Highlights
-
•
The emerging PDCoV broadly infects porcine, human and chicken cells in vitro.
-
•
PDCoV N protein interacts with the IRF7 in a species-specific manner.
-
•
PDCoV N protein induces the porcine IRF7 degradation via ubiquitin-proteasomal pathway.
-
•
The mechanism of PDCoV N protein suppressing the porcine type I IFN is different from those by other CoVs, such as SARS-CoV, MERS-CoV and PEDV.
Keywords: PDCoV, Nucleocapsid, Porcine IRF7, Ubiquitin-proteasome pathway, Species-specific
Abstract
Coronaviruses (CoVs) is showing obvious interspecies transmission, such as the SARS-CoV, MERS-CoV and SARS-CoV-2. Here, the emerging porcine deltacoronavirus (PDCoV) strain, isolated from Shanghai, China, broadly infects porcine, human and chicken cells in vitro. Previously studies by our group and others have confirmed that PDCoV nucleocapsid (N) protein performs an important role in antagonizing retinoic acid-induced gene I-like receptor (RLR) activation. However, the mechanism of PDCoV N protein suppressing porcine type I IFN production remains unclear, especially the downstream of porcine RLR signaling pathway. In the present study, porcine IRF7 (poIRF7) was identified as the interaction protein of PDCoV N protein through LC-MS/MS. The poIRF7 (268-487aa) was the key region of binding PDCoV N protein. Although IRF7 is a conserved functional protein in species, the PDCoV N protein has been confirmed to interact with only poIRF7 and significantly decrease poIRF7-induced type I IFN production, but not human or chicken IRF7. Furthermore, PDCoV N protein can promote poIRF7 degradation via the ubiquitin-proteasome pathway, which directly increased the K6, K11, and K29-linked polyubiquitination of poIRF7. Lysine 359 of poIRF7 was a key site in PDCoV N protein inducing poIRF7 degradation. Taken together, our results reveal a novel mechanism that PDCoV N protein could species-specifically interact with poIRF7 and then promote its degradation to suppress porcine type I IFN production. The novel findings provide a new insight into PDCoV and other zoonotic coronavirus evading the innate immune response of different species.
1. Introduction
Coronaviruses (CoVs) are enveloped, positive-sense RNA viruses that cause severe disease in mammals and birds, such as humans, swine and chickens. CoVs are classified into four genera: Alpha-CoV, Beta-CoV, Gamma-CoV and Delta-CoV. The Alphacoronavirus and Betacoronavirus are isolated from mammals. The evolution analysis of CoVs strains from different regions present obviously clustering characteristics, which present relatively high mutation and recombination rates. This provides a basis for CoVs producing higher virulence strain and adapting to novel hosts, such as the PEDV, SARS-CoV and MERS-CoV(Mingjun et al., 2019; Killerby et al., 2020; Wendong et al., 2005). The recent SARS-CoV-2 has caused a public health emergency, which has infected millions people in globally. SARS-CoV and SARS-CoV-2 has been confirmed interspecises transmission from bat (Liang et al., 2018; Peng et al., 2020). They partly tend to cross-species transmission depending on their spike (S) protein binding to the angiotensin converting enzyme 2 (ACE2) of various mammals, such as the bat and swine (Liang et al., 2018; Peng et al., 2020). The Delta-CoV members are isolated from mammals and birds. Hence, the swine may be the porteinal swine-origin host for inter-species transmission of some CoVs, which is like the transmission of influenza virus in poultry, swine and human (Yi et al., 2014). The emerging porcine daltacoronavirus (PDCoV) was firstly discovered in Hong Kong in 2012 (Patrick et al., 2012). PDCoV infects the porcine intestinal epithelia inducing diarrhoea, vomiting, dehydration and death, especially in new-born piglets. PDCoV has been detected in United States, China, Canada, Korea, Thailand and Laos, which is causing serious economic losses to the swine industry. To date, PDCoV is the only member of the Delta-CoV genus, which could be isolated and cultured in vitro. Therefore, it is very important to better understand the hosts, evolution and the pathogensis of PDCoV.
The innate immune system depends on pattern recognition receptors (PRRs) to recognize viral infection. At least three PRRs that sense CoVs infection may be involved: retinoic acid-inducible gene-I (RIG-I, also known as DDX58), melanoma differentiation-associated gene 5 (MDA5) and toll-like receptor 7 (TLR7) (Jianfeng et al., 2010; Cervantes-Barragan et al., 2007). RIG-I and MDA5 can recognize the different length dsRNA in CoVs genome replication. They can bind to the mitochondrial antiviral protein (MAVS, also known as IPS-1/VISA) depending on their N-terminal domain (CARD). The MAVS recruits and activates the TRAF3/TBK1 to promote phosphorylation of IFN regulator factor (IRF3 or IRF7), which translocate into the nucleus promoting type I IFN production. The chicken MDA5-IRF7 axis may be the most important for chicken to antagonize RNA virus infection (Yuqiang et al., 2015). On the other hand, the viral single-stranded RNA (ssRNA) can be sensed by the TLR7 in cytoplasm after ssRNA virus-infection. TLR7 activation triggers production of the type I IFN through MyD88-mediated IRF7 signaling pathway (Hiroaki et al., 2002).
Most CoVs have been confirmed to antagonize the host immune response with various approaches after infection, such as SARS-CoV, MERS-CoV, MHV-CoV, PEDV and PDCoV (Yong et al., 2017; Zhen et al., 2014; Jingyi et al., 2016). Aside from encapsulating the viral RNA genome, the CoV nucleocapsid has multiple functions in regulating host cell stress responses and signal transduction (McBride et al., 2014). Previously, we had found that the PDCoV N protein was an import antagonist of the porcine RLR signaling pathway by interfering with porcine RIG-I activation, which decreased porcine IFN-β (pIFN-β) production (Likai et al., 2019). This function was also found in the human RLR signaling pathway (Jun et al., 2019). However, the PDCoV N protein also can significantly suppress the porcine RIG-I downstream signaling proteins inducing porcine IFN-β production, but not in human. Hence, there may be another target protein existing in the porcine signaling pathway. The PDCoV N protein expression was subjected to change the expression levels of many host immune proteins in the PDCoV-N-expressing PK-15 (PK-PDCoV-N) cells (Sunhee and Changhee, 2015). However, the changing of these proteins could not clearly explain the mechanism of the PDCoV N protein inhibiting porcine IFN production. Detailed study of the interaction network of the PDCoV N protein with porcine cellular proteins will provide more information to explore its mechanism suppressing porcine type I IFN production.
Thus, we construct the interaction network of the N protein from the emerging PDCoV Shanghai strain, exploring the mechanism in antagonizing the porcine type I IFN production. Although the emerging strain presents potential of cross-species infection, including human and chicken, we have confirmed that the PDCoV N protein can only interact with poIRF7 and actively its degradation through the ubiquitin-proteasome pathway in a species-specific manner to antagonize type I IFN production in porcine cells.
2. Materials and method
2.1. Cell cultures and virus
HEK293 T, PK-15, LLC-PK1, 3D4/21, IPEC-J2 and DF-1 cells were purchased from American Type Culture Collection (ATCC). These cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with a 10% fetal bovine serum (Gibco, USA) and maintained at 37 °C in 5% CO2. The emerging PDCoV Shanghai strain was cultured using LLCPK1 cells as reported previously (Hu et al., 2015). The recombinant VSV-GFP virus was generously provided by Dr. Sun Tao, Shanghai Jiao Tong University, China.
2.2. Genome sequencing and phylogenetic analysis
Virus RNAs were extracted using the TIANGEN viral RNA kit (TIANGEN, China). The cDNA was synthesized using the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, USA). Each sample was first screened by PCR followed by viral isolation or purification, sequenced by sanger sequencing. Total 31 PDCoV strains and one bird deltacoronavirus strain genome and S gene sequences used in this study can be retrieved and available from NCBI GenBank (https://www.ncbi.nlm.nih.gov/) (GenBank accession numbers in Supplementary Table S1). All sequences were aligned using ClustalW and manually adjusted in MEGA7 with the neighbour-joining method. Genome sequences were aligned with the nucleotide, while the S protein coding sequences were aligned with the amino acid.
