Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) global pandemic has led to many health care services, including transplantation, being temporarily suspended. For transplantation to safely recommence, there is a need to understand the effects of SARS-CoV-2 in transplant and waitlist patients.
We identified 21 patients with proven SARS-CoV-2 infection (13 transplant; 8 waitlist) during the first peak of coronavirus disease 2019 in Wales.
Median patient age was 57 years (range, 24-69), 62% were male, and all were white. Median body mass index was 29 kg/m2 (range, 22-42), and 81% had 1 or more significant comorbidities. Median time from transplant to SARS-CoV-2 infection was 135 months (range, 9-356) and median time since being listed was 17.5 months (range, 5-69) for waitlisted patients. Seventeen patients were admitted to the hospital (81%), 18% (n = 3) in intensive care unit, and 5 patients died (4 transplant recipients and 1 waitlist patient; 24%). Two of the 4 transplant patients who died had recent malignancy. Although the mortality of hospitalized transplant patients was high, their infection rate of 0.87% meant that the overall mortality of transplant patients due to SARS-CoV-2 was low and comparable to that of patients on the waitlist.
These data provide confidence in restarting the transplant program, provided that a series of measures aiming to avoid infections in newly transplanted patients are taken.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) global pandemic has presented many challenges in health care delivery. Many health care services, including transplantation have been suspended, as efforts have been focused on reprioritization of staff and resources to meet the demands of the expected waves of SARS-CoV-2 infections. With the predicted peak of SARS-CoV-2 on the horizon in Wales and its resultant constraints (staff redeployment, resource reallocation, reduced theater and ward capacities, etc) and the unknown impact on new patients, in March 2020 we temporarily suspended the kidney and pancreas transplant program.
Given the extent and duration of the pandemic in Wales and the United Kingdom, there is a need to reassess the benefits and risks of continuing to suspend health care services, including transplantation, and plans are being made to resume such programs. For transplantation programs to be safely recommenced, it is necessary to understand the effects of SARS-CoV-2 in an immunocompromised posttransplant patient. This is particularly relevant in the immediate/early posttransplant period, when the diminished T-cell immune effects of the induction immunosuppressive agents are at their peak. Moreover, we need to communicate the risk to the prospective transplant recipient on the waitlist.
There have been a few case reports and case series that have reported outcomes in transplant patients with SARS-CoV-2 [[1], [2], [3], [4], [5], [6], [7]]. These studies have largely shown that the majority of such patients require hospitalization and presenting symptoms are not dissimilar to those observed in the nontransplant population. Furthermore, very few data exist for patients who are on the transplant waitlist and these data are geographically limited.
The Cardiff Transplant Unit is the only transplant center in Wales and serves a population of 2.3 million people over a broad geographic area of over 14,000 km2. We report a series of SARS-CoV-2 infections in our transplant and waitlist population during the time of the first UK-wide peak of the SARS-CoV-2 pandemic.
Patients and Methods
All transplant and waitlist patients who developed proven SARS-CoV-2 infection were identified prospectively. All transplant or waitlist patients who presented to the emergency department in hospitals in South and Mid Wales or to our transplant telephone service with a presumed diagnosis of SARS-CoV-2 between March 1, 2020, and May 31, 2020, were included. A single transplant clinician collected the data prospectively and other dedicated members of the transplant team communicated with the treating team if patients were admitted to any of the surrounding hospitals or called them at home if not. If patients developed symptoms suspicious for SARS-CoV-2, they were initially directed to self-isolate but if they were unwell or if symptoms became worse, they were directed to present to their local hospital.
On March 13, 2020, we ceased the Welsh Kidney (deceased and living donor) and Pancreas Transplant Program in an effort to avoid potential new infections during the expected first peak of the pandemic and as a result of reprioritization of the hospital services for accommodation of a surge of SARS-CoV-2 cases. In addition, we made significant changes to our outpatient service with strict enforcement of social distancing and infection control measures and the intervals between follow-up appointments. Currently, there are 1480 patients with functioning transplants who are being followed, and as of March 1, 2020, there were 149 patients active on the transplant waitlist. New assessments for prospective transplant recipients were also suspended. Given the setup of the transplant service in South and Mid Wales, we are confident that we have captured all of the symptomatic SARS-CoV-2-positive transplant and waitlist patients.
