Abstract
As the severe acute respiratory syndrome coronavirus 2 virus pandemic continues to grow globally, an association is apparent between patients with underlying cardiovascular disease comorbidities and the risk of developing severe COVID-19. Furthermore, there are potential cardiac manifestations of severe acute respiratory syndrome coronavirus 2 including myocyte injury, ventricular dysfunction, coagulopathy, and electrophysiologic abnormalities. Balancing management of the infection and treatment of underlying cardiovascular disease requires further study. Addressing the increasing reports of health care worker exposure and deaths remains paramount. This review summarizes the most contemporary literature on the relationship of the cardiovascular system and COVID-19 and society statements with relevance to protection of health care workers, and provides illustrative case reports in this context.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) appears to have emerged from Wuhan, China, in December of 2019 and has now resulted in pandemic spread. As of May 14, 2020, this virus has resulted in more than 4.4 million documented infections and has exceeded 300,000 deaths worldwide.1 The SARS-CoV-2 virus, although structurally similar to SARS-CoV-1 and Middle East respiratory syndrome (MERS)-CoV, has unique properties distinct from the other coronaviruses and spreads the disease known as COVID-19.2 , 3 This illness can be fatal in those with and without comorbid conditions. It is particularly deadly in vulnerable populations such as the elderly and individuals with comorbidities and in exposed health care workers (HCWs).4 Mortality is often attributed to progressive pulmonary failure with the development of acute respiratory distress syndrome (ARDS). There are increasing reports of cardiac involvement of COVID-19, ranging from myocyte biomarker elevation, electrocardiographic abnormalities, myocardial dysfunction, and arrhythmias.5., 6., 7., 8. With the increasing number of cases, an association is apparent between underlying cardiovascular disease (CVD) comorbidities and risk of developing severe COVID-19. This article will summarize the most contemporary literature on the relationship of the cardiovascular system and manifestations of COVID-19 and society statements addressing HCW safety, and provide illustrative case reports relevant to the topics discussed. The summary of key takeaways from this article is shown in Table I .
Table I.
Summary of key takeaways for COVID-19 and CVD
| Topic | Key takeaways |
|---|---|
| Role of established CVD and comorbidities | • The overall SARS-CoV-2–infected population is young with low rates of comorbidities compared to the general population. • Those patients with a severe clinical course from COVID-19 are often older and have diabetes, hypertension, underlying lung disease, or baseline CVD. • In-hospital outcomes appear worse as the number of comorbid conditions increases. |
| Elevation of cardiac injury biomarkers | • Evidence of myocardial injury in the form of troponin elevation is common in patients hospitalized with COVID-19. • The prevalence of troponin elevation appears to increase with disease severity. • Biomarker elevation may be present without underlying obstructive CAD. • The presence of troponin elevation is an independent predictor of mortality for inpatients. |
| Ventricular dysfunction and myocarditis | • Frank ventricular dysfunction and myocarditis appear to be infrequent when compared to troponin elevation. • The true incidence of ventricular dysfunction appears unclear. • In cases of shock with ventricular failure and/or in refractory pulmonary failure, VA and VV ECMO, respectively, may play a role. |
| Thrombotic events | • Elevation of D-dimer levels is common in patients with COVID-19. • Both macro- and microvascular coagulopathies have been described. • Macrovascular thrombosis in arterial and venous beds is a concern in these patients, and the use of anticoagulation has been associated with improved outcomes in one observational study. |
| Electrophysiologic manifestations | • Diffuse or focal ST-segment elevation can be seen in COVID-19 patients. • The ST-segment elevation can be present even without obstructive CAD. • New-onset supraventricular arrhythmias including atrial fibrillation and flutter are described. • Early interest in the combination therapy of azithromycin and hydroxychloroquine has been tempered by negative observational data. This combination of drugs has been associated with QT interval prolongation and TdP. • Given that fever is central symptom of COVID-19, unmasking or manifestation of Brugada syndrome is a concern in relevant patients. |
| Controversy regarding ACEi and ARB use | • There are theoretical paradigms which propose harm or benefit for the use of RAS blockade in the context of COVID-19. • The data to date suggest no increase in SARS-CoV-2 viral positivity in patients based on baseline RAS blockade use. • Most retrospective analyses suggest no deleterious impact of RAS blockade on outcomes in patients with COVID-19. |
| Protection of HCWs | • The shortages of PPE during the initial period of the pandemic have been well documented and contributed to the deaths of many HCWs. • Although cardiac catheterization is typically not an aerosol-generating procedure, the potential need for CPR or intubation exists—especially in acutely ill patients. The use of PPE for the staff including covering for the head, eyes/face, and body and N95 masks appears as a common recommendation in societal consensus documents for patients with documented and those at risk of SARS-CoV-2. • Treatment delays for ACS (particularly STEMI) on the part of patients fearing exposure and on the health care system related to need for more thorough patient evaluation have been noted. Time delays related to staff donning of PPE and room preparation may be present. • Consensus recommendations still emphasize a goal of minimizing total ischemic time in patients with STEMI. Primary PCI when performed safely (for the staff and patients) remains the reperfusion therapy of choice. • Transesophageal echocardiography risks the performing team to exposure, and PPE along with risk/benefit of the study should be considered. CT imaging may provide an alternative imaging modality for specific anatomical and clinical subsets. • CPR is a high-risk procedure in patients with COVID-19. Emphasis on aggressive PPE protection and early defibrillation (prior to chest compressions) has been recommended. |
Abbreviations: VA (veno‐arterial), VV (veno‐venous), ECMO Extracorporeal membrane oxygenation, CVD Cardiovascular disease, RAS Renin angiotensin system, PPE personal protective equipment, ACS Acute coronary syndrome, STEMI ST segment elevation myocardial infarction.
Cardiac relevance of SARS-CoV-2 infection
Established CVD and incident cardiac abnormalities are both of relevance in the context of infection with SARS and “SARS like” RNA viruses. A systemic analysis of 637 MERS patients demonstrated that 50% of cases had significant risk factors (diabetes and hypertension) for CVD and 30% had established cardiac diseases.9 , 10 In patients with confirmed SARS, diabetes was an independent predictor for both morbidity and mortality.9 , 11 MERS has been associated with troponin elevations and the acute development of myocarditis and congestive heart failure.12 Further, new-onset and reversible subclinical left ventricular (LV) diastolic dysfunction was commonly seen during acute SARS infection, suggesting that LV impairment in the acute phase could be attributed to the inflammatory and cytokine storm induced by acute viral infection.13 The cytokine storm syndrome is characterized by an uncontrolled immune response due to continuous activation and proliferation of lymphocytes and macrophages. Similar evidence is emerging that patients with severe COVID-19 have evidence of cardiac injury and elevated levels of inflammatory markers, such as C-reactive protein, procalcitonin, and leukocytes.14 This increased cytokine milieu may be contributory to the clinical findings in COVID-19 patients.15., 16., 17.