2.3. Plasmids
The porcine IRF7 (poIRF7) was amplified from cDNA generated from PK-15 cells, then cloned into plasmid pcDNA3.1-Flag or pcDNA3.1-HA to generate tagged proteins. Several mutations of poIRF7 were cloned into plasmid pcDNA3.1-Flag and confirmed by sequencing, including amino acid region (1-261, 262-487) and lysine mutated to arginine (K50R, K61R, K119R, K230R, K280R, K325R, K349R, K359R, K428/430R). Human IRF7 (hIRF7) was amplified from cDNA generated from HEK293 T cells, then cloned into plasmid pcDNA3.1-Flag. V5-tagged chicken IRF7 (chIRF7) and pGL3-chIFN-β-Luc were constructed in our laboratory as described previously (Yuqiang et al., 2015). Flag-tagged PDCoV-N, HA-tagged PDCoV-N, Myc-tagged PDCoV-N, and pGL3-pIFN-β-Luc were constructed in our laboratory as described previously (Likai et al., 2019). pGL3-hIFN-α-Luc was constructed according to a previous study (Lin et al., 2000). The pGL3-pIFN-α-Luc (-245 to -1) was amplified from DNA extracted from porcine peripheral red blood cells. HA-tagged ubiquitin (Ub) and Ub mutant (K48R, K6, K11, K27, K29, K33, K48, K63, and K6/11/29O) plasmids were kindly provided by Dr. Yuan Congli, Shanghai Jiao Tong University, China.
2.4. LC-MS/MS
The Flag-tagged PDCoV-N protein expression plasmids were transfected into the PK-15 cells. The whole cell lysates were prepared and subjected to coimmunoprecipitation with anti-Flag affinity gel (Biotool, Germany), SDS-PAGE and Coomassie brilliant blue staining. The resulting separated proteins were excised and digested with trypsin. The peptides were separated and identified with liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Micromass, Inc.). The data were referenced with the Uniprot database for protein identification.
2.5. Co-immunoprecipitation (Co-IP) and Western blot assay
HEK293 T or PK-15 cells in 10 cm culture dishes were co-transfected with the recombination expression plasmid or an empty vector. After 28 h, the cells were lysed on ice for 20 min in 600 μl of lysis buffer (50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 10% glycerin, 0.1% SDS, and 2 mM Na2EDTA) containing a protease inhibitor mixture plus the protease inhibitor PMSF. The cell lysates were then immunoprecipitated at 4 °C with mouse anti-Flag or anti-HA affinity gel (Biotool, USA). The immunoprecipitants were washed four times with 1×Tris-buffered saline and then subjected to Western blot analysis.
The cells were harvested by adding lysis buffer for 30 min at 4 °C supplemented with a protease inhibitor cocktail, phenylmethylsulphonyl fluoride (PMSF), and a phosphatase inhibitor cocktail. The lysates were subjected to SDS-PAGE and electroblotted onto a polyvinylidene difluoride membrane (Bio-Rad, USA). The membranes were then analyzed for the expression proteins by Western blot using primary antibodies overnight at 4 ℃ and HRP-conjugated secondary antibodies for 2 h at room temperature. The enhanced chemiluminescence was used to visualize and was quantified using Image J software.
2.6. Indirect Immunofluorescence Assay (IFA)
LLC-PK1 cells were seeded onto a microscopy cell culture slide, placed into 12 well plates, and allowed to reach approximately 80% confluence. After PDCoV (MOI = 0.5) infection 24 h, the cells were fixed with 4% paraformaldehyde for 10 min and then permeabilized with triton for 10 min at room temperature. After being washed three times with TBST, the cells were blocked with TBST containing 5% bovine serum albumin (BSA) for 1 h and then incubated separately with a mouse monoclonal antibody against PDCoV N protein (1:500), or a rabbit polyclonal antibody against the poIRF7 or HA-tagged PDCoV N protein (1:1000) for 1 h. The cells were then treated with Alexa Fluor 488 or Alexa Fluor 555 secondary antibody for 1 h at room temperature and subsequently treated with 4’, 6-diamidino-2-phenylindole (DAPI) for 15 min at room temperature. The antibody and DAPI used in the present study were purchased from Beyotime, China. Fluorescent images were visualized and examined using an LSM 880 Zeiss confocal microscope (Carl Zeiss, Jena, Germany).
2.7. Dual-Luciferase reporter gene assay
HEK293 T, PK-15 or DF-1 cells were grown in 24-well plates. In selected experiments, the recombination or empty expression plasmids were cotransfected with pGL3-pIFN-β-Luc or pGL3-pIFN-α-Luc and pRL-TK (an internal control for the normalization of transfection efficiency) using Lipofectamine 2000 (InvivoGen, USA). After transfection for 24 h, the cells were lysed. Then the activation levels of firefly luciferase and Renilla luciferase were measured using the Dual-Luciferase reporter assay system (Promega, USA). Data were shown as the relative firefly luciferase activities normalized to the Renilla luciferase activities from three independent experiments.
2.8. RNA extraction and quantitative real-time PCR
Total RNA was extracted from cultured cells using Trizol reagent (Invitrogen) and was reverse transcribed into cDNA using reverse transcriptase (TaKaRa, Japan). Quantitative real-time PCR (qRT-PCR) experiments were performed in triplicate. Relative mRNA expression levels were normalized to the expression level of GAPDH. All qRT-PCR experiments were performed using Low ROX SYBR Green PCR master mix (Vazyme, China) and an ABI 7500 Real-time PCR system.
2.9. Ubiquitin assay
To analyze the ubiquitination of poIRF7 or its mutation, HEK293 T or PK-15 cells were cotransfected with Flag-poIRF7, HA-Ub or HA-Ub mutants (K48R, K6, K11, K27, K29, K33, K48, K63, K6/11/29O) and Myc-PDCoV-N or empty expression plasmids for 28 h. The cells were washed twice in PBS supplemented with 10 mM NEM and lysed with 1% SDS lysis buffer (25 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 1% SDS) containing the protease inhibitor PMSF and 10 mM NEM. The mouse anti-Flag affinity gel was pretreated three times with 1 × TBS, and then the cell lysates were added and incubated for 3 h at 4 °C. After being washed three times with 1 × TBS, the immunoprecipitants were boiled at 100 °C for 10 min and subjected to Western blot analysis.
2.10. Statistical analysis
Data were expressed as means ± standard deviations. Significance was determined with a one-way ANOVA test to analyze the differences in multiple groups (> = 3).
3. Results
3.1. The emerging PDCoV Shanghai strain cross–species infection in vitro
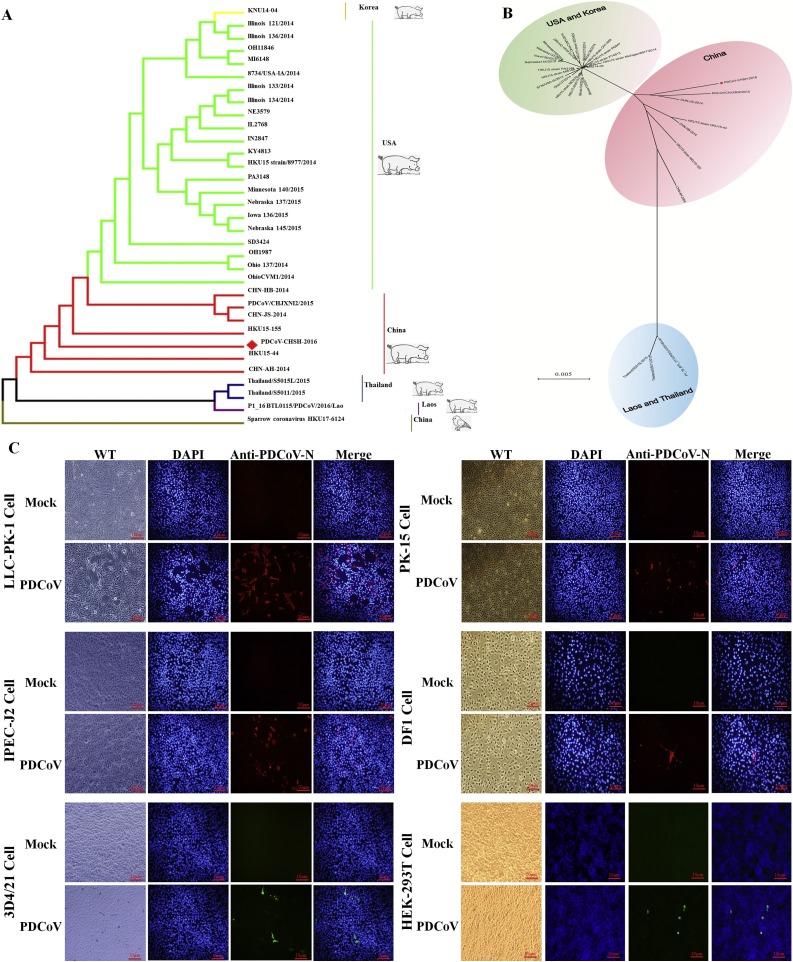
One emerging PDCoV strain was isolated from a suckling piglet with acute diarrhea from China Shanghai in 2016. The phylogeny based on the full genomes of the deltacoronavirus from the pig and bird revealed obvious species and regional differences (Fig. 1 A). The isolated PDCoV strains could be divided into three lineages, which were named as the USA lineage (strains isolated from USA and Korea), China lineage (strains isolated from China) and the Southeast Asia lineage (strains isolated from Thailand and Laos) (Fig. 1B). The emerging isolated Shanghai strain, PDCoV-CHSH-2016, clustered with the China lineage (red dots in Fig. 1A and B). To investigate the susceptibility of PDCoV on different cells, several cell lines of porcine, chicken and human were infected with same dose of PDCoV at a MOI of 1. After 24 h infection, the IFA has been performed and the results show that specific fluorescence in the PDCoV-infected cells but not in the negative control cells (Mock), including porcine cells (LLC-PK1, PK-15, IPEC-J2, 3D4/21), chicken cells (DF-1) and human cells (HEK293 T) (Fig. 1C). It indicated that the emerging PDCoV Shanghai strain has the potential of trans-species infection.
