ABSTRACT
Acquired mutations in anaplastic lymphoma kinase (ALK) gene have been implicated as the major resistance mechanism to ALK inhibitors; however, information on the treatment options after acquiring novel ALK secondary mutations is limited. Herein, we report the efficacy of lorlatinib upon the detection of a novel ALK G1202L after progression on brigatinib. Our patient was a 30-year-old man with ALK-rearranged advanced lung adenocarcinoma. He had a partial clinical response to crizotinib lasting 11 months. Brigatinib was then administered for 12.8 months with stable disease as the best response. Sequencing at progression revealed the retention of EML4-ALK fusion and the emergence of a novel ALK G1202L mutation. With no standard treatment available, lorlatinib was administered, which achieved disease control for 9 months. Our report reveals the efficacy of lorlatinib in targeting ALK G1202L and can serve as an option for the clinical management of patients with ALK-rearranged lung adenocarcinoma after acquiring G1202L-mediated resistance from prior ALK inhibitor therapy. Furthermore, we also demonstrate the sequential use of crizotinib, brigatinib, and lorlatinib in a patient with advanced ALK-rearranged lung adenocarcinoma with an overall progression-free survival of 33.3 months for the sequential ALK inhibitor regimens. His overall survival was 41.5 months inclusive of all regimens.
KEYWORDS: ALK inhibitor resistance, lorlatinib, ALK-rearranged NSCLC, ALK G1202L, sequential ALK inhibitors
Introduction
Gene rearrangements involving the anaplastic lymphoma kinase (ALK), occurring in 3–7% of non-small cell lung cancer (NSCLC), are considered as one of the dominant oncogenic drivers. They are often associated with younger age, never or light smoking history, and adenocarcinoma histology.1–4 Patients with ALK-rearranged NSCLC are highly responsive to crizotinib, a first-generation ALK inhibitor.3,5 Albeit the initial responses, resistance to ALK inhibitors inevitably develops, often within 1 to 2 years after the initiation of therapy.5 The major resistance mechanisms to crizotinib, a first-generation ALK inhibitor, include the acquisition of secondary mutations in the kinase domain (e.g. 1151Tins, L1152R, C1156Y, L1196M, G1202R, S1206Y, G1269A and others),4,6,7 copy number gain of the ALK gene rearrangement,4,6 and activation of bypass signaling pathways such as EGFR,4,6 KIT,4 and KRAS.6 Brigatinib (AP26113), a potent second-generation ALK inhibitor, targeting ALK and EGFR, has been demonstrated to be effective even after treatment failure with first- and other second-generation ALK inhibitors.8 Moreover, a third-generation ALK inhibitor, lorlatinib (PF-06463922), was shown to be effective against all known ALK mutants, including G1202R.9,10 In this report, we present the first clinical evidence of the efficacy generated by lorlatinib targeting a novel acquired ALK G1202L mutation after brigatinib treatment. Furthermore, we also demonstrate the sequential use of three generations of ALK inhibitors, crizotinib, brigatinib, and lorlatinib, in a patient with advanced ALK-rearranged lung adenocarcinoma with an overall progression-free survival (PFS) of 33.3 months considering only the three generations of ALK inhibitors and overall survival (OS) of 41.5 months inclusive of all chemotherapy and ALK inhibitor regimens.