Established CVD and comorbidities in patients with COVID-19
The current COVID-19 literature is significantly limited by inconsistent data reporting, variable definitions of CVD, and study data overlap.18 SARS-CoV-2–infected patients are mostly young (<65 years of age) with low rates (compared to pre–COVID-19 population data) of established CVD, diabetes, hypertension, or cerebrovascular disease.19 , 20 A severe clinical course such as requiring intensive care or intubation, or progressing to ARDS is more common in older individuals and those with multiple underlying comorbidities.21 Similarly, mortality rates are consistently higher in older patients who have age-associated increases in CVD risk factors.21
An analysis limited to fatal cases of COVID-19 from the Chinese health authorities demonstrated a mean age of 70.6 years with a prevalence of 26.2% for diabetes, 40.5% for hypertension, and 23.3% for established CVD or cerebrovascular disease.22 The specific contribution of established coronary heart disease (CAD), prior myocardial infarction (MI), or pre-existing heart failure (HF) as direct causative factors to adverse outcomes remains unclear. A publication by Wu et al described the characteristics of 201 patients with SARS-CoV-2 pneumonia who required admission to the hospital.21 In this study, risk factors for development of ARDS were older age (median 58.5 vs 48.0 years), presentation with higher fevers, male gender, and the presence of hypertension and diabetes. Patients who died were significantly older than those who lived (median 68.5 vs 50.0 years) with numerically higher rates of hypertension and diabetes. The largest Chinese analysis of CVD and mortality was obtained by examination of more than 44,000 confirmed cases of COVID-19 from China's Infectious Disease Information System.23 Those who died were older with an age-associated rise in mortality—exceeding 30% death rate in patients more than the age of 60 years. Mortality was also associated with diabetes, hypertension, and pre-existent CVD. Although distribution between genders was similar, mortality rate was higher in men versus women. Importantly, the case fatality rate was markedly increased by comorbidities: 0.9% for those without risk factors and 10.5%, 7.3%, and 6.0% for those with established CVD, diabetes, or hypertension, respectively.
Beyond China, relevant data are now emerging from the United States and Italy. In one of the earliest published reports from a single hospital in Washington State, Arentz et al described the characteristics of 21 SARS-Cov-2–infected patients treated in the ICU and demonstrated an overall clinical profile similar to the Chinese experience.24 Subsequent to this publication, more robust evidence from the United States is now available.24., 25., 26., 27. A key limitation of the broader COVID-19 literature is a lack of granular information about CVD details—specifically of CAD. Advancing our understanding of these conditions in this context, Richardson et al and Goyal et al reported patient characteristics and outcomes from the Northwell Health System and from 2 hospitals in New York City, respectively. In the former data set, CAD was present in 11.1% (and HF in 6.9%) of patients, whereas in the latter analysis, CAD was present in 13.7% of all individuals.25 , 26
As of the submission of the present manuscript, there have been more than 1.7 million cases and more than 100,000 deaths from COVID-19 in the United States. Reflecting data up to May 30, 2020, an analysis of 1,320,488 COVID-19 cases in the United States was reported by the Centers for Disease Control and Prevention (CDC). Of these, 287,320 (22%) had information on clinical characteristics and risk factors published in the CDC's Morbidity and Mortality Weekly Report.20 Nearly 22% of all of these patients had at least 1 comorbid condition, most commonly CVD (92,546 patients, 32.2%) diabetes mellitus (86,737 patients, 30.2%), and chronic lung disease (50,148 patients, 17.5%). Furthermore, hospitlizations were six times higher in patients with at least 1 underlying condition compared to patients without any reported underlying condition (45.4% vs 7.6), ICU admissions were increased (8.5% vs 1.5%) and death was 12 times higher compared to patients without co-morbidities (19.5% vs 1.6%).
The Italian experience provides additional support to the themes highlighted above for China and the United States. An analysis of 3,200 mortality cases from 19 regions in Italy as of March 20, 2020, demonstrated that patient deaths were largely in older individuals with on average 2.7 comorbidities.28 Overall, 1.2% of the deceased patients presented with no comorbidities, 23.5% with 1 comorbidity, 26.6% with 2, and 48.6% with 3 or more. With respect to CVD risk factors, ischemic heart disease was present in 30.1%, atrial fibrillation in 22.0%, stroke in 11.2%, hypertension in 73.8%, and diabetes in 33.9% of patients. Comparable data were also published from the Lombardy ICU Network which included 1,591 critically ill patients (mean age 63 years) from 72 hospitals.29
A key point emphasized in the Morbidity and Mortality Weekly Report is that the prevalence of underlying diseases increases your risk of developing severe outcomes including increased hospitalization(s), admission to the ICU, and death.20 However, there appears to be an important association between pre-existing health conditions and the development of severe COVID-19 disease. The specific mechanism and degree by which underlying heart disease or diabetes impacts the clinical course of COVID-19 patients remain to be determined.