Fig. 1.
The PDCoV Shanghai strain cross-sepecises infection in vitro. (A) Phylogeny reconstructed using 32 PDCoV and 1 bird deltacoronavirus (HKU17-6124) genomes, or (B) the amino acid of 32 PDCoV S protein by the MEGA7 software with the neabor-jioning algorithm. Strains in red regions were from China. Strains in blue regions were from Thailand and Laos. (C) IFA identification of PDCoV with the PDCoV-N antibody in uninfected or infected cells, including porcine (LLC-PK1, PK-15, IPEC-J2, 3D4/21), chicken (DF-1) and human (HEK293 T) cell lines.
3.2. PDCoV N protein interacted with poIRF7
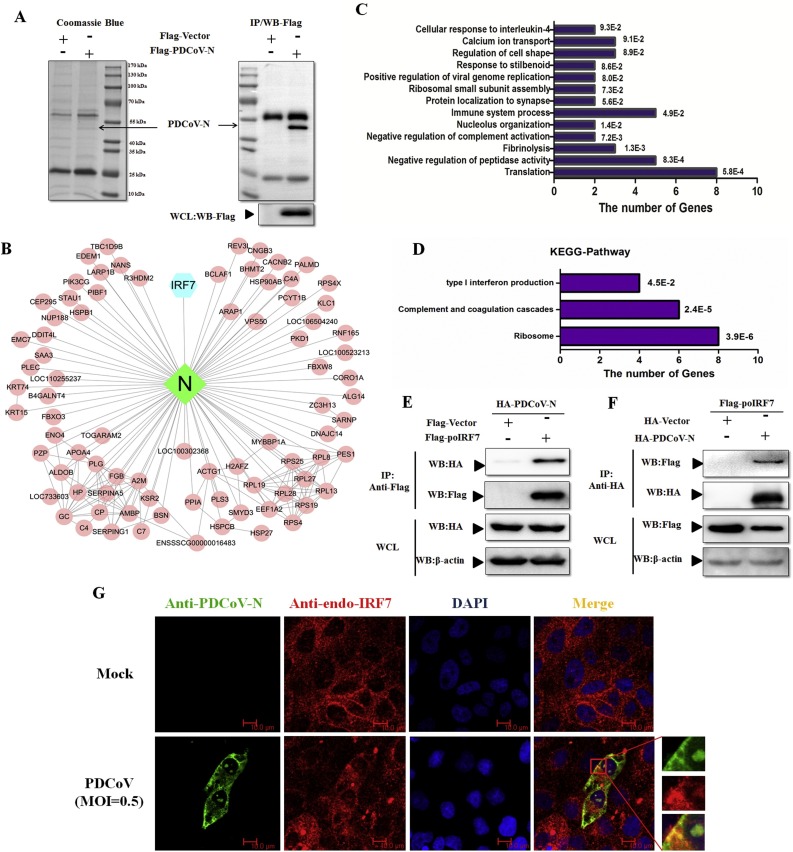
PDCoV infection could antagonize porcine type I IFN production in LLC-PK1 cells (Supplementary Fig. S1). The PDCoV N protein is the important antagonist (Likai et al., 2019; Jun et al., 2019). Thus, to investigate the intracellular interaction proteins of the PDCoV N protein in porcine cells, Flag-tagged PDCoV-N, or Flag-vector as control, were transfected into PK-15 cells. Their whole cell lysates were co-immunoprecipitated with anti-Flag affinity gel. The immunoprecipitates were analyzed by Coomassie blue staining and Western blot with anti-Flag antibody (Fig. 2 A). Compared with the Flag-Vector, there were 77 specific proteins identified in the Flag-PDCoV-N immunoprecipitates by LC-MS/MS analysis (Fig. 2B). We highlighted statistical enrichment for specific biological processes (BP) or cellular components (CC) as determined by gene ontology (GO) analysis. The results of the GO enrichment analysis showed that these proteins were clustered into 13 groups representing prominent cellular modules, including translation, immune system processes, and negative regulation of peptidase activity (Fig. 2C). The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis results found that three important pathways were identified: type I IFN production, ribosome, complement and coagulation cascades (Fig. 2D). In these data, the poIRF7 was identified as a conditional interaction protein of the PDCoV N protein (Fig. 2B).
Fig. 2.
PDCoV N protein interacts with poIRF7. (A) The immunoprecipitates of PDCoV N protein in PK-15 cells were separated with SDS-PAGE, and then detected by Coomassie blue staining or Western blot with anti-Flag antibody. (B) The interaction net-work was built with Cytoscape software. (C, D) Results of analysis of (C) Gene ontology (GO) enrichments and (D) the Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichments of the specific proteins identified in the Flag-PDCoV N immunoprecipitates. The Y-axis is the name of each category, while the X-axis is the number of genes enriched in each category. Their p values were shown at the end of each bar. (E, F) HEK293 T cells were co-transfected with Flag-poIRF7 or Flag-Vector and HA-PDCoV-N or HA-Vector expression plasmids for 28 h. Cells were lysed for co-immunoprecipitation (Co-IP) assay with anti-Flag (IP: anti-Flag) or anti-HA (IP: anti-HA) affinity gel. The whole cell lysates (WCLs) and immunoprecipitants were detected by Western blot with anti-Flag, anti-HA or anti-β-action antibody. (G) The confocal microscope assay was used to detect the localization of PDCoV-N (green) and endogenous poIRF7 (endo-poIRF7, red) in the mock or PDCoV infected LLC-PK1 cells, respectively. DAPI (blue) stained the cell nuclei. Fluorescent images were acquired with a confocal laser scanning microscope (scar bar: 10 μm).
To verify PDCoV N protein interaction with poIRF7, HEK293T cells were used to express the Flag-tagged poIRF7 and HA-tagged PDCoV N proteins. The PDCoV N proteins were clearly coimmunoprecipitated with Flag-poIRF7 but not the Flag-vector by anti-Flag affinity gel (Fig. 2E). Moreover, the interaction between PDCoV N protein and poIRF7 was remarkably observed by immunoblotting the anti-HA affinity gel immunoprecipitates with anti-Flag antibody (Fig. 2F). Furthermore, the IFA results showed that the PDCoV N protein and endogenous poIRF7 (endo-poIRF7) were significantly colocalized in the cytoplasm of PDCoV-infected LLC-PK1 cells (Fig. 2G). These data collectively suggest that poIRF7 is an interaction partner of the PDCoV N protein.
