Y. Thiopurine analogs and mycophenolic acid synergistically inhibit the papain-like protease of Middle East respiratory syndrome coronavirus. Antiviral Res. 2015;115:9–16. doi: 10.1016/j.antiviral.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brunink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin M.M., Prabhakar B.S. Neutralizing human monoclonal antibodies to severe acute respiratory syndrome coronavirus: target, mechanism of action, and therapeutic potential. Rev. Med. Virol. 2012;22:2–17. doi: 10.1002/rmv.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Li G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016;29:695–747. doi: 10.1128/CMR.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T., Scott D., Cihlar T., Feldmann H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. U. S. A. 2020;117:6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., He Y., Zhou Y., Liu S., Zheng B.J., Jiang S. The spike protein of SARS-CoV--a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009;7:226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Kou Z., Ma C., Tao X., Wang L., Zhao G., Chen Y., Yu F., Tseng C.T., Zhou Y., Jiang S. A truncated receptor-binding domain of MERS-CoV spike protein potently inhibits MERS-CoV infection and induces strong neutralizing antibody responses: implication for developing therapeutics and vaccines. PLoS One. 2013;8 doi: 10.1371/journal.pone.0081587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Y., Peng C., Yuan M., Huang J., Wang Z., Yu J., Gao X., Wang D., Yu X., Li L., Zhang J., Wu X., Li B., Xu Y., Chen W., Peng Y., Hu Y., Lin L., Liu X., Huang S., Zhou Z., Zhang L., Wang Y., Zhang Z., Deng K., Xia Z., Gong Q., Zhang W., Zheng X., Liu Y., Yang H., Zhou D., Yu D., Hou J., Shi Z., Chen S., Chen Z., Zhang X., Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. U. S. A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert D.M., Kim P.S. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 2001;70:777–810. doi: 10.1146/annurev.biochem.70.1.777. [DOI] [PubMed] [Google Scholar]
- Fan H.H., Wang L.Q., Liu W.L., An X.P., Liu Z.D., He X.Q., Song L.H., Tong Y.G. Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus-related coronavirus model. Chin Med J (Engl) 2020;133:1051–1056. doi: 10.1097/CM9.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S.G., Shen H., Wang J., Tay F.P., Liu D.X. Proteolytic processing of polyproteins 1a and 1ab between non-structural proteins 10 and 11/12 of Coronavirus infectious bronchitis virus is dispensable for viral replication in cultured cells. Virology. 2008;379:175–180. doi: 10.1016/j.virol.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima A., Fukuda N., Lai Y., Ueno T., Moriyama M., Taguchi F., Iguchi A., Shimizu K., Kuroda K. Development of a chimeric DNA-RNA hammerhead ribozyme targeting SARS virus. Intervirology. 2009;52:92–99. doi: 10.1159/000215946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung T.S., Liu D.X. Coronavirus infection, ER stress, apoptosis and innate immunity. Front. Microbiol. 2014;5:296. doi: 10.3389/fmicb.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017;93:449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Lu G., Qi J., Li Y., Wu Y., Deng Y., Geng H., Li H., Wang Q., Xiao H., Tan W., Yan J., Gao G.F. Structure of the fusion core and inhibition of fusion by a heptad repeat peptide derived from the S protein of Middle East respiratory syndrome coronavirus. J. Virol. 2013;87:13134–13140. doi: 10.1128/JVI.02433-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A.K., Xi K., Ratia K., Santarsiero B.D., Fu W., Harcourt B.H., Rota P.A., Baker S.C., Johnson M.E., Mesecar A.D. Design and synthesis of peptidomimetic severe acute respiratory syndrome chymotrypsin-like protease inhibitors. J. Med. Chem. 2005;48:6767–6771. doi: 10.1021/jm050548m. [DOI] [PubMed] [Google Scholar]