Cardiovascular biomarker elevation in patients with COVID-19
Multiple nonspecific laboratory and biochemical abnormalities have been described in COVID-19 patients, including hypoalbuminemia, elevated C-reactive protein, high lactate dehydrogenase, lymphopenia, and increased erythrocyte sedimentation rate. Increasing evidence suggests that a cytokine cascade or “storm” may play a pathophysiological role in severe illness with increases in interleukin-2, interleukin-6, granulocyte-colony stimulating factor, interferon-γ inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1-α, and tumor necrosis factor-α.16 , 17
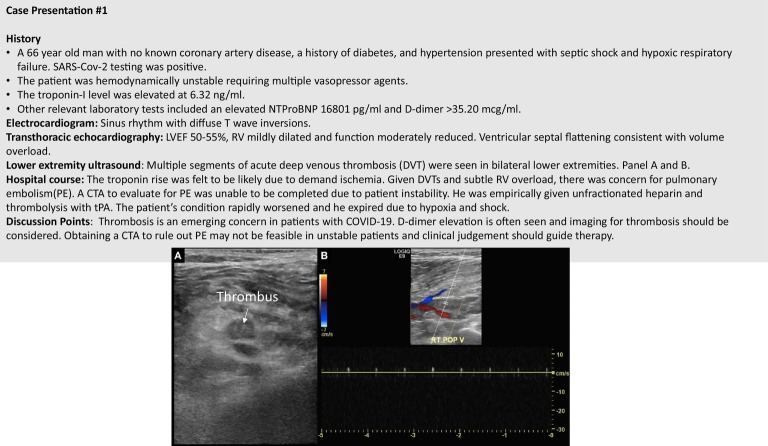
D-dimer elevation has also been described and appears to be a common abnormality.19 Finding of an elevated D-dimer level in the presence of overt sepsis and disseminated intravascular coagulopathy has been reported.30 , 31 The association between the severity of SARS-CoV-2 infection and D-dimer levels has been studied in several retrospective studies from China. In a study of 140 patients investigating the severity of COVID-19 (with severity defined based on the degree of hypoxemia and respiratory rate), more severe patients had higher D-dimer levels compared to nonsevere patients.32 Elevated D-dimer levels may also be associated with the development of ARDS. Furthermore, among patients with ARDS, higher D-dimer levels appear to be related to an increase in in-hospital mortality rate.31 In a cohort of 191 patients from Wuhan, D-dimer levels greater than 1 μg/mL at admission were associated with higher odds of in-hospital death.31 The authors of this latter study speculated that causative factors for D-dimer elevation included a reflection of systemic inflammatory cytokine responses and potentially induction of procoagulant factors—both of which predispose to tissue ischemia and vascular thrombosis. Coagulopathy and micro- and macrovascular thrombosis have been described in patients with COVID-19.33 Specifically, there are now numerous case reports of thrombosis involving a diverse array of vascular beds. Thrombus formation has been described in lower extremity arterial and venous vessels, abdominal veins, bypass grafts, coronary arteries, cerebral veins, pulmonary arteries, large vessel cerebral and carotid arteries, and small distal arteries with vaso-occlusive inflammatory clots causing a chilblains-like presentation.34., 35., 36., 37., 38., 39., 40., 41. Standard of care venous thromboembolism prophylaxis for critically ill individuals with COVID-19 appears reasonable with close monitoring of markers of coagulation and blood counts. However, the tendency to develop thrombotic events appears to be greater than what would be expected from otherwise critically ill patients. This concern is centered on the growing reports of clotting coupled with common findings of abnormal coagulopathy including elevated D-dimer, high lactate dehydrogenase, prothrombin time and partiral thromboplastin time, and frequent antiphospholipid positivity.42 , 43 Whether more aggressive anticoagulation (AC) is warranted in COVID-19 patients remains an area of investigation.98 Paranjpe et al examined data from 2,773 patients hospitalized with COVID-19 (28% of whom received anticoagulation) at Mount Sinai Hospital in New York.44 Survival was greater in the overall patient cohort and more so in those that were mechanically ventilated (doubling of survival) with administration of systemic AC. More prolonged duration of AC was also associated with a reduced risk of death (adjusted hazard ratio of 0.86 per day, 95% CI 0.82-0.89, P < .001). Bleeding events were not significantly elevated in the AC group as compared to the non-AC group (3% vs 1.9%, P = .20). These data provide important hypothesis generation that aggressive AC may play a role in this disease. Figure 1 outlines a challenging case of thrombosis complicating the clinical course of a COVID-19 patient.
Figure 1.
A case of deep venous thrombosis in a patient with COVID-19.
Cardiac biomarker elevation is now being recognized as a common occurrence in COVID-19. Complicating clinical decision making is the need to differentiate between an evolving acute coronary syndrome (ACS), a type 2 non–ST-segment elevation myocardial infarction (NSTEMI), and myocarditis. Infection with SARS-CoV-2 can cause new-onset myocardial dysfunction or trigger an exacerbation of pre-existing CVD. One proposed mechanism of direct cardiac injury is via the severe cytokine-induced inflammatory response described earlier which could trigger unstable plaque rupture and coronary thrombosis (type 1 NSTEMI). This process has been documented in other viral illnesses such as influenza.45 Similarly, increased myocardial oxygen demand due to inflammatory stress or frank sepsis can cause a type 2 NSTEMI seen with demand ischemia.
Myocardial injury, as evidenced by elevated troponin levels, is associated with acute COVID-19 disease in several studies. A single-center observational study from Wuhan, China, found that 26% (36 of 138) of COVID-19 patients treated in the ICU had significant elevation of cardiac enzymes.46 The National Health Commission of China also reported that 11.8% of patients who died of COVID-19 disease had elevation of troponin-I levels despite no prior history of known underlying CVD.47 In the early Washington State experience from Evergreen Hospital, troponin elevation was present in 14% of patients admitted to the ICU.24
The central question is whether cardiac biomarkers have predictive value for the clinical outcomes in COVID-19 patients. A meta-analysis of studies from China which included 341 patients demonstrated that severe SARS-CoV-2 infection was related to a larger rise in troponin levels as compared to milder cases.48 A cohort study from Wuhan that included 416 COVID-19 hospitalized patients with a median age of 64 years found that 82 patients (19.7%) had cardiac injury. This analysis demonstrated that patients with myocyte injury with biomarker elevation had a significantly higher mortality than those without cardiac injury (51.2% vs 4.5%).14 Similar observations were seen in another Wuhan case series study of 187 inpatients of whom 27.8% had myocardial injury shown by elevated troponin T (TnT) levels. Biomarker-positive patients had a higher death rate compared to patients with normal TnT levels (59.6% vs 8.9%).6 Patients with elevated TnT levels also had greater rates of malignant arrhythmias and more mechanical ventilation as compared to patients with normal TnT levels. This study also reported that the highest mortality rates were seen in patients with elevated TnT levels with underlying CVD compared to patients with elevated TnT levels and no underlying CVD (69.4% vs 37.5%). It was also demonstrated that TnT levels were associated with increased C-reactive protein and NT-proBNP levels, which implicate a link between myocardial injury and the degree of systemic inflammation and ventricular stress.49 A recent study of 671 patients in China demonstrated that elevation of troponin I was independently predictive of mortality with an area under the curve of 0.92 (CI 0.87-0.96, sensitivity 0.86 and specificity 0.86), P < .001.50 Taken together, these studies strongly suggest that myocardial injury can be precipitated by COVID-19 infection and is related to adverse outcomes.