3.3. PDCoV N protein interacted with poIRF7 in a species-specific manner
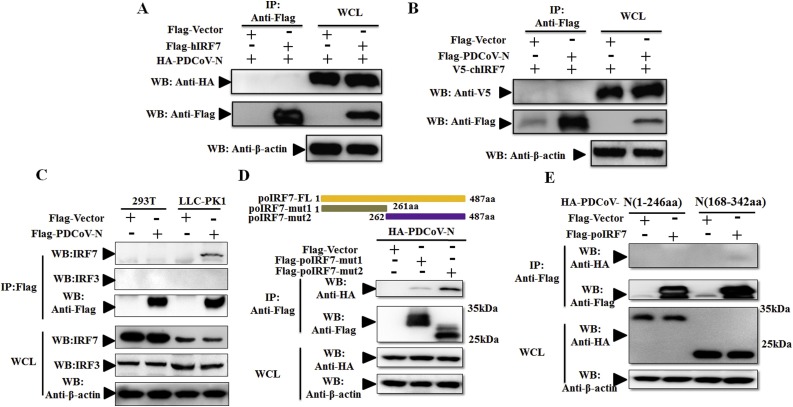
Unlike IRF3, IRF7 as a conservative protein is expressed in mammals and chickens, which is necessary to promote type I IFN production. On the other hand, PDCoV could infect the human and chicken cells (Fig. 2). Thus, whether IRF7 is the target partner of the PDCoV N protein in humans and chickens, we tested the ability of the PDCoV N protein to interact with their homologous protein of poIRF7. Co-immunoprecipitation (Co-IP) studies in transfected HEK293 T cells have shown that PDCoV N protein interacts with neither human IRF7 (hIRF7) nor chicken IRF7 (chIRF7) (Fig. 3 A and B). To further explore the existing interaction in a species-specific manner, the commercial hIRF7 and hIRF3 antibodies were used to respectively detect the endo-IRF7 and endo-IRF3 protein in HEK293 T and LLC-PK1 cells. The results showed that as a homologous protein of hIRF7 and hIRF3, poIRF7 and poIRF3 could be respectively recognized by hIRF7 or hIRF3 antibody (Fig. 3C). However, PDCoV N could be co-immunioprecipitated with endo-poIRF7, but not endo-hIRF7 (Fig. 3C). Neither porcine nor human endo-IRF3 protein was detected in the co-immunoprecipitants of the PDCoV N protein (Fig. 3C). To determine which region of poIRF7 facilitates its interaction with the PDCoV N protein, two Flag-tagged poIRF7 mutant plasmids (the N-terminal region 1-261aa and C-terminal region 262-487aa), as described in Fig. 3D (up), were respectively expressed with HA-tagged PDCoV N protein in HEK293 T cells. The C-terminal region of poIRF7 mainly co-immunoprecipitated with PDCoV N, suggesting that poIRF7 (262-487aa) might be essential for the association of poIRF7 with the PDCoV N protein (Fig. 3D). To investigate the major region of the interaction between the PDCoV N protein and poIRF7, two truncated mutants of the PDCoV N protein (1-246aa, and 168-342aa) were constructed and then cotransfected with Flag-poIRF7 into HEK293 T cells. The Co-IP results showed that neither PDCoV N (1-246aa) nor N (168-342aa) could clearly co-precipitate with poIRF7 (Fig. 3E). These results collectively demonstrate that the PDCoV N protein might depend on its complete protein to interact with IRF7 in a species-specific manner.
Fig. 3.
PDCoV N protein interacted with poIRF7, but not hIRF7 or chIRF7. (A, B) HEK293 T cells were co-transfected with HA-tagged PDCoV-N and Flag-tagged hIRF7 (A) or V5-tagged chIRF7 and Flag-tagged PDCoV-N (B), respectively. (C) HEK293 T cells or LLC-PK1 cells were transfected with Flag-tagged PDCoV-N for 28 h. The porcine or human endo-IRF7 and endo-IRF3 were respectively detected by the commercialized human IRF7 or IRF3 antibody. (D) Schematic representation of PDCoV N protein and poIRF7 fragments used for Co-IP analysis. HEK293 T cells were co-transfected with HA-tagged PDCoV-N and truncated fragments of Flag-tagged poIRF7. (E) HEK293 T cells were co-transfected with Flag-tagged poIRF7 and HA-tagged truncated fragments of the PDCoV N protein. After 28 h of transfection, cells were lysed for co-immunoprecipitation (Co-IP) assay with anti-Flag (IP: anti-Flag) affinity gel. The WCLs and immunoprecipitants were detected by Western blot with an-ti-Flag, anti-HA or anti-β-action antibody.
3.4. PDCoV N protein specifically suppressed poIRF7-induced type I IFN production
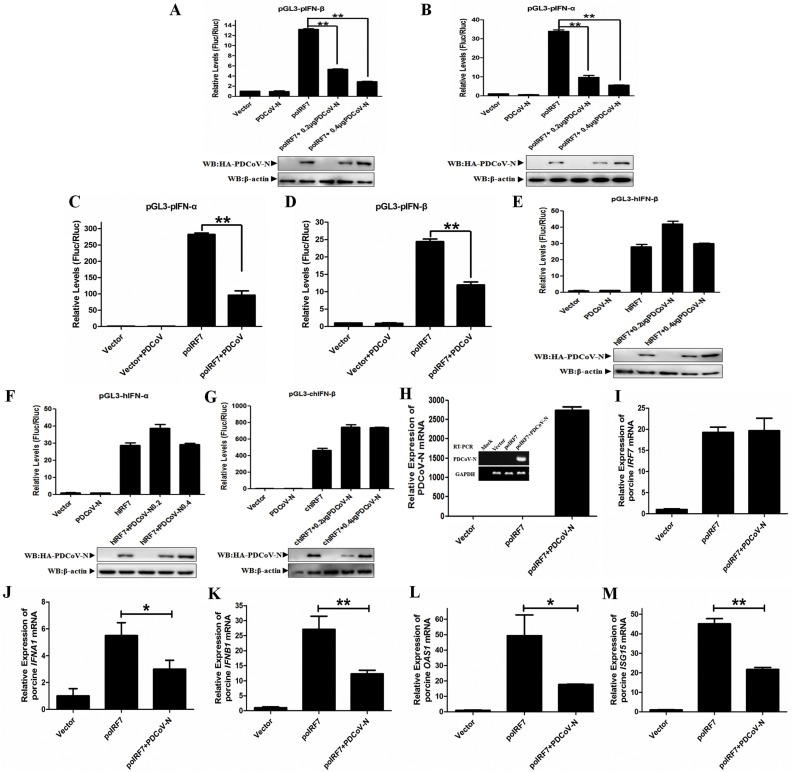
IRF7 is an essential transcription factor for type I IFN production. To investigate the effects of PDCoV N protein interaction with poIRF7 on type I IFN production, dual-luciferase reporter assays demonstrated that eukaryotic-expression of the PDCoV N protein suppressed both poIRF7-induce pIFN-β and pIFN-α promotor activation, which was dose-dependent (Fig. 4 A and B). It was consistent with the PDCoV infection on poIRF7-induced type I IFN, which significantly suppressed the poIRF7-induced pIFN-α and pIFN-β promoter activity in LLC-PK1 cells (Fig. 4C and D). However, eukaryotic-expression PDCoV N protein could not affect the activation level of hIRF7-induced hIFN-β nor hIFN-α in HEK293 T cells (Fig. 4E and F). The chIRF7-induced chIFN-β transcription activation also could not be interfered by the PDCoV N protein in DF-1 cells (Fig. 4G). To further confirm that PDCoV N inhibited poIRF7-induced type I IFN production, the qRT-PCR was subjected to detect the expression level of porcine type I IFN and several IFN-induced genes (ISGs) in PK-15 cells. The qRT-PCR and RT-PCR results showed that PDCoV N protein expression cannot interfere with poIRF7 transcription in PK-15 cells (Fig. 4H and I). However, the poIRF7-induced porcine type I IFN (porcine IFNB1 and IFNA1), porcine OAS1, and porcine ISG15 mRNA expression were significantly decreased by eukaryotic-expression of the PDCoV N protein (Figs. 4J to M). Together, these data demonstrate that poIRF7 is the key target of the PDCoV N protein to suppress porcine type I IFN production.
Fig. 4.