- Golden E.B., Cho H.Y., Hofman F.M., Louie S.G., Schonthal A.H., Chen T.C. Quinoline-based antimalarial drugs: a novel class of autophagy inhibitors. Neurosurg. Focus. 2015;38:E12. doi: 10.3171/2014.12.FOCUS14748. [DOI] [PubMed] [Google Scholar]
- Gong S.R., Bao L.L. The battle against SARS and MERS coronaviruses: reservoirs and animal models. Animal Model Exp Med. 2018;1:125–133. doi: 10.1002/ame2.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.X., Nicastri E., Oda R., Yo K., Quiros-Roldan E., Studemeister A., Redinski J., Ahmed S., Bernett J., Chelliah D., Chen D., Chihara S., Cohen S.H., Cunningham J., D'Arminio Monforte A., Ismail S., Kato H., Lapadula G., L'Her E., Maeno T., Majumder S., Massari M., Mora-Rillo M., Mutoh Y., Nguyen D., Verweij E., Zoufaly A., Osinusi A.O., DeZure A., Zhao Y., Zhong L., Chokkalingam A., Elboudwarej E., Telep L., Timbs L., Henne I., Sellers S., Cao H., Tan S.K., Winterbourne L., Desai P., Mera R., Gaggar A., Myers R.P., Brainard D.M., Childs R., Flanigan T. Compassionate use of remdesivir for patients with severe covid-19. N. Engl. J. Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D.P., Penn-Nicholson A., Cho M.W. Identification of critical determinants on ACE2 for SARS-CoV entry and development of a potent entry inhibitor. Virology. 2006;350:15–25. doi: 10.1016/j.virol.2006.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y.S., Chang G.G., Juo C.G., Lee H.J., Yeh S.H., Hsu J.T., Chen X. Papain-like protease 2 (PLP2) from severe acute respiratory syndrome coronavirus (SARS-CoV): expression, purification, characterization, and inhibition. Biochemistry. 2005;44:10349–10359. doi: 10.1021/bi0504761. [DOI] [PubMed] [Google Scholar]
- Hart B.J., Dyall J., Postnikova E., Zhou H., Kindrachuk J., Johnson R.F., Olinger G.G., Frieman M.B., Holbrook M.R., Jahrling P.B., Hensley L. Interferon-beta and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. J. Gen. Virol. 2014;95:571–577. doi: 10.1099/vir.0.061911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Li J., Li W., Lustigman S., Farzan M., Jiang S. Cross-neutralization of human and palm civet severe acute respiratory syndrome coronaviruses by antibodies targeting the receptor-binding domain of spike protein. J. Immunol. 2006;176:6085–6092. doi: 10.4049/jimmunol.176.10.6085. [DOI] [PubMed] [Google Scholar]
- He Y., Lu H., Siddiqui P., Zhou Y., Jiang S. Receptor-binding domain of severe acute respiratory syndrome coronavirus spike protein contains multiple conformation-dependent epitopes that induce highly potent neutralizing antibodies. J. Immunol. 2005;174:4908–4915. doi: 10.4049/jimmunol.174.8.4908. [DOI] [PubMed] [Google Scholar]
- Ho T.Y., Wu S.L., Chen J.C., Li C.C., Hsiang C.Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Res. 2007;74:92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Li L., Kao R.Y., Kou B., Wang Z., Zhang L., Zhang H., Hao Z., Tsui W.H., Ni A., Cui L., Fan B., Guo F., Rao S., Jiang C., Li Q., Sun M., He W., Liu G. Screening and identification of linear B-cell epitopes and entry-blocking peptide of severe acute respiratory syndrome (SARS)-associated coronavirus using synthetic overlapping peptide library. J. Comb. Chem. 2005;7:648–656. doi: 10.1021/cc0500607. [DOI] [PubMed] [Google Scholar]
- Hung I.F., Lung K.C., Tso E.Y., Liu R., Chung T.W., Chu M.Y., Ng Y.Y., Lo J., Chan J., Tam A.R., Shum H.P., Chan V., Wu A.K., Sin K.M., Leung W.S., Law W.L., Lung D.C., Sin S., Yeung P., Yip C.C., Zhang R.R., Fung A.Y., Yan E.Y., Leung K.H., Ip J.D., Chu A.W., Chan W.M., Ng A.C., Lee R., Fung K., Yeung A., Wu T.C., Chan J.W., Yan W.W., Chan W.M., Chan J.F., Lie A.K., Tsang O.T., Cheng V.C., Que T.L., Lau C.S., Chan K.H., To K.K., Yuen K.Y. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J., Grum-Tokars V., Zhou Y., Turlington M., Saldanha S.A., Chase P., Eggler A., Dawson E.S., Baez-Santos Y.M., Tomar S., Mielech A.M., Baker S.C., Lindsley C.W., Hodder P., Mesecar A., Stauffer S.R. Discovery, synthesis, and structure-based optimization of a series of N-(tert-butyl)-2-(N-arylamido)-2-(pyridin-3-yl) acetamides (ML188) as potent noncovalent small molecule inhibitors of the severe acute respiratory syndrome coronavirus (SARS-CoV) 3CL protease. J. Med. Chem. 2013;56:534–546. doi: 10.1021/jm301580n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Yang H., Ji W., Wu W., Chen S., Zhang W., Duan G. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12 doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam R.U., Wilson I.A. Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc. Natl. Acad. Sci. U. S. A. 2017;114:206–214. doi: 10.1073/pnas.1617020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao R.Y., Tsui W.H., Lee T.S., Tanner J.A., Watt R.M., Huang J.D., Hu L., Chen G., Chen Z., Zhang L., He T., Chan K.H., Tse H., To A.P., Ng L.W., Wong B.C., Tsoi H.W., Yang D., Ho D.D., Yuen K.Y. Identification of novel small-molecule inhibitors of severe acute respiratory syndrome-associated coronavirus by chemical genetics. Chem Biol. 2004;11:1293–1299. doi: 10.1016/j.chembiol.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck Z.Y., Wang Y., Lau P., Foung S.K.H. Isolation of HCV neutralizing antibodies by yeast display. Methods Mol. Biol. 2019;1911:395–419. doi: 10.1007/978-1-4939-8976-8_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.Y., Kim Y.I., Park S.J., Kim I.K., Choi Y.K., Kim S.H. Safe, high-throughput screening of natural compounds of MERS-CoV entry inhibitors using a pseudovirus expressing MERS-CoV spike protein. Int. J. Antimicrob. Agents. 2018;52:730–732. doi: 10.1016/j.ijantimicag.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse R.L. Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China. 2020;F1000Res 9:72. doi: 10.12688/f1000research.22211.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo L., Godeke G.J., Raamsman M.J., Masters P.S., Rottier P.J. Retargeting of coronavirus by substitution of the spike glycoprotein ectodomain: crossing the host cell species barrier. J. Virol. 2000;74:1393–1406. doi: 10.1128/jvi.74.3.1393-1406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K., Woo P.C., Li K.S., Huang Y., Tsoi H.W., Wong B.H., Wong S.S., Leung S.Y., Chan K.H., Yuen K.Y. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc. Natl. Acad. Sci. U. S. A. 2005;102:14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.Y., Chen C., Zhang H.Q., Guo H.Y., Wang H., Wang L., Zhang X., Hua S.N., Yu J., Xiao P.G., Li R.S., Tan X. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res. 2005;67:18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H., Wang H., Crameri G., Hu Z., Zhang H., Zhang J., McEachern J., Field H., Daszak P., Eaton B.T., Zhang S., Wang L.F. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- Li Y., Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. AJR Am. J. Roentgenol. 2020;214:1280–1286. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- Lim J., Jeon S., Shin H.Y., Kim M.J., Seong Y.M., Lee W.J., Choe K.W., Kang Y.M., Lee B., Park S.J. Case of the index patient who caused tertiary transmission of COVID-19 infection in korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Korean Med Sci. 2020;35:e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.C., Ho C.T., Chuo W.H., Li S., Wang T.T., Lin C.C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017;17:144. doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Xiao G., Chen Y., He Y., Niu J., Escalante C.R., Xiong H., Farmar J., Debnath A.K., Tien P., Jiang S. Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: implications for virus fusogenic mechanism and identification of fusion inhibitors. Lancet. 2004;363:938–947. doi: 10.1016/S0140-6736(04)15788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo M.K., Feldmann F., Gary J.M., Jordan R., Bannister R., Cronin J., Patel N.R., Klena J.D., Nichol S.T., Cihlar T., Zaki S.R., Feldmann H., Spiropoulou C.F., de Wit E. Remdesivir (GS-5734) protects African green monkeys from Nipah virus challenge. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aau9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin A., Dijkman R., Bergstrom T., Kann N., Adamiak B., Hannoun C., Kindler E., Jonsdottir H.R., Muth D., Kint J., Forlenza M., Muller M.A., Drosten C., Thiel V., Trybala E. Targeting membrane-bound viral RNA synthesis reveals potent inhibition of diverse coronaviruses including the middle East respiratory syndrome virus. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifsud E.J., Hayden F.G., Hurt A.C. Antivirals targeting the polymerase complex of influenza viruses. Antiviral Res. 2019;169:104545. doi: 10.1016/j.antiviral.2019.104545. [DOI] [PubMed] [Google Scholar]
- Munster V.J., Koopmans M., van Doremalen N., van Riel D., de Wit E. A novel coronavirus emerging in China - key questions for impact assessment. N. Engl. J. Med. 2020;382:692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- O'Keefe B.R., Giomarelli B., Barnard D.L., Shenoy S.R., Chan P.K., McMahon J.B., Palmer K.E., Barnett B.W., Meyerholz D.K., Wohlford-Lenane C.L., McCray P.B., Jr. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. J. Virol. 2010;84:2511–2521. doi: 10.1128/JVI.02322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omrani A.S., Saad M.M., Baig K., Bahloul A., Abdul-Matin M., Alaidaroos A.Y., Almakhlafi G.A., Albarrak M.M., Memish Z.A., Albarrak A.M. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect. Dis. 2014;14:1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.Y., Jeong H.J., Kim J.H., Kim Y.M., Park S.J., Kim D., Park K.H., Lee W.S., Ryu Y.B. Diarylheptanoids from Alnus japonica inhibit papain-like protease of severe acute respiratory syndrome coronavirus. Biol. Pharm. Bull. 2012;35:2036–2042. doi: 10.1248/bpb.b12-00623. [DOI] [PubMed] [Google Scholar]
- Park J.Y., Kim J.H., Kim Y.M., Jeong H.J., Kim D.W., Park K.H., Kwon H.J., Park S.J., Lee W.S., Ryu Y.B. Tanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteases. Bioorg. Med. Chem. 2012;20:5928–5935. doi: 10.1016/j.bmc.2012.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters H.L., Jochmans D., de Wilde A.H., Posthuma C.C., Snijder E.J., Neyts J., Seley-Radtke K.L. Design, synthesis and evaluation of a series of acyclic fleximer nucleoside analogues with anti-coronavirus activity. Bioorg. Med. Chem. Lett. 2015;25:2923–2926. doi: 10.1016/j.bmcl.2015.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin. Microbiol. Infect. 2020;26:729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L.L., Wang Y.M., Wu Z.Q., Xiang Z.C., Guo L., Xu T., Jiang Y.Z., Xiong Y., Li Y.J., Li X.W., Li H., Fan G.H., Gu X.Y., Xiao Y., Gao H., Xu J.Y., Yang F., Wang X.M., Wu C., Chen L., Liu Y.W., Liu B., Yang J., Wang X.R., Dong J., Li L., Huang C.L., Zhao J.P., Hu Y., Cheng Z.S., Liu L.L., Qian Z.H., Qin C., Jin Q., Cao B., Wang J.W. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl) 2020;133:1015–1024. doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider T.H., Zook C.E., Boettcher T.L., Wick S.T., Pancoast J.S., Zusman B.D. Broad-spectrum antiviral therapeutics. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas M., Rodriguez Y., Monsalve D.M., Acosta-Ampudia Y., Camacho B., Gallo J.E., Rojas-Villarraga A., Ramirez-Santana C., Diaz-Coronado J.C., Manrique R., Mantilla R.D., Shoenfeld Y., Anaya J.M. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu Y.B., Park S.J., Kim Y.M., Lee J.Y., Seo W.D., Chang J.S., Park K.H., Rho M.C., Lee W.S. SARS-CoV 3CLpro inhibitory effects of quinone-methide triterpenes from Tripterygium regelii. Bioorg. Med. Chem. Lett. 2010;20:1873–1876. doi: 10.1016/j.bmcl.2010.01.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo M., Morikawa S., Fukushi S., Mizutani T., Hasegawa H., Nagata N., Iwata N., Kurane I. Inhibitory effect of mizoribine and ribavirin on the replication of severe acute respiratory syndrome (SARS)-associated coronavirus. Antiviral Res. 2005;66:159–163. doi: 10.1016/j.antiviral.2005.