SARS-CoV-2 positivity was defined as a positive result on real-time polymerase chain reaction (PCR) assay of nasal and/or oropharyngeal swab specimens. Data on clinical status were obtained daily from the treating team. Laboratory data were obtained from the Welsh Clinical Portal, an all-Wales online information technology system. Status of the transplant or waitlist, as well as original diagnosis and comorbidities, was verified by VitalData, a dedicated database for renal and transplant patients. Patients provide consent for clinical data to be anonymously collected at the point of listing for transplantation.
Data collected included demographic and background transplant data (age, sex, ethnicity, body mass index [BMI], comorbidities, type of transplant, immunosuppression, etc) and data related to the SARS-CoV-2 infection episode, including outcomes such as graft function, hospital stay, intensive care unit (ICU) admission, and mortality. Deidentified participant data or other prespecified data are available subject to a written proposal and a signed data sharing agreement.
Results
Patient Demographics
Thirteen functioning kidney transplant patients (0.87%) and 8 waitlisted patients were found to be SARS-CoV-2 positive (5.4%). Median age was 57 years (range, 24-69), and 13 (62%) were male. All patients in this cohort were white, which represents approximately 95% of the Welsh transplant and waitlist population. Median BMI was 29 kg/m2 (range, 22-42). The median time since transplant to SARS-CoV-2 infection was 135 months (range, 9-356) for transplant patients and 17.5 months (range, 5-69) since being listed for transplantation for waitlist patients. Seventeen (81%) patients had at least 1 or more significant comorbidities: 10 had hypertension (48%), 5 had diabetes (24%), 5 had malignancies (24%), and 5 had lung disease (24%), including 1 with a previous lung transplant. Patient demographic characteristics are described in Table 1 .
Table 1.
Patient Demographic Characteristics
| Patient Characteristic | Transplant Patients | Waitlist Patients |
|---|---|---|
| Median age, years (range) | 58 (24-69) | 54 (44-67) |
| Gender | ||
| Male | 8 (62%) | 5 (62.5%) |
| Female | 5 (38%) | 3 (37.5%) |
| Median BMI (range) | 29 (22-42) | 29 (24-37) |
| Cause of renal failure | ||
| APKD | 2 | 3 |
| Diabetes | 1 | 1 |
| GN | 2 | 2 |
| IgA nephropathy | 1 | 2 |
| Other/unknown | 7 | 0 |
| Comorbidities | ||
| CVD | 1 (8%) | 2 (25%) |
| Hypertension | 5 (38%) | 5 (62.5%) |
| Diabetes | 2 (15%) | 3 (37.5%) |
| Malignancy | 4 (31%) | 1 (12.5%) |
| Lung disease | 3 (23%) | 3 (37.5%) |
| Prev. transplant | 3 (23%) | 4 (50%) |
| Dialysis status | ||
| HD | --- | 6 |
| PD | --- | 1 |
| Pre-dialysis | --- | 1 |
Abbreviations: APKD, adult polycystic kidney disease; BMI, body mass index; CVD, cardiovascular disease; GN, glomerulonephritis; HD, hemodialysis; PD, peritoneal dialysis.
Immunosuppression in the Transplant Cohort
Induction immunosuppression included methylprednisolone for all patients, antithymocyte globulin for the 4 kidney transplant recipients with deceased after circulatory death donors, and basiliximab for the kidney transplant recipients with living donors and deceased after brainstem death donors. In 2 patients (both with deceased after brainstem death donors), no monoclonal or polyclonal antibody was administered. These patients received their transplants 17 and 20 years ago. Maintenance immunosuppression at the time of diagnosis included calcineurin inhibitor (CNI) in 12 (92%), antimetabolite (mycophenolate mofetil [MMF]/azathioprine) in 11 (85%), and steroids in 8 (62%) patients in the transplant cohort. Median tacrolimus trough level at time of admission was 11 ng/mL (range, 4.5-18.7). Of note, of the waitlist patients, 4 had a previous transplant (50%), with 1 was still on maintenance immunosuppression (CNI and steroids). See Table 2 for details of immunosuppression in the transplant cohort.
Table 2.