Case report
In June 2015, a 30-year-old Chinese male smoker was referred to our clinic with complaints of idiopathic chronic cough for 3 months. Figure 1a illustrates his clinical summary. Chest computed tomography (CT) scan revealed the presence of multiple small nodules, with the largest diameter measuring 1.9 cm along the bronchi and bronchioles of the right upper lobe of the lung extending into the parietal pleura and multiple lymph node involvement around the right hilum and mediastinum (Figure 1b). Head CT did not reveal any abnormality. Cytology examination of the mediastinal lymph node biopsy sample revealed poorly differentiated adenocarcinoma (Figure 1a). In addition, immunohistochemistry results showed positive for cytokeratin, TTF-1, and NapsinA, and negative for villin. The patient was diagnosed with stage IV (T1bN2M1a) lung adenocarcinoma. He was initially treated with a chemotherapy regimen of 500 mg/m2 pemetrexed plus carboplatin AUC = 5 but had progressive disease (PD) after 1.8 months. Docetaxel was then administered as the second-line treatment for a total of three cycles until PD (Figure 1b). Fluorescence in situ hybridization (FISH) of the mediastinal lymph node biopsy sample at PD revealed 60% positive rate for chromosomal translocation in ALK (2p23), but negative for MET and ROS1. Crizotinib at a dose of 250 mg twice daily was administered. Within two months from the start of crizotinib therapy, partial response (PR) was achieved based on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (Figure 1c). His response lasted for 11 months until the detection of new metastatic lesions on the right lung (Figure 1d). His treatment was then switched to brigatinib at a dose of 180 mg once daily. His disease was stable for 12.8 months until it eventually progressed (Figure 1e). Tissue biopsy samples collected at PD were submitted for capture-based targeted sequencing using a panel consisting of 520 cancer-related genes covering 1.64 megabases of the human genome (OncoScreen Plus, Burning Rock Biotech). Sequencing analysis revealed the retention of EML4-ALK variant 3 (E6, A20) gene fusion and the emergence of a novel dinucleotide substitution in the exon 23 of the ALK gene resulting in a missense mutation, ALK G1202L (NM_004304.4:c.3604_3605delGGinsCT p.Gly1202Leu), with an allelic fraction of 11.6% (Figure 2). No mutations in other oncogenic drivers were detected. ALK G1202L mutation has not been previously reported but is in the same position as G1202R, a previously reported crizotinib and brigatinib resistance mutation that can be effectively targeted with lorlatinib.9,10 Due to the lack of standard of care, we decided to administer lorlatinib at a dose of 100 mg once a day. Upon lorlatinib treatment, his clinical symptoms improved and his disease was evaluated to be stable with a maximum tumor diameter reduction of 10.7% (figure 1f). At 9 months, PD was evaluated after review of the CT revealed enlargement of the primary lung lesions, mediastinal lymphadenopathy, increased pleural and pericardial effusion as well as metastasis to the liver and abdomen (Figure 1g). After PD, he received paclitaxel with cisplatin and bevacizumab with no benefit. The patient succumbed to complications from metastatic disease soon after.
Figure 1.
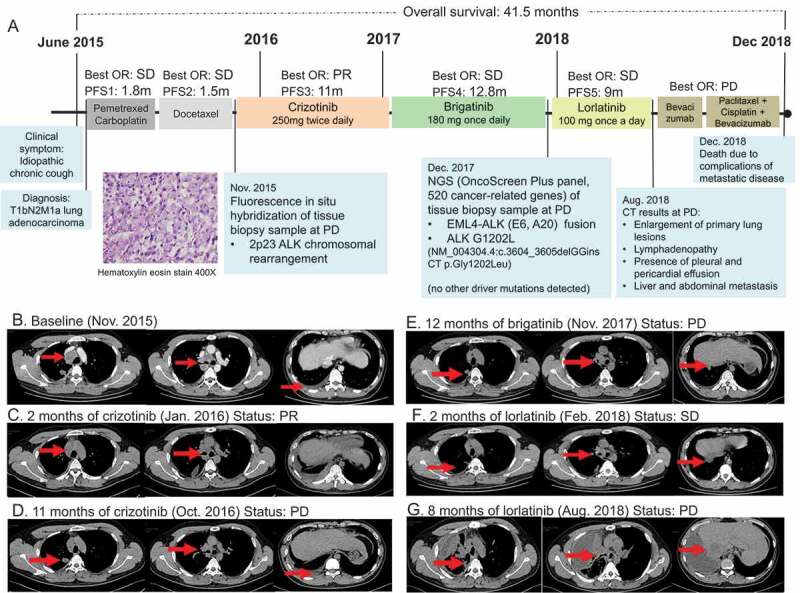
Clinical, molecular, and radiological summary of our patient. (a). An illustrated summary of the treatment regimen received by the patient including best response and progression-free survival in each line of treatment. Hematoxylin-eosin staining illustrating the diagnosis of adenocarcinoma (magnification 400X). Chest computed tomography (CT) scans of the patient from baseline (b), at evaluation of partial response (PR) within 2 months of crizotinib therapy (c), at PD after 11 months of crizotinib therapy (d), at PD after 12 months of brigatinib therapy (e), at SD after 2 months of lorlatinib therapy (f) and at PD after 9 months of lorlatinib therapy (g)
Figure 2.