Ventricular function and COVID-19
Frank ventricular dysfunction and clinical HF have also been described. From the 191-patient case series from Wuhan, the incidence of acute HF was 23% and significantly higher among nonsurvivors (52% vs 21%).31 Ruan et al in a review of 150 critically ill patients noted that of 68 mortalities, the cause of death was reported as cardiac damage and HF in 7% of patients and combined respiratory failure and HF in 33% of patients.51 In the early Washington State experience of 21 critically ill patients, 7 (33%) developed new cardiomyopathy.24
The mechanisms of myocardial injury in COVID-19 are likely multifactorial. Direct viral infection of the cardiac muscle leading to myocarditis has been described.47 The affinity of SARS-CoV-2 for the angiotensin-converting enzyme 2 (ACE-2) receptor, which is abundantly found in myocytes, may play a role in direct viral infection of the myocardium.52 The infection can lead to focal or global myocardial involvement, in some cases mimicking an ST-segment elevation myocardial infarction (STEMI), and may result in severe LV dysfunction.53 , 54 Noninvasive imaging modalities may be helpful in evaluation of these patients. Myocardial interstitial edema with diffuse late gadolinium enhancement on cardiac magnetic resonance imaging has been reported in SARS-CoV-2 myocarditis.53
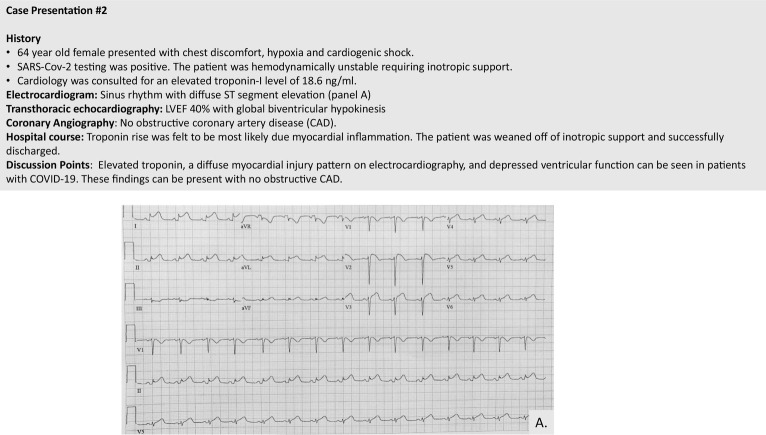
The manifestation of myocardial involvement appears highly variable, ranging from isolated troponin elevation to autopsy-confirmed interstitial inflammation to frank ventricular dysfunction and shock.55 Figure 2 outlines a case example of biomarker elevation and LV dysfunction in a SARS-CoV-2–infected patient. In patients with isolated cardiac biomarker elevation or subclinical ventricular dysfunction, there is no current consensus on treatment. In patients with hemodynamic instability or pulmonary failure, mechanical support may be required. The mortality in COVID-19 patients who require mechanical ventilation is high, and venovenous (VV) extracorporeal membrane oxygenation (ECMO) may play a role in this condition. VV ECMO can be lifesaving in patients with severe forms of ARDS.56 , 57 Beyond pulmonary support, venoarterial (VA) ECMO may additionally be a relevant modality in patients with COVID and shock. The Extracorporeal Life Support Organization has an ongoing registry study looking at the use of and outcomes for ECMO in COVID-19 patients. As of June 20th, 2020, this registry has 1,511 patients with confirmed SARS-CoV-2 infection who have received ECMO primarily for respiratory failure with a 56% discharged-alive rate.46 The full outcome data from this registry are not yet available. For reference, an overall framework for the use of ECMO in COVID-19 patients has been published by Pham et al.58
Figure 2.
Case example of diffuse ST-segment elevation with no obstructive coronary disease.
Electrocardiographic findings and arrhythmias
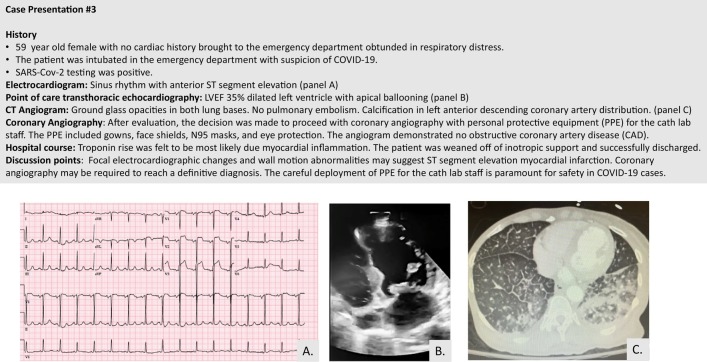
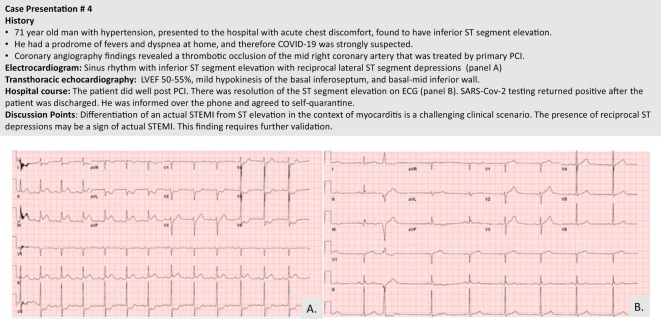
Patients infected with the SARS-CoV-2 virus can develop electrophysiologic manifestations ranging from ST-segment changes to rhythm abnormalities with increased risk of sudden cardiac death. ST-segment elevation may be diffuse or regional, mimicking a STEMI without an angiographic culprit lesion. These changes may be indicative of myopericardial inflammation.53 Figure 3 provides a case example of focal ST-segment elevation in an injury pattern with no evidence of obstructive CAD. This latter case is in contrast to an actual STEMI in a patient with COVID-19, as shown in Figure 4 . In a case series of 18 patients from New York, a wide variety of ST segment elevation patterns can be present with or without underlying obstructive CAD or actual MI.59 Of note, diffuse ST segment elevation (n = 4 patients) was associated with noncoronary myocardial injury in all cases.
Figure 3.
Case example of a patient with COVID-19 and regional ST-segment elevation mimicking a STEMI.
Figure 4.
Case example of a patient with COVID-19 and a true inferior STEMI.