Immunosuppression in Transplant Cohort
| Patient | Donor Type | Time from Transplant to SARS-CoV-2 (mo) | Induction Immunosuppression | Maintenance Immunosuppression |
||||
|---|---|---|---|---|---|---|---|---|
| CNI | mTORi | Antimetabolite | Pred | CNI Level at Time of Presentation | ||||
| 1 | DCD | 122 | ATG, MP | Siro | MMF | Yes | ||
| 2 | DCD | 10 | ATG, MP | Tac | MMF | Yes | 11 | |
| 3 | DBD | 135 | Basiliximab, MP | Tac | Aza | 7 | ||
| 4 | LD | 218 | Basiliximab, MP | Tac | Aza | 18 | ||
| 5 | LD | 122 | Basiliximab, MP | Tac | MMF | Yes | 4.5 | |
| 6 | LD | 64 | Basiliximab, MP | Tac | MMF | 12.5 | ||
| 7 | DBD | 356 | MP | Cicl | 61 | |||
| 8 | DBD | 243 | Basiliximab, MP | Tac | Yes | 15 | ||
| 9 | DBD | 204 | Basiliximab, MP | Tac | Aza | Yes | 18.7 | |
| 10 | DBD | 317 | MP | Tac | Aza | Yes | 18 | |
| 11 | DCD | 9 | ATG, MP | Tac | MMF | 8.4 | ||
| 12 | LD | 86 | Basiliximab, MP | Tac | MMF | Yes | 7.3 | |
| 13 | DCD | 153 | ATG, MP | Tac | Aza | Yes | 9 | |
Abbreviations: ATG, antithymocyte globulin; Aza, azathioprine; Cicl, ciclosporin; CNI, calcineurin inhibitor; DBD, deceased after brainstem death; DCD, deceased after circulatory death; LD, living donor; MMF, mycophenolate mofetil; MP, methylprednisolone; mTORi, mammalian target of rapamycin inhibitor; Pred, prednisolone; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; Siro, sirolimus; Tac, tacrolimus.
SARS-CoV-2 Presentation and Treatment
Fourteen of the overall cohort presented with 1 or more symptoms (67%), including fever, shortness of breath, cough, and diarrhea. Eight, 10, and 8 of the patients were reported to have fever (38%), shortness of breath (48%), and cough (38%), respectively, with 1 patient presenting with exacerbation of a groin hernia secondary to persistent coughing. Two of the patients presented with diarrhea (10%), without any respiratory symptoms. Seven of the patients had either general malaise or their symptoms were unrecorded (33%). One asymptomatic patient acquired the SARS-CoV-2 infection during an unrelated admission for a fractured hip.
Overall, 17 of the patients required hospital admission (81%; 12 patients in the transplant cohort [92%] and 5 in the waitlist cohort [62.5%]), with a median length of hospital stay of 15 days (range, 4-43). Of the 17 patients who were admitted, 15 had radiological imaging confirming suspicious appearances suggestive of SARS-CoV-2 infection (chest radiograph and/or thorax computed tomography; 88%). Fourteen of those admitted received oxygen therapy for part of their hospital stay (82%), 3 received noninvasive ventilation with continuous positive airway pressure (18%), and 2 received invasive ventilation in the ICU (12%). Fifteen of the admitted patients received antibiotic therapy according to local hospital guidelines for community- or hospital-acquired pneumonia (88%; 1 patient received azithromycin). No patients were recruited into any trials and therefore did not receive any antiviral therapy or dexamethasone.
In all admitted patients, the antimetabolite agent (MMF or azathioprine) was withdrawn until discharge and then reinstated approximately 10 days postdischarge, tailored to the needs of the patient. One patient, in whom MMF was resumed relatively early, required readmission to the hospital with ongoing respiratory symptoms. In addition, CNI doses were adjusted where needed. In particular, 5 out of 12 transplant patients had significantly elevated CNI levels that prompted reduction of CNI dose (42%).