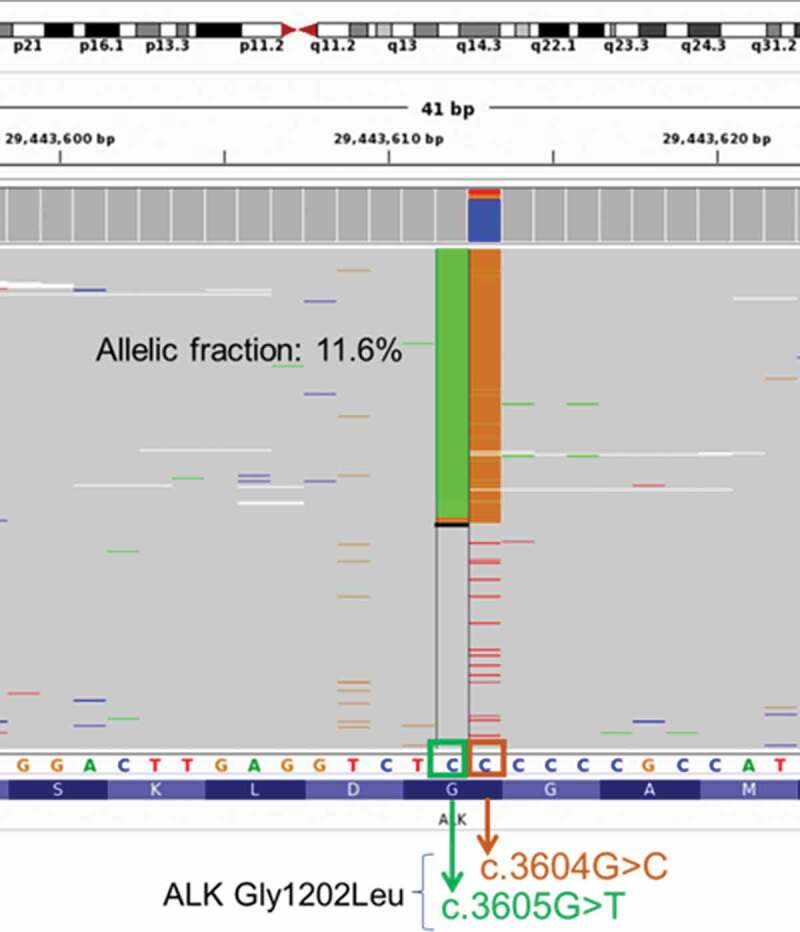
Illustration of the novel ALK G1202L mutation detected from the patient at PD from brigatinib therapy. ALK G1202L is a missense mutations resulting from dinucleotide substitution in the exon 23 of the ALK gene (NM_004304.4:c.3604_3605delGGinsCT p.Gly1202Leu). Green column represents reads with c.3605 G > T, while orange column represents reads with c.3604 G > C. Each gray row represents the sequencing read from a DNA fragment. Bottom bar shows the protein sequence annotation of ALK
Discussion
ALK inhibitors have now been the standard of care for patients with ALK-rearranged advanced-stage NSCLC and the availability of different generations of ALK inhibitors allows the sequential use of these inhibitors after treatment failure from prior generations. However, the resistance mechanisms, as well as patient treatment responses with the sequential use of ALK inhibitors after the emergence of resistance to prior generations of ALK inhibitors remain limited.11,12 Despite numerous studies demonstrating treatment outcomes on the sequential use of crizotinib followed by second-generation ALK inhibitors including ceritinib,9 alectinib13 or any,12,14 limited study has reported on the sequential use of three generations of ALK inhibitor. Efforts to elucidate these mechanisms and treatment outcomes are paramount to developing novel therapeutic strategies. Using capture-based targeted sequencing, we have detected a previously unreported ALK G1202L mutation after brigatinib treatment, which has not been recorded in COSMIC, cBioportal, or any other somatic variation database. Although G1202L affects the glycine residue in position 1202 of the ALK protein in the same fashion as G1202R, the glycine residue in G1202R was substituted with arginine, a polar amino acid; whereas in G1202L it was replaced by leucine, a nonpolar amino acid. Being located at the kinase domain, mutations in G1202 would likely alter the binding of ALK inhibitors. Hence, we speculate that G1202L can also be a potential resistance mechanism to either crizotinib or brigatinib similar to G1202R. Since our patient did not undergo mutational profiling at baseline and any other time point during the treatment process, we cannot pinpoint the exact time of emergence of G1202L.