PDCoV N protein only suppressed poIRF7 induced-type I IFN production. (A, B) PK-15 cells were co-transfected with HA-PDCoV-N (0 ng, 200 ng, 400 ng), Flag-poIRF7 or empty vector expression plasmids, along with pGL3-pIFN-β or pGL3-pIFN-α, pRL-TK (as a normalizing control). (C, D) LLC-PK1 cells were uninfected or infected with PDCoV at a MOI of 0.5 for 6 h, and then the cells were co-transfected with the pIFN-α (C) or pIFN-β (D) promoter luciferase reporter and the Flag-tagged poIRF7 or an empty vector plasmids for 24 h. The cell lysates were harvested and subjected to a dual-luciferase assay. (E, F) HEK293 T cells were co-transfected with HA-PDCoV-N (0 ng, 200 ng, 400 ng), Flag-hIRF7, or empty vector, along with pGL3-hIFN-β or pGL3-hIFN-α, pRL-TK. (G) DF1 cells were co-transfected with HA-PDCoV-N (0 ng, 200 ng, 400 ng), V5-chIRF7 or empty vector expression plasmids, along with pGL3-chIFN-β, pRL-TK. Dual-luciferase assays were performed at 24 h post-transfection. The relative firefly luciferase activity was relative to that of an empty vector control. Western blot was used to detect the protein expression of PDCoV N with HA antibody. The β-actin was the loading control. (H-M) PK-15 cells cultured in 12-well plates were transfected with HA-PDCoV-N or empty expression plasmids together with the Flag-poIRF7 for 24 h. The cells were lysed with TRIZOL to extract the total RNA. The qRT-PCR or PCR was used to detect the relative expression of PDCoV-N (H), poIRF7 (I), porcine type I IFN genes (including IFNA1 and IFNB1) (J, K) and ISGs (L, M) mRNA. All data are presented as means ± SD of three independent experiments (*p < 0.05 and **p < 0.01).
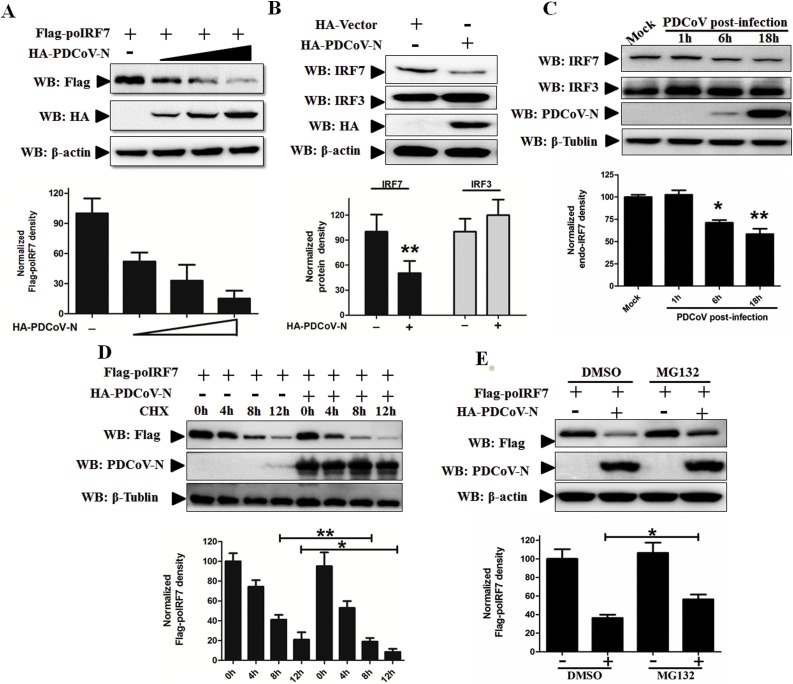
3.5. PDCoV N protein increased proteasomal degradation of poIRF7
To explore the mechanism of PDCoV N protein target poIRF7 in decreasing type I IFN production, the poIRF7 expression plasmids were cotransfected with the empty vector or PDCoV N expression plasmids in PK-15 cells. Western blot analysis indicated that the poIRF7 protein was remarkably and dose-dependently decreased by the expression of the PDCoV N protein (Fig. 5 A). Moreover, the endo-poIRF7 was also degraded in PDCoV N protein-overexperession or PDCoV-infected cells (Fig. 5B and C). As the control, we detected the endo-poIRF3 expression levels and found that the PDCoV N protein had no effect on poIRF3 expression (Fig. 5B). Then, we performed cycloheximide (CHX) chase assay to analyse the poIRF7 half-life, and found that the PDCoV N protein increased the rate of poIRF7 protein turnover (Fig. 5D). These results indicate that eukaryotic expression of PDCoV N protein decreased the poIRF7 protein. Furthermore, compared with the DMSO treated cells, the proteasome inhibitor MG132 was added to prevent PDCoV N protein-mediated poIRF7 degradation (Fig. 5E). These results indicate that the PDCoV N protein could specifically promote poIRF7 proteasomal degradation.
Fig. 5.
PDCoV N protein promoted the degradation of poIRF7. (A) PK-15 cells were transfected with 200 ng of Flag-poIRF7 with increasing amounts of HA-PDCoV-N plasmid (200 ng, 400 ng, or 800 ng) for 24 h. (B) PK-15 cells were transfected with 1 μg HA-PDCoV-N or empty vector plasmid for 24 h. (C) LLC-PK1 cells were mock-infected or infected by PDCoV at MOI of 0.5. The whole cell proteins were extracted at the indicated post-infection hours. (B, C) The whole cell lysates were analyzed through Western blot with the commercialized hIRF7 or hIRF3 antibody. (D) PK-15 cells were co-transfected with 300 ng Flag-tagged poIRF7 and 700 ng HA-tagged PDCoV-N or empty vector plasmids. After 24 h, cells were treated with protein synthesis inhibitor cycloheximide (CHX) 200 μg/ml for the indicated time before analysis of the protein levels by Western blot. (E) PK-15 cells were co-transfected with 300 ng Flag-tagged IRF7 and 700 ng HA-tagged PDCoV-N or empty vector plasmids for 18 h. Then, the cells were treated with MG132 (25 μM) or DMSO as a control for 6 h before harvesting. The ex-pression level of poIRF7, which was normalized to the β-actin, was determined by densitometry analysis with ImageJ software. All of the experiments were performed independently three times.
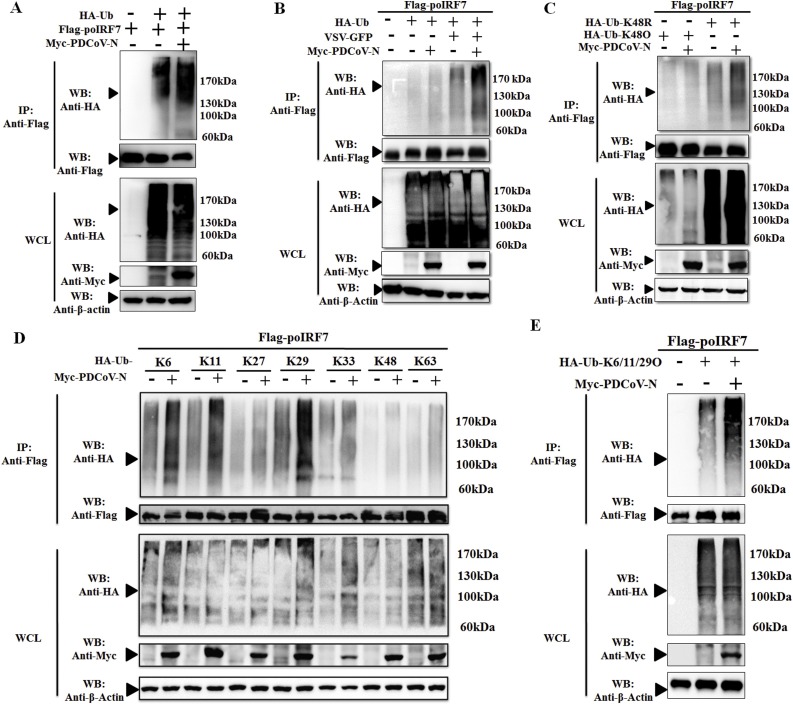
3.6. PDCoV N protein promoted poIRF7 polyubiquitination
Ubiquitination is an important post-translational modification to regulate protein activation or degradation. Hence, we investigated whether the degradation of poIRF7 induced by the PDCoV N protein was due to ubiquitination. PK-15 or HEK293T cells were co-transfected with Flag-tagged poIRF7, Myc-tagged PDCoV N or empty vector expression plasmids, together with the HA-tagged ubiquitination plasmids for 24 h. The results revealed that eukaryotic expression of the PDCoV N protein increased the polyubiquitination of poIRF7 (Fig. 6 A and B). When the cells were infected or uninfected with a recombinant vesicular stomatitis virus (VSV-GFP) for 6 h, the ubiquitination level was more obvious under virus infection (Fig. 6B). However, PDCoV N protein could antagonize the VSV-induced pIFN-β production in LLC-PK1 cells (Likai et al., 2019). Hence, we speculated that if PDCoV N protein could induce the poIRF7 degradation by promoting its K48-linked polyubiquitination to suppress the VSV-GFP induced pIFN-β production. To explore this conjecture, HEK293 T cells were co-transfected with the expression plasmids for Flag-poIRF7, HA-Ub-K48R or K48O (K48 only) in the presence or absence of the Myc-PDCoV N protein. The results show that the PDCoV N protein could not affect the K48O-linked polyubiquitination of poIRF7, but increased the K48R-linked polyubiquitination (Fig. 6C). These data suggest that other types of ubiquitination might be performed by the PDCoV N protein. To achieve this hypothesis, plasmids expressing different types of ubiquitin mutants retaining only a single lysine residue (K6, K11, K27, K29, K33, K63) were used. The co-IP and immunoblot results indicated that K6, K11 and K29-linked polyubiquitination of poIRF7 remarkably increased after PDCoV N protein expression, but with no effect on other linkages (Fig. 6D). To further demonstrate this result, the ubiquitin mutants retaining K6, K11 and K29 (K6/11/29O) was constructed and performed above experiment. As shown in the Fig. 6E, PDCoV N protein could obviously increase the K6/K11/K29-linked polyubiquitination of poIRF7 in PK-15 cells. Collectively, these data demonstrated that the PDCoV N protein induces the ubiquitination of poIRF7, which is mostly through K6, K11 and K29-linked polyubiquitination.