Clinical Outcomes
Table 3 provides details for the clinical outcomes for the whole cohort. Of the 17 hospital admissions (81%), 3 required ICU admission (18%; 2 in the transplant cohort [17%] and 1 in the waitlist cohort [20%]). Five patients died (23.8%; 4 in the transplant cohort [31%] and 1 in the waitlist cohort [12.5%]). Of the deaths within the transplant cohort, 1 patient had a BMI of 42, 1 had small bowel posttransplant lymphoproliferative disorder with recent (3 months earlier) bowel resection of the obstructing lesion and rituximab infusions during this period, 1 had bronchiectasis and a previous lung transplant with a recent history of a recurrent bladder cancer, and 1 was admitted for a fractured hip and acquired the SARS-CoV-2 infection during that admission. Within the waitlist cohort, the patient who died was obese (BMI = 33) and had both hypertension and diabetes. This patient had also previously (>15 years ago) been treated for colon cancer with bowel resection and chemotherapy.
Table 3.
Clinical Outcomes of SARS-CoV-2-Positive Transplant and Waitlist Patients
| Pt | Tx or W/L Pt | Time From Tx or W/L to SARS-CoV-2 (mo) | Hospital Admission | LOS (days) | ICU Admission | Death | Graft Dysfunction | Graft Failure | WCC (×103/μL) | Lymphocyte count (×103/μL) | CRP (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Tx | 122 | Yes | 6 | Yes | Yes | 6.5 | 0.2 | 229 | ||
| 2 | Tx | 10 | Yes | 43 | 5.4 | 0.2 | 42 | ||||
| 3 | Tx | 135 | |||||||||
| 4 | Tx | 218 | Yes | 9 | Yes | 9.6 | 0.7 | 97 | |||
| 5 | Tx | 122 | Yes | 6 | Yes | 4.3 | 0.9 | 48 | |||
| 6 | Tx | 64 | Yes | 22 | Yes | Yes | 8.6 | 0.7 | 42 | ||
| 7 | Tx | 356 | Yes | 15 | Yes | Yes | 3.4 | 0.7 | 47 | ||
| 8 | Tx | 243 | Yes | 6 | Yes | Yes | 10.5 | 2.5 | 122 | ||
| 9 | Tx | 204 | Yes | 17 | Yes | Yes | 4.8 | 0.5 | 77 | ||
| 10 | Tx | 317 | Yes | 16 | Yes | Yes | 1.2 | 0.3 | 152 | ||
| 11 | Tx | 9 | Yes | 4 | Yes | 6.2 | 0.1 | 47 | |||
| 12 | Tx | 86 | Yes | 12 | Yes | Yes | Yes | 9.3 | 2.1 | 60 | |
| 13 | Tx | 153 | Yes | 24 | Yes | 6.3 | 0.9 | 16 | |||
| 14 | W/L | 8 | Yes | 16 | Yes | Yes | 9 | 0.7 | 220 | ||
| 15 | W/L | 24 | Yes | 24 | 7.8 | 0.7 | 255 | ||||
| 16 | W/L | 12 | Yes | 27 | 2.7 | 0.7 | 42 | ||||
| 17 | W/L | 33 | Yes | 12 | 2.1 | 0.4 | 135 | ||||
| 18 | W/L | 22 | 7.2 | 1 | 7 | ||||||
| 19 | W/L | 5 | Yes | 5 | 5.6 | 0.9 | 39 | ||||
| 20 | W/L | 69 | 4.7 | 1 | |||||||
| 21 | W/L | 13 |
Abbreviations: CRP, C-reactive protein; ICU, intensive care unit; LOS, length of stay; Pt, patient; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; Tx, transplant; WCC, white cell count; W/L, waitlist.
Of the transplant cohort, 11 had acute kidney injury/graft dysfunction (85%), of whom 2 developed graft failure. Both of these patients had graft failure, with estimated glomerular filtration rates of 12 and 10 mL/min before the onset of their SARS-CoV-2 infection. The laboratory results of the admitted patients revealed lymphopenia and elevated C-reactive protein, with a mean white cell count of 6.3 × 103 (±0.80)/μL and 5.6 × 103 (±0.98)/μL, lymphocyte count of 0.8 × 103 (±0.2)/μL and 0.8 × 103 (±0.1)/μL, and C-reactive protein of 82 (±17) mg/L and 100 (±39) mg/L in the transplant and waitlist cohorts, respectively.