However, based on the remarkable clinical response of our patient to brigatinib, we believe that ALK G1202L emerged as a resistance mechanism to crizotinib. ALK G1202R is one of the most common secondary mutations acquired not only with crizotinib but also brigatinib and has been shown to be highly resistant to crizotinib, alectinib and ceritinib.9 However, G1202R acquired after crizotinib was reported to be sensitive to brigatinib10 and lorlatinib.9,10 Interestingly, patients who harbor variant 3 EML4-ALK rearrangement were more likely to develop secondary ALK mutations, particularly G1202R as resistance mechanisms from first- or second-generation ALK inhibitor therapy.15 Moreover, patients with variant 3 EML4-ALK treated with lorlatinib also had significantly longer overall survival than patients with variant 1.15 Consistently, our patient who harbored variant 3 EML4-ALK rearrangement acquired G1202L at either crizotinib or brigatinib therapy, benefited from lorlatinib, and had a longer overall survival of 41.5 months inclusive of the short chemotherapy regimens and a total of 33.3 months of sequential ALK inhibitor therapy. These findings suggest that lorlatinib can be a treatment option in patients with acquired ALK G1202L. In vitro and clinical evidence are required to further understand the resistance mediated by ALK G1202L and its sensitivity profile with various ALK inhibitors.
Conclusion
To the best of our knowledge, this is the first clinical evidence of efficacy generated by lorlatinib targeting a novel acquired ALK G1202L mutation, suggesting that lorlatinib can be a treatment option for patients with ALK-rearranged NSCLC who acquired ALK G1202L. Furthermore, we also demonstrate the sequential use of crizotinib, brigatinib, and lorlatinib in a patient with advanced ALK-rearranged lung adenocarcinoma with an overall PFS of 33.3 months of sequential ALK inhibitor regimens and an overall survival of 41.5 months inclusive of chemotherapy and ALK inhibitor regimens.
Acknowledgments
The authors thank the patient and his family. We also thank the investigators, study coordinators, operation staff and the whole project team who worked on this case.
Funding Statement
This work was supported by National Natural Science Foundation of China [grant number 81572321 to DH] and the Wu Jieping Medical Foundation [grant number 320.6750.18158 to ZM].
Abbreviations
- ALK
anaplastic lymphoma kinase
- PD
disease progression
- PFS
progression-free survival
- PR
partial response
- SD
stable disease
Disclosure of potential conflicts of interest
A. Lizaso is an employee of Burning Rock Biotech. All the other authors report no conflict of interest.
Ethical consent
The patient’s next of kin provided written informed consent for the use of biopsies and publication of case details of the patient.
Consent for publication
The patient’s next of kin provided consent for the publication of case details of the patient.