It is expected that sinus tachycardia will be a common rhythm—particularly in critically ill COVID-19 patients. During the 2003 SARS-CoV-1 coronavirus outbreak, 71.9% of patients presenting with respiratory distress had sinus tachycardia which persisted during follow-up in 39% of recovered patients.60 Arrhythmias, mainly supraventricular tachycardia, were present in 17% of admitted COVID-19 patients and were more common in ICU setting.46 In another report, malignant arrhythmias including ventricular tachycardia and fibrillation were more common among those with elevated troponin levels (11.5% vs 5.2%).6 From the coauthor's experiences, atrial arrhythmias can be seen in these patients. We report 5 patients (age range 31-73 years) presenting with new-onset atrial arrhythmias (4 with atrial fibrillation with rapid ventricular rate, 1 with atypical flutter). Four of these individuals presented to the hospital with the arrhythmia, 2 with chest pain, 1 with shock, and 3 with fever and dyspnea. Three of the patients required antiarrhythmic therapy, and 4 converted to sinus rhythm over their hospitalization. In this cohort, the arrhythmias did not appear to predict a worse outcome, as 4 of them recovered to discharge. However, similar to prior reports, 3 patients who had myocardial injury endured a complicated course, requiring ICU admission and inotropic support.
There are electrophysiological considerations with respect to proposed therapies for COVID-19. Preliminary research has suggested that hydroxychloroquine alone or in combination with azithromycin may prove to be an effective treatment for COVID-19.61 Since initial reports, there was an empiric increase in use of those drugs in management of COVID-19 patients despite equipoise regarding benefit.62 There is concern that these drugs, especially in combination, can prolong the QT interval and may lead to torsades de pointes (TdP) and sudden death.63 Factors that increase the risk of drug-induced TdP include congenital long-QT syndromes, female sex, structural heart disease, electrolyte abnormalities, hepatic/renal failure, and concomitant QT-prolonging medications. In a cohort of 90 COVID-19 patients, Mercuro et al demonstrated that QT interval prolongation was common with the use of hydroxychloroquine alone or in combination with azithromycin.64 More recent observation data suggest a lack of efficacy of this treatment regimen—with randomized trial data required for a more definite conclusion.65 , 66
Fever is a well-known trigger for ventricular arrhythmias in patients with Brugada syndrome, with more than 50% in some cohort experiencing syncope or arrest during fever.67 , 68 Because fever is one of the most common presenting signs of COVID-19, it is not surprising that known Brugada syndrome patients, especially those with spontaneous type I pattern, could be at risk for arrhythmias and sudden death. In one case report, COVID-19 unmasked a Brugada pattern on the electrocardiogram during the initial presentation in someone with no known Brugada syndrome.69 Therefore, it is recommended to treat fever aggressively in all patients with Brugada syndrome— including those with concomitant COVID-19.
Controversies regarding ACE inhibitors/angiotensin receptor blockers
Significant controversy exists as to the benefit versus harm of renin-angiotensin system (RAS) blockade via ACE inhibitors (ACEi) or angiotensin receptor blockers (ARBs) in patients with COVID-19. Similar to SARS-CoV-1 but with much greater affinity, SARS-CoV-2 uses the cell entry receptor ACE-2 in the lungs to gain entry into host cells. ACE-2 is a counterregulator of RAS and converts angiotensin II to Ang-. Ang- decreases inflammation and causes vasodilation. Two competing theories of RAS modulation based largely on preclinical studies have been forwarded.70 The scenarios include one of harm by which ACEi/ARBs lead to ACE-2 increase and thus enhanced viral entry into cells, and alternatively, ACEi/ARBs prevent lung injury by reducing production/receptor binding of angiotensin II to the type 1 angiotensin receptor (which is responsible for inflammation), downregulation of ACE-2 expression, and activation of antifibrosis pathways.70 , 71
To determine if ACEi or ARB use might predispose to SARS-CoV-2 infection, Reynolds et al examined medication use in 12,594 patients tested for the virus and found no relationship of RAS blocking medications and test positivity.72 The aggregate of published outcome data demonstrates neutral or potential benefit of baseline ACEi and/or ARB use in hospitalized patients. A retrospective analysis of RAS blockade in SARS-CoV-2–positive patients suggested less severe disease in those who took an ARB prior to hospitalization.73 A larger analysis of 1,178 patients (31.8% with ACEi/ARB use) from Wuhan found no association between ACEi/ARB use and disease severity or mortality.74 In 6,272 patients from Italy, ACEi and ARB use was more common in COVID-19 patients (due to pre-existing CVD), but there was no relationship with disease outcome.72 A retrospective analysis of 4480 patients found prior use of ACEi or ARB was not associated with the diagnosis of COVID-19, severity of disease, or mortality.103 However, another retrospective multi-instiution study of 1,128 patients found the inpatient use of ACEi/ARB for hypertension lowered the risk of all-cause mortality compared to patients who did not receive ACEI/ARB.99 At present, there are not enough prospective human data to support either paradigm. Given the known benefit of RAS blockade in patients with CVD, there is no evidence-based rationale to withhold (or initiate) these agents for COVID-19 specific indications.
Protection of HCWs
One of the most visible aspects of the SARS-CoV-2 pandemic is the impact on HCWs. The virus has a well-documented transmission among those providing care and has resulted in numerous deaths. The risk to HCWs is not novel and resulted in more than 1,700 deaths in the 2002 SARS outbreak.75 In the current COVID-19 pandemic, Ran et al analyzed the symptoms, characteristics, and outcomes of frontline HCWs with acute respiratory illness treating COVID-19 patients at a tertiary hospital in Wuhan.76 The key findings were a symptom profile in HCWs that mirrored the general COVID-19 patient population—including fever, cough, chest discomfort, headache, and diarrhea. The identified etiologies for transmission included inadequate hand hygiene and lack of appropriate personal protective equipment (PPE) use.76 These modalities for transmission remain important for all HCWs regardless of specialty. For cardiovascular HCWs, exposure can come in any context of providing clinical care. Some key relevant scenarios highlighted in society consensus statements include minimizing exposure in the cardiac catheterization laboratory, during noninvasive imaging, and during cardiopulmonary resuscitation (CPR).7 , 77., 78., 79.