Comparison of SARS-CoV-2 Cases With Wales
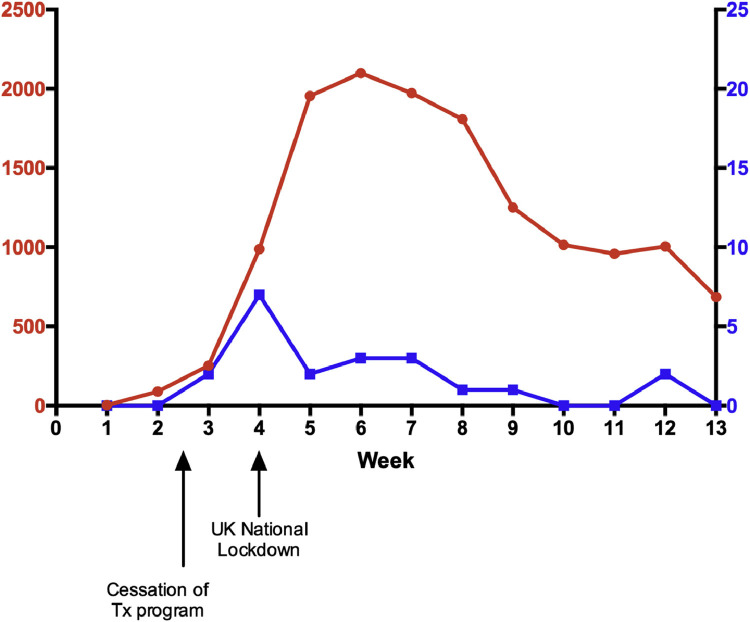
Figure 1 shows the new weekly infection rates of SARS-CoV-2 between March and May 2020 within the transplant and waitlisted cohort in comparison to the national infection rate in Wales.
Fig 1.
Weekly (March-May 2020) number of new severe acute respiratory syndrome coronavirus 2 infections in Wales (red) and in the Welsh transplanted and waitlist cohorts (blue).
Discussion
This is a full report of the direct impact of SARS-CoV-2 on transplant and waitlisted patients during the first peak of the disease in Wales.
Given that the center stopped transplantation and took specific infection control measures early in the course of the pandemic, no new transplant recipients were infected. Of note, no infections occurred among patients who received transplants in the 6 months preceding this period.
There is a low incidence of SARS-CoV-2 symptomatic infections among the transplant patients (0.87%) compared to an estimated 0.47% of the national population in Wales [8] and 1.4% among English patients with a functioning kidney transplant [9]. Given the testing policy in the United Kingdom, in the early months of the outbreak, the real numbers of the general population infected with SARS-CoV-2 are likely to be higher. This number is estimated to be nearly 7% according to a SARS-CoV-2 antibody survey performed toward the end of the study period [10].
There is a high mortality rate among transplant recipients admitted with SARS-CoV-2 (31%), similar that reported for hospitalized patients overall in the United Kingdom (26%) [11], in English kidney transplant recipients (26.4%) [9], and in transplant patients in the United States and Europe [6,12,13]. The hospitalization rate among the transplant and waitlist cohorts in our study is higher than that of the general nontransplant population and is comparable to that of other transplant cohorts [6,13]. In the United States, of the total number of transplant patients with SARS-CoV-2, >70% were hospitalized, 30% were admitted to the ICU, and 22% were intubated (unpublished report of the University of Washington coronavirus disease 2019 solid organ transplantation case report series, presented in ATC 2020) [14]. One of the known features of SARS-CoV-2 is that it disproportionally leads to adverse outcomes in certain groups of patients [11,[15], [16], [17]]. Although our patients in general belong to such a group, a high proportion of the patients who died had a previous/recent malignancy (3/5; 60%). The in-hospital mortality in our group was no different from that reported by other groups [6,13], despite the fact that none of our patients received any antivirals or dexamethasone.
The mortality among waitlisted patients was 12.5% but the incidence of infection was much higher than that among transplanted patients (5.4% vs 0.87%). Therefore, the chance of dying with a functioning transplant is 4/1480 (0.27%) and compares favorably with the mortality of 1/149 (0.67%) among the waitlisted cohort.
A high proportion of this transplant cohort had mild or severe graft dysfunction, and most patients recovered. A potential explanation for this, other than the septic consequences of the disease, is the possible direct effect of SARS-CoV-2 on the renal tubules [18]. In this cohort, a high proportion of the patients had elevated tacrolimus levels. Although 2 patients had diarrhea which may explain their results, we can postulate that SARS-CoV-2 might be associated with decreased small bowel transit time and therefore increased enterohepatic reabsorption of tacrolimus. This will need to be confirmed in a larger group of patients.