References
- 1.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S-I, Watanabe H, Kurashina K, Hatanaka H, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 2.Solomon B, Varella-Garcia M, Camidge DR.. ALK gene rearrangements: a new therapeutic target in a molecularly defined subset of non-small cell lung cancer. J Thorac Oncol. 2009;4:1450–1454. doi: 10.1097/JTO.0b013e3181c4dedb. [DOI] [PubMed] [Google Scholar]
- 3.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SHI, Dezube BJ, Jänne PA, Costa DB, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katayama R, Shaw AT, Khan TM, Mino-Kenudson M, Solomon BJ, Halmos B, Jessop NA, Wain JC, Yeo AT, Benes C, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med. 2012;4:120ra17. doi: 10.1126/scitranslmed.3003316 [DOI] [PMC free article] [PubMed]
- 5.Camidge DR, Bang YJ, Kwak EL, Iafrate AJ, Varella-Garcia M, Fox SB, Riely GJ, Solomon B, Ou SH, Kim DW, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13: 1011–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doebele RC, Pilling AB, Aisner DL, Kutateladze TG, Le AT, Weickhardt AJ, Kondo KL, Linderman DJ, Heasley LE, Franklin WA, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18:1472–1482. doi: 10.1158/1078-0432.CCR-11-2906 [DOI] [PMC free article] [PubMed]
- 7.Awad MM, Engelman JA, Shaw AT. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N Engl J Med. 2013;369:1173. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S, Anjum R, Squillace R, Nadworny S, Zhou T, Keats J, Ning Y, Wardwell SD, Miller D, Song Y, et al. The potent ALK inhibitor brigatinib (AP26113) overcomes mechanisms of resistance to first- and second-generation ALK inhibitors in preclinical models. Clin Cancer Res. 2016;22:5527–5538. doi: 10.1158/1078-0432.CCR-16-0569. [DOI] [PubMed] [Google Scholar]
- 9.Gainor JF, Dardaei L, Yoda S, Friboulet L, Leshchiner I, Katayama R, Dagogo-Jack I, Gadgeel S, Schultz K, Singh M, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6:1118–1133. doi: 10.1158/2159-8290.CD-16-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katayama R. Drug resistance in anaplastic lymphoma kinase-rearranged lung cancer. Cancer Sci. 2018;109:572–580. doi: 10.1111/cas.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asao T, Fujiwara Y, Itahashi K, Kitahara S, Goto Y, Horinouchi H, Kanda S, Nokihara H, Yamamoto N, Takahashi K, et al. Sequential use of anaplastic lymphoma kinase inhibitors in japanese patients with ALK-rearranged non-small-cell lung cancer: a retrospective analysis. Clin Lung Cancer. 2017;18:e251–e8. doi: 10.1016/j.cllc.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Barrows SM, Wright K, Copley-Merriman C, Kaye JA, Chioda M, Wiltshire R, Torgersen KM, Masters ET. Systematic review of sequencing of ALK inhibitors in ALK-positive non-small-cell lung cancer. Lung Cancer (Auckl). 2019;10:11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito K, Hataji O, Kobayashi H, Fujiwara A, Yoshida M, D’Alessandro-Gabazza CN, Itani H, Tanigawa M, Ikeda T, Fujiwara K, et al. Sequential therapy with crizotinib and alectinib in ALK-rearranged non-small cell lung cancer-a multicenter retrospective study. J Thorac Oncol. 2017;12:390–396. doi: 10.1016/j.jtho.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Chiari R, Metro G, Iacono D, Bellezza G, Rebonato A, Dubini A, Sperduti I, Bennati C, Paglialunga L, Burgio MA, et al. Clinical impact of sequential treatment with ALK-TKIs in patients with advanced ALK-positive non-small cell lung cancer: results of a multicenter analysis. Lung Cancer (Amsterdam, Netherlands). 2015;90(2):255–260. doi: 10.1016/j.lungcan.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Lin JJ, Zhu VW, Yoda S, Yeap BY, Schrock AB, Dagogo-Jack I, Jessop NA, Jiang GY, Le LP, Gowen K, et al. Impact of EML4-ALK variant on resistance mechanisms and clinical outcomes in ALK-positive lung cancer. J Clin Oncol. 2018;36: 1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]