Cardiac catheterization
For patients who are SARS-CoV-2 positive or persons under investigation (PUIs), awareness and preparation on the part of HCWs are important. Determination of which procedures require which PPE has been an ongoing area of controversy. In many regions, the lack of adequate numbers of N95 respirator masks or full respirators further influences this decision-making process. The CDC issued their Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 in Healthcare Settings on March 10, 2020. In this guidance, contact and airborne precautions using an N95 respirator, gown, gloves, and eye protection (goggles or face shield) were recommended for bronchoscopy, CPR, endotracheal intubation, nebulizer therapy, noninvasive positive pressure ventilation, suctioning, sputum induction, tracheostomy placement, and management of a ventilated patient with dislodged tubing.
Strictly speaking, a routine cardiac catheterization procedure is nonaerosolizing and therefore would fall under standard droplet precautions. However, pulmonary, hemodynamic, or electrical instability requiring mechanical ventilatory support or CPR can occur during cardiac procedures. This conversion from a nonaerosolizing procedure to one with increased risk to HCWs is of particular concern when taking unstable patients to the cardiac catheterization laboratory such as those with a STEMI, nonintubated post return of spontaneous circulation patients, and patients in cardiogenic shock. This latter group of patients is further difficult to manage because there are often a limited history available and inadequate time for preprocedure SARS-CoV-2 testing. However, given the high false-negative rate of current SARS-CoV-2 reverse-transcription polymerase chain reaction testing, this approach is unlikely to negate the risk to HCWs in this and many other clinical environments.80 This constellation of challenges has led many centers from a catheterization standpoint to recommend an initial conservative approach with rule out SARS-CoV-2 testing for patients with a stable clinical picture including those with effort angina, compensated heart failure, and MI without shock or ST elevation. Others have advocated an even more conservative approach with thrombolysis as first-line therapy for SARS-CoV-2/PUI patients presenting with STEMI.7 , 81 Given the uncertainty of infection status and often unpredictable conversion to an aerosol scenario, the American College of Cardiology and the Society of Cardiovascular Angiography and Interventions have issued recommendations which support strong protections for catheterization laboratory staff.7 These guidelines have also been further augmented by supporting expert opinion with recent statements favoring primary percutaneous coronary intervention (PCI) as standard of care when done with proper PPE.78 , 79 , 82 , 83 A further area of uncertainty is selection of specific mechanical support devices in patients with shock. Intra-aortic balloon pumps and transvalvular support devices (Impella) are commonly used in cardiogenic shock and generally require involvement of a limited team of HCWs. In contrast, VA ECMO necessitates more in-room maintenance and therefore may be less attractive as initial therapy from a HCW protection standpoint.
Noninvasive imaging
Investigation of ventricular function normally requires noninvasive imaging. However, the high transmissibility and virulence of SARS-CoV-2 place physicians, nurses, and sonographers at risk. In an effort to protect health care providers, the American Society of Echocardiography has issued a consensus statement noting that transthoracic echocardiogram, stress echocardiography, and transesophageal echocardiography (TEE) should only be performed if they are expected to change management.84 Studies should be prioritized in those more vulnerable for significant morbidity or mortality if echocardiographic examination is not performed; following previously published appropriate use criteria indications.85 , 86 Studies that are rarely appropriate or maybe appropriate should be avoided at least until the SARS-CoV-2 status of the patient is clear. Any elective studies should be rescheduled for a later date. Limited physical interaction should also take place among sonographers, the echocardiographer, and other consultants in the reading room with heavier emphasis on phone or webinar resources for any questions/review of images. Of note, when appropriate infection control strategies are used, potential use of pharmacologic nuclear stress testing rather than exercise stress or echo-based imaging may be attractive in this context to limit aerosolization risk and proximity of direct patient contact to the HCW performing the imaging.
Additionally, point-of-care ultrasounds should be used by those trained clinicians that are already taking care of the suspected/confirmed SARS-CoV-2 patient to avoid exposing others. Sonographers should also aim to obtain limited but sufficient images to answer the question at hand and decrease contact time with the patient. Similar to cardiac catheterization, HCWs should follow droplet and airborne precautions with adherence to a higher level of protection dictated by patient's SARS-CoV-2 status and use of invasive and noninvasive ventilation. The highest degree of protection is needed for a TEE because this procedure carries a significant risk of viral transmission due to aerosolization of the virus. Therefore, TEE should only be done if it will absolutely alter the patient's care plan.
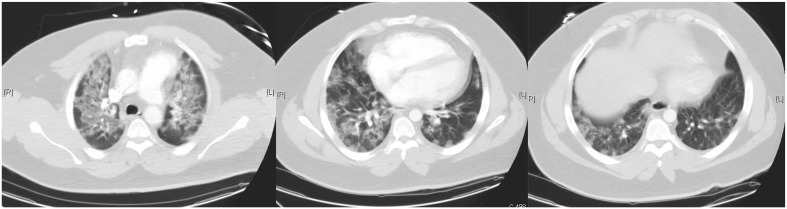
Computed tomography (CT) has 2 potential focus areas in the context of COVID-19. First, CT may serve as an alternative to other imaging modalities. Specifically, cardiac CT can be considered an alternative to TEE or cardiac catheterization for (1) ruling out left atrial appendage and intracardiac thrombus prior to cardioversion; (2) symptomatic prosthetic valve dysfunction, endocarditis, perivalvular extension of endocarditis, or possible valve abscess; (3) excluding CAD or high-risk anatomy in symptomatic stable patients with chest pain; and (4) evaluating cardiac masses suspected to be malignant, if necessary to plan biopsy or surgery. The second use of CT is as a primary lung imaging modality. The pulmonary CT findings of SARS-CoV-2 infection include often bilateral and peripheral ground-glass and consolidative pulmonary opacities. An example of CT findings from a patient who presented with troponin elevation, myocardial dysfunction, and respiratory distress is shown in Figure 5 . The presence of these findings may evolve over the hospital course and progress in concert with the clinical picture.87 The prognostic utility of CT in COVID-19 patients remains to be determined. A complex clinical scenario is differentiating pulmonary edema/HF from COVID-19, and it is even more challenging when both entities are present in a given patient. The ground-glass opacities and consolidation can be seen with both diseases. Compared to COVID-19 patients, HF patients more commonly have peribronchovascular thickening, fissural thickening, a more central distribution of findings, pulmonary vein enlargement, cardiac enlargement, and subpleural effusions.88 , 89 The logistics of having SARS-CoV-2–positive/PUI patients undergo CT increase risk to HCWs and potentially to other patients who use the scanner. The Society of Cardiovascular Computer Tomography has also issued guidance for use of CT during this COVID-19 pandemic.90 For those patients suspected/confirmed to have COVID-19 that are felt necessary to undergo CT scanning, proper PPE should be worn by all HCWs involved in the acquisition, preparation, and reading of the study. The patient should also wear a surgical mask during imaging to ensure strict droplet precautions. All rooms should be appropriately decontaminated after the study. As with echocardiographic studies, all elective cardiac CT should be postponed.