It is notable in this cohort the ICU admission rate was low despite the severity of disease. This ICU admission rate is lower than the ICU admission rate reported in the United States (unpublished report of the University of Washington coronavirus disease 2019 sold organ transplantation case report series, presented at ATC 2020), but similar to other European countries [12,13]. This could reflect ICU admission criteria, ICU availability and capacity, and advanced decision directives in place.
Pattern of disease followed the national Welsh infection rate [8], as presented in Fig 1. This most likely confirms that the temporary cessation of the program and infection control measures taken in the clinic have had a positive effect. We have established that, at least in Wales, the overall risk of death among transplant patients from SARS-CoV-2 is not higher than that among waitlist patients, with the caveat that no transplants were performed during the acute peak. One of the concerns when resuming the transplant program is the potential increased risk of acquiring SARS-CoV-2 at the time of transplantation. Therefore, a series of measures including regular testing while on the waitlist, testing on the day of transplant, regular staff testing, and strict infection control measures that also restrict movement of nontransplant patients to the transplant unit were taken for the program to be restarted safely. The social distancing measures in the clinic also continued to include avoiding other means of intrahospital transmission. Patients were also advised to take strict social distancing measures for the first 3 months posttransplant. It remains to be seen whether this intervention bundle will keep infection rates for new transplant recipients low, avoid cancellations on the day of transplant and intrahospital transmission, and allow most patients to be reactivated on the transplant waitlist.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu L., Xu X., Ma K., Yang J., Guan H., Chen S., et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant. 2020;20:1859–1863. doi: 10.1111/ajt.15869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guillen E., Pineiro G.J., Revuelta I., Rodriguez D., Bodro M., Moreno A., et al. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? Am J Transplant. 2020;20:1875–1878. doi: 10.1111/ajt.15874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang J., Lin H., Wu Y., Fang Y., Kumar R., Chen G., et al. COVID-19 in posttransplant patients---report of 2 cases. Am J Transplant. 2020;20:1879–1881. doi: 10.1111/ajt.15896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nair V., Jandovitz N., Hirsch J.S., Nair G., Abate M., Bhaskaran M., et al. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20:1819–1825. doi: 10.1111/ajt.15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akalin E., Azzi Y., Bartash R., Seethamraju H., Parides M., Hemmige V., et al. COVID-19 and kidney transplantation. N Engl J Med. 2020;382:2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Public Health Wales Public Health Wales rapid COVID-19 surveillance. https://public.tableau.com/views/RapidCOVID-19virology-Mobilefriendly/Summary?%3AshowVizHome=no&%3Aembed=true#2 2020 [accessed 20.06.17]
- 9.Ravanan R., Callaghan C.J., Mumford L., Ushiro-Lumb I., Thorburn D., Casey J., et al. SARS-CoV-2 infection and early mortality of wait-listed and solid organ transplant recipients in England: a national cohort study. Am J Transplant. 2020;20:3008–3018. doi: 10.1111/ajt.16247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Office for National Statistics, Coronavirus (COVID-19) infection survey pilot. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveypilot/12june2020 2020 [accessed 20.06.17]
- 11.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., et al. Features of 20,133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crespo M., José Pérez-Sáez M., Redondo-Pachón D., Llinàs-Mallol L., Montero M.M., Villar J., et al. COVID-19 in elderly kidney transplant recipients. Am J Transplant. 2020;20:2883–2889. doi: 10.1111/ajt.16096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoek R.A.S., Manintveld O.C., Betjes M.G.H., Hellemons M.E., Seghers L., van Kampen J.A.A., et al. COVID-19 in solid organ transplant recipients: a single center experience. Transpl Int. 2020;33:1099–1105. doi: 10.1111/tri.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kates O. COVID-19: Clinical Presentation, Complications and Outcomes in Transplant Recipients. Oral presentation at Virtual American Transplant Congress (ACT), May 30, 2020.
- 15.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang W., Guan W., Chen R., Wang W., Li J., Xu K., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CDC COVID-19 Response Team Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019---United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su H., Yang M., Wan C., Yi L.X., Tang F., Zhu H.Y., et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]