Fig. 6.
PDCoV N protein promoted the ubiquitination of poIRF7. (A) PK-15 cells were cotrans-fected with Flag-tagged poIRF7 and Myc-tagged PDCoV N or empty expression plasmids with HA-tagged ubiquitin (HA-Ub) or empty control plasmids. After 28 h of post-transfection, the cells were lysed for Co-IP by Flag-affinity gel. (B) HEK293 T cells were cotransfected with Flag-tagged poIRF7 and Myc-tagged PDCoV N or empty expression plasmids with HA-tagged ubiquitin (HA-Ub) or empty control plasmids. After 24 h, the cells were infected or uninfected with VSV-GFP strain for another 8 h. The cells were lysed for Co-IP by Flag-affinity gel. (C) HEK293 T cells were co-transfected with Flag-tagged poIRF7 and Myc-tagged PDCoV N or an empty control plasmid with HA-Ub K48R, or (D) K48 only, K6 only, K11 only, K27 only, K29 only, K33 only, K63 only, or (E) K6/11/29 only or empty control plasmids, respectively. After 28 h of transfection, cells were lysed for Co-IP with Flag-affinity gel. Western blot was used to detect the immunoprecipitants and WCLs protein.
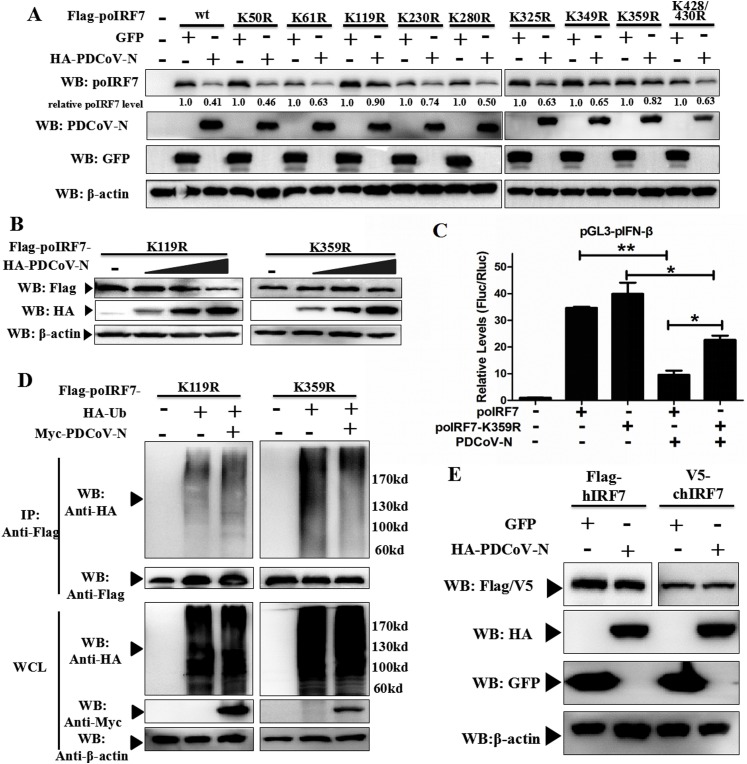
3.7. Lysine 359 was the key site for poIRF7 activation
To identify the ubiquitination site(s) in poIRF7, ten lysine sites were mutated with aspartate. PK-15 cells were co-transfected with HA-tagged PDCoV-N or GFP expression plasmid and either wild-type Flag-tagged poIRF7 (wt) or Lys residues mutant Flag-tagged poIRF7 (K50R, K61R, K119R, K230R, K280R, K325R, K349R, K359R, K428/430R). As shown in Fig. 7 A, the K119R or K359R mutant could significantly decrease the level of PDCoV N protein mediated-poIRF7 degradation. The result suggested that poIRF7 K119 and K359 might be the conditional ubiquitination sites in the presence of the PDCoV N protein. Moreover, when Flag-tagged poIRF7 and different doses of HA-tagged PDCoV N protein expression plasmids were cotransfected in PK-15 cells, we found that poIRF7 K359R could not be degraded by PDCoV N protein at any dose, but poIRF7 K119R could (Fig. 7B). Furthermore, the poIRF7-K359R mutation could significantly alleviate the PDCoV N protein-mediated pIFN-β promoter activity (Fig. 7C). Only the K359R mutant of poIRF7 was not ubiquitinated by the PDCoV N protein (Fig. 7D). These results indicate that the K359 of poIRF7 was the key site which induced ubiquitination by the PDCoV N protein. The K359 of poIRF7 were conserved lysine sites in human, chicken and pig by multi-sequence alignment (supplementary Fig. S2). However, PDCoV N protein coexpression with hIRF7 or chIRF7 could not induce their degradation in PK-15 cells (Fig. 7E). These results indicate that poIRF7 degradation depends on direct interaction with the PDCoV N protein.
Fig. 7.
PDCoV N protein promoted the ubiquitination of poIRF7 K359 site. (A) PK-15 cells were co-transfected with Flag-tagged wild-type poIRF7 (wt) or its lysine mutants expression plasmids and constructs HA-tagged PDCoV N or GPF protein. Western blot was used to analyze thier expression after 28 h of transfection. The relative expression level of poIRF7 was normalized to the corresponding control group through Image J software analysis. (B) PK-15 cells were co-transfected with Flag-tagged poIRF7 K119R or K359R and dose-dependent HA-tagged PDCoV N protein expression plasmids. (C) HEK293 T cells were co-transfected with HA-tagged Ub, Flag-tagged poIRF7 mutants (K119R, K359R), and Myc-tagged PDCoV N or empty expression plasmids. After 28 h of transfection, the cells were lysed and immunoprecipitated with anti-Flag affinity gel. The WCL and immunoprecipitants were analyzed by Western blot with anti-Flag, anti-HA, anti-Myc and anti-β-actin antibodies. (D) PK-15 cells were co-transfected with Flag-poIRF7 or Flag-poIRF7-K359R or empty vector expression plasmids, along with pGL3-pIFN-β, pRL-TK and HA-PDCoV-N or empty vector expression plasmids. After 24 h transfection, the dual-luciferase assay was performed. All data are presented as means ± SD of three independent experiments (*p < 0.05, **p < 0.01). (E) PK-15 cells were co-transfected with Flag-tagged hIRF7 or V5-tagged chIRF7 and HA-tagged PDCoV N or GFP expression plasmids. At 28 h post-transfection, the cells were lysed to analyze the hIRF7 or chIRF7 expression by Western blot with anti-Flag or anti-V5 antibodies.