Figure 5.
Example of computed tomographic (CT)Wo images from a patient with COVID-19, myocardial dysfunction, troponin elevation, and respiratory distress. This patient presented with a combination of CT signs of both infection and cardiac failure. The ground-glass opacities and consolidation can be seen with both diseases. Patients in isolated heart failure often have peribronchovascular thickening, fissural thickening, a more central distribution of findings, pulmonary vein enlargement, cardiac enlargement, and subpleural effusions.
Cardiopulmonary resuscitation
CPR remains a concerning aerosol-generating therapy. The European Society of Intensive Care Medicine/Society of Critical Care of Medicine gave the highest recommendation to place ICU SARS-CoV-2–positive patients undergoing aerosol-generating procedures in a negative pressure room to decrease cross-contamination and keep pathogens confined to the patient's room.91 The Resuscitation Council of United Kingdom released recommendations which emphasize complete PPE (respirator, gown, gloves, eye protection) and use of palpation of carotid pulses but otherwise avoiding close physical examination during resuscitation. Initial CPR should focus on chest compressions with ventilation focused on establishment of an advanced airway, and consideration is given to defibrillation as soon as possible for ventricular arrhythmias, even before chest compressions, to limit aerosol generation.92 There may be a potential role for automated chest compression devices or wearable defibrillators in hospitalized patients to limit direct contact; however, this approach remains to be studied. Recently, the American Heart Association released a statement for the guidance of CPR for COVID-19 patients.77 This guideline is concordant with the UK recommendations, outlines methods to limit aerosol risk and management of out-of-hospital and in-hospital arrest, and discusses prone patient CPR.77 , 93 Potential ethical challenges that will need broader discussion are CPR candidacy and duration weighing HCW exposure risk, likelihood of immediate resuscitation success in particularly hypoxic patients, and overall prognosis.
Impact of COVID-19 on cardiovascular admissions and procedure volumes
A noticeable trend has emerged with a decrease in cardiovascular related admissions, including ACS (particularly STEMI) presentations to hospitals during the COVID-19 pandemic.100., 101., 102. Indeed, a telematics survey of 81 centers involved within a STEMI network in Spain equipped with cardiac catheterization laboratories demonstrated a dramatic decline in procedural volumes during the peak of the COVID-19 pandemic.94 Data obtained from 71 centers over a 7-day period before the start of the pandemic and during the pandemic revealed a 57% decline in diagnostic procedures, a 48% decline in PCIs, a 81% decline in structural procedures, and a 40% decline in the use of PCI for STEMI. The decline in elective cardiac procedures is likely due to restrictions placed by hospitals and governmental agencies. However, the etiology behind the drop in ACS and specifically STEMI volume remains unclear. A similar decline in STEMI volume in the United States and ACS volume in Northern Italy has been demonstrated during the pandemic.95 , 96 A potential explanation raised may be hesitancy on the part of patients to seek health care. The Society for Cardiovascular Angiography and Interventions and Canadian Association of Interventional Cardiology are collaborating to form the North American COVID-19 ST-Segment Elevation Myocardial Infarction Registry. The registry will collect data on COVID-19/PUI with STEMI and provide some insights into ACS care during the COVID-19 pandemic. Beyond initial admission, postdischarge care of cardiac patients with and without COVID-19 has likely been disrupted by the pandemic, and therefore, the impact on clinic follow-up and unplanned readmissions will need to be studied. An initial expert opinion–generated document on reopening of cardiovascular services has been provided by Wood et al.97 This document highlights the ethical principles behind triage of testing and management of patients who have been deferred by the pandemic and emphasizes the maintenance of protection of patients and HCWs—including use of routine viral screening prior to cardiovascular testing.
Conclusions
The COVID-19 pandemic continues to grow globally. The aggregate data thus far would suggest that severity of clinical course and mortality risk are linked to baseline comorbidities—including established CVD. This disease directly affects multiple aspects of the cardiovascular system. Specific management strategies, protection of HCWs, and impact of COVID-19 on cardiovascular health care delivery will need further study.
Sources of funding
This work is supported by the Freeman Heart Association Endowment in Cardiovascular Disease.
Disclosures/declaration of interest
None.
Acknowledgements
None to be made.
Footnotes
None of the authors has relevant conflicts of interest.
No extramural funding was used to support this work.
References
- 1.World Health Organization 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2.Petrosillo N., Viceconte G., Ergonul O. 2020. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ou X., Liu Y., Lei X. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang Z, Yi F, Wu K, Lai K, Sun X, Zhong N, et al. Clinical characteristics of coronavirus pneumonia 2019 (COVID-19): an updated systematic review. medRxiv. 2020:2020.03.07.20032573.