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect. Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki S.G., Sawicki D.L., Siddell S.G. A contemporary view of coronavirus transcription. J. Virol. 2007;81:20–29. doi: 10.1128/JVI.01358-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethuraman N., Jeremiah S.S., Ryo A. Interpreting diagnostic tests for SARS-CoV-2. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M.O., Mackman R.L., Spahn J.E., Palmiotti C.A., Siegel D., Ray A.S., Cihlar T., Jordan R., Denison M.R., Baric R.S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S., Nguyen P.K., Cabral H.J., Diez-Barroso R., Derry P.J., Kanahara S.M., Kumar V.A. Development of peptide inhibitors of HIV transmission. Bioact Mater. 2016;1:109–121. doi: 10.1016/j.bioactmat.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y.W., Chang K.H., Hong G.W., Yeo S.G., Jee Y., Kim J.H., Oh M.D., Cho D.H., Kim S.H. Selection of vaccinia virus-neutralizing antibody from a phage-display human-antibody library. J. Microbiol. Biotechnol. 2019;29:651–657. doi: 10.4014/jmb.1812.12024. [DOI] [PubMed] [Google Scholar]
- Siu Y.L., Teoh K.T., Lo J., Chan C.M., Kien F., Escriou N., Tsao S.W., Nicholls J.M., Altmeyer R., Peiris J.S., Bruzzone R., Nal B. The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. J. Virol. 2008;82:11318–11330. doi: 10.1128/JVI.01052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalin Raj V., Okba N.M.A., Gutierrez-Alvarez J., Drabek D., van Dieren B., Widagdo W., Lamers M.M., Widjaja I., Fernandez-Delgado R., Sola I., Bensaid A., Koopmans M.P., Segales J., Osterhaus A., Bosch B.J., Enjuanes L., Haagmans B.L. Chimeric camel/human heavy-chain antibodies protect against MERS-CoV infection. Sci Adv. 2018;4 doi: 10.1126/sciadv.aas9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J., Li W., Murakami A., Tamin A., Matthews L.J., Wong S.K., Moore M.J., Tallarico A.S., Olurinde M., Choe H., Anderson L.J., Bellini W.J., Farzan M., Marasco W.A. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc. Natl. Acad. Sci. U. S. A. 2004;101:2536–2541. doi: 10.1073/pnas.0307140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J., Li W., Roberts A., Matthews L.J., Murakami A., Vogel L., Wong S.K., Subbarao K., Farzan M., Marasco W.A. Evaluation of human monoclonal antibody 80R for immunoprophylaxis of severe acute respiratory syndrome by an animal study, epitope mapping, and analysis of spike variants. J. Virol. 2005;79:5900–5906. doi: 10.1128/JVI.79.10.5900-5906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.W., Schmitz J.E., Persing D.H., Stratton C.W. Laboratory diagnosis of COVID-19: current issues and challenges. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., Lu L., Jiang S., Yang Z., Wu Y., Ying T. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect. 2020;9:382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- Traggiai E., Becker S., Subbarao K., Kolesnikova L., Uematsu Y., Gismondo M.R., Murphy B.R., Rappuoli R., Lanzavecchia A. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat. Med. 2004;10:871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlington M., Chun A., Tomar S., Eggler A., Grum-Tokars V., Jacobs J., Daniels J.S., Dawson E., Saldanha A., Chase P., Baez-Santos Y.M., Lindsley C.W., Hodder P., Mesecar A.D., Stauffer S.R. Discovery of N-(benzo[1,2,3]triazol-1-yl)-N-(benzyl)acetamido)phenyl) carboxamides as severe acute respiratory syndrome coronavirus (SARS-CoV) 3CLpro inhibitors: identification of ML300 and noncovalent nanomolar inhibitors with an induced-fit binding. Bioorg. Med. Chem. Lett. 2013;23:6172–6177. doi: 10.1016/j.bmcl.2013.08.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahedi H.M., Ahmad S., Abbasi S.W. Stilbene-based natural compounds as promising drug candidates against COVID-19. J. Biomol. Struct. Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1762743. [DOI] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020 doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292 e286. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94 doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Shi W., Chappell J.D., Joyce M.G., Zhang Y., Kanekiyo M., Becker M.M., van Doremalen N., Fischer R., Wang N., Corbett K.S., Choe M., Mason R.D., Van Galen J.G., Zhou T., Saunders K.O., Tatti K.M., Haynes L.M., Kwong P.D., Modjarrad K., Kong W.P., McLellan J.S., Denison M.R., Munster V.J., Mascola J.R., Graham B.S. Importance of neutralizing monoclonal antibodies targeting multiple antigenic sites on the Middle East respiratory syndrome coronavirus spike glycoprotein to avoid neutralization escape. J. Virol. 2018;92 doi: 10.1128/JVI.02002-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Cao R., Zhang H., Liu J., Xu M., Hu H., Li Y., Zhao L., Li W., Sun X., Yang X., Shi Z., Deng F., Hu Z., Zhong W., Wang M. The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discov. 2020;6:28. doi: 10.1038/s41421-020-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y., Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Y., Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V., Siegel D., Perron M., Bannister R., Hui H.C., Larson N., Strickley R., Wells J., Stuthman K.S., Van Tongeren S.A., Garza N.L., Donnelly G., Shurtleff A.C., Retterer C.J., Gharaibeh D., Zamani R., Kenny T., Eaton B.P., Grimes E., Welch L.S., Gomba L., Wilhelmsen C.L., Nichols D.K., Nuss J.E., Nagle E.R., Kugelman J.R., Palacios G., Doerffler E., Neville S., Carra E., Clarke M.O., Zhang L., Lew W., Ross B., Wang Q., Chun K., Wolfe L., Babusis D., Park Y., Stray K.M., Trancheva I., Feng J.Y., Barauskas O., Xu Y., Wong P., Braun M.R., Flint M., McMullan L.K., Chen S.S., Fearns R., Swaminathan S., Mayers D.L., Spiropoulou C.F., Lee W.A., Nichol S.T., Cihlar T., Bavari S. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Takada A., Watanabe T., Ito H., Kida H., Kawaoka Y. Functional importance of the coiled-coil of the Ebola virus glycoprotein. J. Virol. 2000;74:10194–10201. doi: 10.1128/jvi.74.21.10194-10201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartl H., Czub M., Czub S., Neufeld J., Marszal P., Gren J., Smith G., Jones S., Proulx R., Deschambault Y., Grudeski E., Andonov A., He R., Li Y., Copps J., Grolla A., Dick D., Berry J., Ganske S., Manning L., Cao J. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J. Virol. 2004;78:12672–12676. doi: 10.1128/JVI.78.22.12672-12676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.K., Li W., Moore M.J., Choe H., Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J. Biol. Chem. 2004;279:3197–3201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M., Chen L., Li H. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J., Sheng J., Quan L., Xia Z., Tan W., Cheng G., Jiang T. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.J., Huang H.W., Liu C.Y., Hong C.F., Chan Y.L. Inhibition of SARS-CoV replication by siRNA. Antiviral Res. 2005;65:45–48. doi: 10.1016/j.antiviral.2004.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.Y., Jan J.T., Ma S.H., Kuo C.J., Juan H.F., Cheng Y.S., Hsu H.H., Huang H.C., Wu D., Brik A., Liang F.S., Liu R.S., Fang J.M., Chen S.T., Liang P.H., Wong C.H. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc. Natl. Acad. Sci. U. S. A. 2004;101:10012–10017. doi: 10.1073/pnas.0403596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.Y., Brian D.A. Subgenomic messenger RNA amplification in coronaviruses. Proc. Natl. Acad. Sci. U. S. A. 2010;107:12257–12262. doi: 10.1073/pnas.1000378107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S., Qi F., Bao L., Du L., Liu S., Qin C., Sun F., Shi Z., Zhu Y., Jiang S., Lu L. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Yan L., Xu W., Agrawal A.S., Algaissi A., Tseng C.K., Wang Q., Du L., Tan W., Wilson I.A., Jiang S., Yang B., Lu L. A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike. Sci Adv. 2019;5 doi: 10.1126/sciadv.aav4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang F., Wang X., He X., Peng Z., Yang B., Zhang J., Zhou Q., Ye H., Ma Y., Li H., Wei X., Cai P., Ma W.L. Antibody detection and dynamic characteristics in patients with COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Xie W., Xue X., Yang K., Ma J., Liang W., Zhao Q., Zhou Z., Pei D., Ziebuhr J., Hilgenfeld R., Yuen K.Y., Wong L., Gao G., Chen S., Chen Z., Ma D., Bartlam M., Rao Z. Design of wide-spectrum inhibitors targeting coronavirus main proteases. PLoS Biol. 2005;3:e324. doi: 10.1371/journal.pbio.0030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q.Y., Tian X.Y., Fang W.S. Bioactive coumarins from Boenninghausenia sessilicarpa. J. Asian Nat. Prod. Res. 2007;9:59–65. doi: 10.1080/10286020500382397. [DOI] [PubMed] [Google Scholar]
- Yang Z.Y., Werner H.C., Kong W.P., Leung K., Traggiai E., Lanzavecchia A., Nabel G.J. Evasion of antibody neutralization in emerging severe acute respiratory syndrome coronaviruses. Proc. Natl. Acad. Sci. U. S. A. 2005;102:797–801. doi: 10.1073/pnas.0409065102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao T.T., Qian J.D., Zhu W.Y., Wang Y., Wang G.Q. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus-A possible reference for coronavirus disease-19 treatment option. J. Med. Virol. 2020;92:556–563. doi: 10.1002/jmv.25729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L., Li Z., Yuan K., Qu X., Chen J., Wang G., Zhang H., Luo H., Zhu L., Jiang P., Chen L., Shen Y., Luo M., Zuo G., Hu J., Duan D., Nie Y., Shi X., Wang W., Han Y., Li T., Liu Y., Ding M., Deng H., Xu X. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J. Virol. 2004;78:11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J., Ng O.T., Marimuthu K., Ang L.W., Mak T.M., Lau S.K., Anderson D.E., Chan K.S., Tan T.Y., Ng T.Y., Cui L., Said Z., Kurupatham L., Chen M.I., Chan M., Vasoo S., Wang L.F., Tan B.H., Lin R.T.P., Lee V.J.M., Leo Y.S., Lye D.C. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. J. Am. Med. Assoc. 2020;323:1488–1494. doi: 10.1001/jama.2020.3204. Singapore novel coronavirus outbreak research, T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Jiang S., Du L. Current advancements and potential strategies in the development of MERS-CoV vaccines. Expert Rev. Vaccines. 2014;13:761–774. doi: 10.1586/14760584.2014.912134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Wu Q., Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 2020;30:1346–1351 e1342. doi: 10.1016/j.cub.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B.J., Guan Y., Hez M.L., Sun H., Du L., Zheng Y., Wong K.L., Chen H., Chen Y., Lu L., Tanner J.A., Watt R.M., Niccolai N., Bernini A., Spiga O., Woo P.C., Kung H.F., Yuen K.Y., Huang J.D. Synthetic peptides outside the spike protein heptad repeat regions as potent inhibitors of SARS-associated coronavirus. Antivir. Ther. 2005;10:393–403. [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Yang Y., Huang J., Jiang S., Du L. Advances in MERS-CoV vaccines and therapeutics based on the receptor-binding domain. Viruses. 2019;11 doi: 10.3390/v11010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Chakraborti S., He Y., Roberts A., Sheahan T., Xiao X., Hensley L.E., Prabakaran P., Rockx B., Sidorov I.A., Corti D., Vogel L., Feng Y., Kim J.O., Wang L.F., Baric R., Lanzavecchia A., Curtis K.M., Nabel G.J., Subbarao K., Jiang S., Dimitrov D.S. Potent cross-reactive neutralization of SARS coronavirus isolates by human monoclonal antibodies. Proc. Natl. Acad. Sci. U. S. A. 2007;104:12123–12128. doi: 10.1073/pnas.0701000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.Y. Coronaviruses - drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]