4. Discussion
The swine as the wildly domesticated animals is the most important host of zoonosis viruses, such as the swine-origin AIV, Japanese bencephahlitis virus (JEV). The re-emerging swine acute diarrhea syndrome coronavirus (SADS-CoV), SARS-CoV and SARS-CoV-2 have the potential ability of interspecies transmission in different animals, including the swine, bats and human (Liang et al., 2018; Peng et al., 2020; Yong-Le et al., 2019). PDCoV is the emerging deltacoronavirus from swine and has spread in most countries. Under natural conditions, PDCoV is also positive in some cats, which was detected through sera reaction (Zhao et al., 2019). The newly emerged PDCoV also could infect the calves, but without lesions or clinical disease (Kwonil et al., 2017). In the present study, the emerging epidemic PDCoV strain isolated from in Shanghai could widely infect porcine, human and chicken cells in vitro (Fig. 1). PDCoV cross-species infection might depend on aminopeptidase N (APN) from a host cell surface (Wentao et al., 2018). It indicated that PDCoV might be a potential zoonosis virus to threat the swine industry and human public health.
The HCoV-229E and TGEV typically induced type I IFN and inflammatory cytokines production (Mutsuo et al., 2020; Zhen et al., 2017a,2017b). Whereas, most CoVs employ numerous mechanisms were to suppress the activation of host innate immune response. In the present study, we proved that PDCoV could significantly suppress the VSV and poIRF7-induced type I IFN production (supplementary Fig. S1 and Fig. 4). PDCoV was also inhibited the IFN-β production stimulated by poIRF3 or its upstream molecules (RIG-I, MDA5, IPS-1, TBK1 and IKKε) (Jianfeng et al., 2010). The CoV N protein is one of the most important type I IFN antagonists. For example, the PEDV N protein was confirmed to suppress IFN-β production by sequestering the formation of human TBK1/IRF3 complex (Zhen et al., 2014). The SARS-CoV, MHV and PDCoV N protein antagonized IFN-β production through interference with the dsRNA transferring binding process from the protein activator of protein kinase R (PACT) to RIG-I and MDA5 at the initial step of human RLR (Jun et al., 2019; Zhen et al., 2017a,b; Xiaolu et al., 2011). SARS-CoV and the MERS-CoV N protein could target TRIM25 to decrease the K63-linked polyubiquitination of human RIG-I activation (Yong et al., 2017). To our interesting, RIG-I downstream signal molecular inducing pIFN-β production was also suppressed by the PDCoV N protein in porcine cells, but not in human cells (Likai et al., 2019; Jun et al., 2019). Hence, we further identified an interaction protein of the PDCoV N protein in porcine cells by LC-MS/MS. The poIRF7 was found and confirmed as an interaction protein of the PDCoV N protein (Fig. 2). Although IRF7 was an important and conservative protein in a broad range of species, PDCoV N protein only interacted with poIRF7 to suppress its induced pIFN-α and pIFN-β production in a species-specific manner (Fig. 3, Fig. 4). These results suggest that the species-specific binding of PDCoV N protein to IRF7 might be responsible for the virus-specific inhibition of the downstream RIG-I signaling pathway in swine. All these results indicate that CoV N protein as an inhibitor of host IFN-β production, which suppresses RLR signaling pathway activation, exists in various mechanisms in different types of the CoVs and hosts.
Protein ubiquitination is an important mechanism for regulating host immune response, and the levels and types of protein post-transcriptional modification may be changed following virus infection. In the present study, we found that PDCoV N protein-mediated degradation of poIRF7 is not associated with host transcription shutoff (Fig. 4I), but is suppressed by the proteasome inhibitor MG-132 (Fig. 5E). It indicates that PDCoV N protein promotes the degradation of poIRF7 through the ubiquitin-proteasomal pathway. In a previous study, we also found that the PDCoV N protein could inhibit the IRF3-induced pIFN-β activation (Likai et al., 2019). However, PDCoV N protein could not directly interact with poIRF3 and promote its degradation (Figs. 3C and 5 B). IRF7 could interact with IRF3 to form heterodimerization in uninfected or virus-infected cells (Honda et al., 2006). The interaction also increased after virus infection, which increased IFN production (Wan et al., 2017). These results demonstrate that IRF7/IRF3 heterodimerization may be necessary for the host to promote type I IFN production during a virus infection. Hence, PDCoV N protein mediated IRF7 degradation might decrease the dose of IRF7/IRF3 heterodimerization, which might be the reason for PDCoV N protein inhibiting IRF3-induced pIFN-β activation.
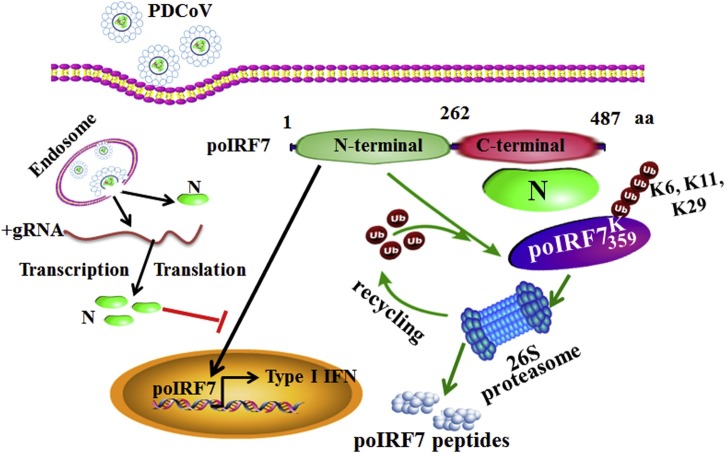
The degradation of IRF7 is medicated by multiple E3 ubiquitin ligases. The Kaposi’s sarcoma-associated herpesvirus (KSHV or HHV8) could encode RTA protein, which has ubiquitin E3 ligase activity, to interact with IRF7 for K48-linked polyubiquitination proteasome-mediated degradation (Yu et al., 2005). However, there is no evidence proving that CoV N protein has an ubiquitin ligase or a deubiquintinase function. On the other hand, we found that the PDCoV N protein could promote the K6, K11, K29-linked polyubiquitination of poIRF7, but not the classical K48-linked polyubiquitination (Fig. 6C). The K6, K11, K27, and K29-linked polyubiquitination also promoted the target protein degradation in mammals (Xu et al., 2009). Three different types of polyubiquitination predicted that several ubiquitination sites might be affected in poIRF7. Hence, we constructed ten conditional lysine mutants of poIRF7, which was substituted with aspartate. The Lys359 of poIRF7 is the key site for the PDCoV N protein to promote poIRF7 polyubquitination degradation (Fig. 8 ). To our interest in this study, although the Lys359 of poIRF7 was also conserved in hIRF7 (Lys375) and chIRF7 (Lys368) according to the multi-sequence analysis (supplementary Fig. S2), the PDCoV N protein could not induce the hIRF7 and chIRF7 degradation (Fig. 7E). The influenza A virus NS1 protein could species-specifically interact with TRIM25 to inhibit host RIG-I ubiquitination (Rajsbaum et al., 2012). The E protein of the porcine reproductive and respiratory syndrome virus (PRRSV) could degrade porcine CH25H, but not human CH25H (Ke and Fang, 2019). The PRRSV nsp4 also could cleave the porcine mRNA-decapping enzyme 1a (DCP1a), but not the human or monkey DCP1a (Xu et al., 2009; Tao et al., 2018). These data indicate that viral protein specifically interacts and changes the host protein in a species-specific manner. In the present study, we suggest that the PDCoV N protein directly interacting with IRF7 might be the first step in promoting its degradation (Fig. 8). The PDCoV N protein interacted with poIRF7 and increased its ubiquitination, which might require recruitment of E3 ubiquitin ligase. Hence, it will be necessary to identify the E3 ubiquitin ligase in further investigations.
Fig. 8.
PDCoV N protein degrade the poIRF7 via ubiquitin-proteasomal pathway. PDCoV infect and release its genome and N protein into the porcine cytoplasm. The N protein could bind to the C-terminal rigion (262-487aa) of poIRF7. Then it promotes the K6, K11 and K29-linked polyubiquitination of poIRF7 at K359 site to dregrade it via 26S proteasome, which results in suppressing the type I IFN production.
In summary, we have showed that the PDCoV N protein degraded poIRF7 through the ubiquitin-proteasome pathway in a species-specific manner, which resulted in significantly antagonizing the porcine type I IFN production. Although PDCoV has a broad infection range of species, PDCoV could adopt different strategies to interfere with a host’s innate immunity response.
Author contributions
Yaxian Yan, Jianhe Sun, Likai Ji conceived and designed the experiments. Likai Ji, Na Wang, Yuqiang Cheng performed the experiments and analyzed data. Likai Ji, Jingjiao Ma, Hengan Wang wrote the manuscript. All authors reviewed, revised, and approved the final manuscript.
Funding
This work was supported by funding from the National Key Research and Development Program of China (No. 2018YFD0500100), S hanghai Agriculture Applied Technology Development Program (No. T20170110),National Natural Science Foundation of China (Nos. 31571932 and31772744).
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.vetmic.2020.108853.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Mingjun S., Chunqiu L., Shanshan Q., Dan Y. A molecular epidemiological investigation of PEDV in China: characterization of co-infection and genetic diversity of S1-based genes. Transbound Emerg. Dis. 2019 doi: 10.1111/tbed.13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killerby M.E., Biggs H.M., Midgley C.M. Middle east respiratory syndrome coronavirus transmission. Emerg. Infect. Dis. 2020;26(2):191–198. doi: 10.3201/eid2602.190697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendong L., Zhengli S., Meng Y. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310(5748):676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- Liang W., Shuo S., Yuhai B. Bat-rigin coronaviruses expand their host range to pigs. Trends Microbiol. 2018;26(6):466–470. doi: 10.1016/j.tim.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z., Xinglou Y., Xianguang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S., Ying W., Wei Z. Enabling the’ host jump’: structural determinants of receptor-binding specificity in influenza A viruses. Nat Rev Microbiol. 2014;12(12):822–831. doi: 10.1038/nrmicro3362. [DOI] [PubMed] [Google Scholar]
- Patrick C.Y.W., Susanna K.P.L., Carol S.F.L. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012;86(7):3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jianfeng L., Yin L., Xuming Z. Murine coronavirus induces type I interferon in oligodendrocytes through recognition by RIG-I and MDA5. J. Virol. 2010;84(13):6472–6482. doi: 10.1128/JVI.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Barragan L., Zust R., Weber F. Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood. 2007;109(3):1131–1137. doi: 10.1182/blood-2006-05-023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuqiang C., Yingjie S., Hengan W. Chicken STING mediates activation of the IFN gene independently of the RIG-I gene. J. Immunol. 2015;195(8):3922–3936. doi: 10.4049/jimmunol.1500638. [DOI] [PubMed] [Google Scholar]
- Hiroaki H., Tsuneyasu K., Osamu T. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 2002;3(2):196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- Yong H., Wei L., Ting G. The severe acute respiratory syndrome coronavirus nucleocapsid inhibits type I interferon production by interfering with TRIM25-mediated RIG-I ubiquitination. J. Virol. 2017;91(8) doi: 10.1128/JVI.02143-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen D., Liurong F., Huiyuan J. Porcine epidemic diarrhea virus nucleocapsid protein antagonizes beta interferon production by sequestering the interaction between IRF3 and TBK1. J. Virol. 2014;88(16):8936–8945. doi: 10.1128/JVI.00700-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jingyi L., Liurong F., Nan D. Porcine deltacoronavirus (PDCoV) infection suppresses RIG-I-mediated interferon-β production. Virology. 2016;495:10–17. doi: 10.1016/j.virol.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride R., van Zyl M., Fielding B.C. The coronavirus nucleocapsid is a multifunctional protein. Viruses. 2014;6(8):2991–3018. doi: 10.3390/v6082991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likai J., Shasha L., Wenxian Z. Porcine deltacoronavirus nucleocapsid protein suppressed IFN-β production by interfering porcine RIG-I dsRNA-binding and K63-linked polyubiquitination. Front. Immunol. 2019;10:1024. doi: 10.3389/fimmu.2019.01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun C., Puxian F., Mohan W. Porcine deltacoronavirus nucleocapsid protein antagonizes IFN-β production by impairing dsRNA and PACT binding to RIG-I. Virus Genes. 2019;55(4):520–531. doi: 10.1007/s11262-019-01673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunhee L., Changhee L. Functional characterization and proteomic analysis of the nucleocapsid protein of porcine deltacoronavirus. Virus Res. 2015;208:136–145. doi: 10.1016/j.virusres.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Kwonil J., Anastasia N.V. Isolation and characterization of porcine deltacoronavirus from pigs with diarrhea in the United States. J. Clin. Microbiol. 2015;53(5):1537–1548. doi: 10.1128/JCM.00031-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R., Mamane Y., Hiscott J. Multiple regulatory domains control IRF-7 activity in response to virus infection. J. Biol. Chem. 2000;275(44):34320–34327. doi: 10.1074/jbc.M002814200. [DOI] [PubMed] [Google Scholar]
- Yong-Le Y., Pan Q., Bin W. In vivobroad cross-species infection of cultured cells by bat HKU2-related swine acute diarrhea syndrome coronavirus and identification of its replication in murine dendritic cells highlight its potential for diverse interspecies transmission. J. Virol. 2019;93(24) doi: 10.1128/JVI.01448-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Li W., Schuurman N. Serological screening for coronavirus infections in cats. Viruses. 2019;11(8) doi: 10.3390/v11080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwonil J., Hui H., Linda J.S. Calves are susceptible to infection with the newly emerged porcine deltacoronavirus, but not with the swine enteric alphacoronavirus, porcine epidemic diarrhea virus. Arch. Virol. 2017;162(8):2357–2362. doi: 10.1007/s00705-017-3351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentao L., Ruben J.G.H., Scott P.K. Broad receptor engagement of an emerging global coronavirus may potentiate its diverse cross-species transmissibility. PNAS. 2018;115(22):E5135–E5143. doi: 10.1073/pnas.1802879115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutsuo Y., Hidekazu N., Xue D. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir. Investig. 2020;58(3):155–168. doi: 10.1016/j.resinv.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen D., Kang A., Lilan X. Transmissible gastroenteritis virus infection induces NF-κB activation through RLR-mediated signaling. Virology. 2017;507:170–178. doi: 10.1016/j.virol.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen D., Liurong F., Shuangling Y. The nucleocapsid proteins of mouse hepatitis virus and severe acute respiratory syndrome coronavirus share the same IFN-β antagonizing mechanism: attenuation of PACT-mediated RIG-I/MDA5 activation. Oncotarget. 2017;8(30):49655–49670. doi: 10.18632/oncotarget.17912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiaolu L., Jian P., Jiali T. SARS-CoV nucleocapsid protein antagonizes IFN-β response by targeting initial step of IFN-β induction pathway, and its C-terminal region is critical for the antagonism. Virus Genes. 2011;42(1):37–45. doi: 10.1007/s11262-010-0544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K., Takaoka A., Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25(3):349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Wan Q., Yang C., Rao Y. MDA5 induces a stronger interferon response than RIG-I to GCRV infection through a mechanism involving the phosphorylation and dimerization of IRF3 and IRF7 in CIK cells. Front. Immunol. 2017;8:189. doi: 10.3389/fimmu.2017.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y.X., Wang S.Z.E., Hayward G.S. The KSHV immediate-early transcription factor RTA encodes ubiquitin E3 ligase activity that targets IRF7 for proteosome-mediated degradation. Immunity. 2005;22:59–70. doi: 10.1016/j.immuni.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Xu P., Duong D.M., Seyfried N.T. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell. 2009;137(1):133–145. doi: 10.1016/j.cell.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajsbaum R., Albrecht R.A., Wang M.K. Species-specific inhibition of RIG-I ubiquitination and IFN induction by the influenza A virus NS1 protein. Plos Pathog. 2012;8(11) doi: 10.1371/journal.ppat.1003059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke W., Fang L. Porcine reproductive and respiratory syndrome virus E protein degrades porcine vholesterol 25-hydroxylase via the ubiquitin-proteasome pathway. J. Virol. 2019;93(20) doi: 10.1128/JVI.00767-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R., Fang L., Bai D. Porcine reproductive and respiratory syndrome nirus nonstructural protein 4 cleaves porcine DCP1a to attenuate its antiviral activity. J. Immunol. 2018;201(8):2345–2353. doi: 10.4049/jimmunol.1701773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.