- 5.Guo J., Huang Z., Lin L. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc. 2020;9(7) doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clerkin K.J., Fried J.A., Raikhelkar J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;141(20):1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 9.von Scheidt W, Welz A, Pauschinger M, Fischlein T, Schachinger V, Treede H, et al. Interdisciplinary consensus on indications for transfemoral transcatheter aortic valve implantation (TF-TAVI): joint consensus document of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausarzte e.V. (ALKK) and cooperating cardiac surgery departments. Clin Res Cardiol 2020;109(1):1–12. [DOI] [PubMed]
- 10.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J.K., Feng Y., Yuan M.Y. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23(6):623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 12.Alhogbani T. Acute myocarditis associated with novel Middle east respiratory syndrome coronavirus. Ann Saudi Med. 2016;36(1):78–80. doi: 10.5144/0256-4947.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S.S., Cheng C.W., Fu C.L. Left ventricular performance in patients with severe acute respiratory syndrome: a 30-day echocardiographic follow-up study. Circulation. 2003;108(15):1798–1803. doi: 10.1161/01.CIR.0000094737.21775.32. [DOI] [PubMed] [Google Scholar]
- 14.Shi S., Qin M., Shen B. China; JAMA Cardiol: 2020. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poyiadji N., Shahin G., Noujaim D. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020;201187 doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130(5):2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauchner H., Golub R.M., Zylke J. Editorial concern-possible reporting of the same patients With COVID-19 in different reports [published online ahead of print, 2020 Mar 16] JAMA. 2020;JAMA doi: 10.1001/jama.2020.3980. [DOI] [PubMed] [Google Scholar]
- 19.Guan W.J., Ni Z.Y., Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stokes E.K., Zambrano L.D., Anderson K.N. Coronavirus Disease 2019 Case Surveillance — United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759–765. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu C., Chen X., Cai Y. China; JAMA Intern Med: 2020. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung C. Clinical features of deaths in the novel coronavirus epidemic in China. Rev Med Virol. 2020:e2103. [DOI] [PMC free article] [PubMed]
- 23.The Novel Coronavirus Pneumonia Emergency Response Epidemiology T. Vital surveillances: the epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) China CDC Weekly. 2020;2(8):113–22. [PMC free article] [PubMed]
- 24.Arentz M., Yim E., Klaff L. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State [published online ahead of print, 2020 Mar 19] JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goyal P., Choi J.J., Pinheiro L.C. Clinical Characteristics of Covid-19 in New York City. N. Engl. J. Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area [published online ahead of print, 2020 Apr 22] JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [published correction appears in doi: 10.1001/jama.2020.7681] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberg ES, Dufort EM, Blog DS, Hall EW, Hoefer D, Backenson BP, et al. COVID-19 testing, epidemic features, hospital outcomes, and household prevalence, New York State—March 2020. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed]
- 28.Luigi Palmieri XA, Antonino Bella, Stefania Bellino, Stefano Boros, Marco Canevelli, Maria Rita Castrucci, Alessandra Ciervo, Fortunato D'Ancona, Martina Del Manso, Chiara Donfrancesco, Massimo Fabiani, Antonietta Filia, Cinzia Lo Noce, Alberto Mateo Urdiales, Graziano Onder, Patrizio Pezzotti, Ornella Punzo, Valeria Raparelli, Giovanni Rezza, Flavia Riccardo, Maria Cristina Rota, Andrea Siddu, Paola Stefanelli, Brigid Unim, Nicola Vanacore, Silvio Brusaferro. COVID-19 Surveillance Group. Characteristics of COVID-19 patients dying in Italy: report based on available data on Rome, Italy. Instituto Superiore Di Sanita; 2020.
- 29.Grasselli G., Zangrillo A., Zanella A. Italy; JAMA: 2020. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang N., Li D., Wang X. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J.J., Dong X., Cao Y.Y. China; Allergy: 2020. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan. [DOI] [PubMed] [Google Scholar]
- 33.Mei H, Hu Y. [Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19]. Zhonghua Xue Ye Xue Za Zhi. 2020;41(0):E002. [DOI] [PMC free article] [PubMed]
- 34.Zhou B., She J., Wang Y. Venous thrombosis and arteriosclerosis obliterans of lower extremities in a very severe patient with 2019 novel coronavirus disease: a case report. J Thromb Thrombolysis. 2020 doi: 10.1007/s11239-020-02084-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Barry O., Mekki A., Diffre C. Arterial and venous abdominal thrombosis in a 79-year-old woman with COVID-19 pneumonia. Radiol Case Rep. 2020;15(7):1054–1057. doi: 10.1016/j.radcr.2020.04.055. [published online ahead of print, 2020 Apr 29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giacomelli E., Dorigo W., Fargion A. Acute Thrombosis of an Aortic Prosthetic Graft in a Patient with Severe COVID-19-Related Pneumonia. Ann Vasc Surg. 2020;66:8–10. doi: 10.1016/j.avsg.2020.04.040. [published online ahead of print, 2020 Apr 29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dahl-Cruz F., Guevara-Dalrymple N., Lopez-Hernandez N. Cerebral venous thrombosis and SARS-CoV-2 infection. Rev Neurol. 2020;70(10):391–392. doi: 10.33588/rn.7010.2020204. [DOI] [PubMed] [Google Scholar]
- 38.Le Berre A., Marteau V., Emmerich J. Concomitant acute aortic thrombosis and pulmonary embolism complicating COVID-19 pneumonia. Diagn Interv Imaging. 2020;101(5):321–322. doi: 10.1016/j.diii.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viguier A., Delamarre L., Duplantier J. Acute ischemic stroke complicating common carotid artery thrombosis during a severe COVID-19 infection. J Neuroradiol. 2020 doi: 10.1016/j.neurad.2020.04.003. [published online ahead of print, 2020 May 4] S0150-9861(20)30159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colonna C., Monzani N.A., Rocchi A. Chilblain-like lesions in children following suspected COVID-19 infection. Pediatr Dermatol. 2020 doi: 10.1111/pde.14210. [published online ahead of print, 2020 May 6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dominguez-Erquicia P., Dobarro D., Raposeiras-Roubín S. Multivessel coronary thrombosis in a patient with COVID-19 pneumonia. Eur Heart J. 2020;41(22):2132. doi: 10.1093/eurheartj/ehaa393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowles L., Platton S., Yartey N. Lupus Anticoagulant and Abnormal Coagulation Tests in Patients with Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMc2013656. [published online ahead of print, 2020 May 5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y., Xiao M., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17) doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paranjpe I., Fuster V., Lala A. Association of Treatment Dose Anticoagulation with In-Hospital Survival Among Hospitalized Patients with COVID-19. J Am Coll Cardiol. 2020;S0735-1097(20):35218–35219. doi: 10.1016/j.jacc.2020.05.001. [published online ahead of print, 2020 May 5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwong J.C., Schwartz K.L., Campitelli M.A. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378(4):345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 46.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel coronavirus-infected pneumonia in Wuhan, China JAMA 2020. [DOI] [PMC free article] [PubMed]
- 47.Zheng Y.Y., Ma Y.T., Zhang J.Y. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lippi G., Lavie C.J., Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog Cardiovasc Dis. 2020 doi: 10.1016/j.pcad.2020.03.001. [published online ahead of print, 2020 Mar 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonow R.O., Fonarow G.C., O'Gara P.T. Association of Coronavirus Disease 2019 (COVID-19) With Myocardial Injury and Mortality. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1105. [published online ahead of print, 2020 Mar 27] [DOI] [PubMed] [Google Scholar]
- 50.Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J 2020. [DOI] [PMC free article] [PubMed]
- 51.Ruan Q., Yang K., Wang W. China; Intensive Care Med: 2020. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oudit G.Y., Kassiri Z., Jiang C. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39(7):618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inciardi R.M., Lupi L., Zaccone G. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1096. [published online ahead of print, 2020 Mar 27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu H., Ma F., Wei X. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa190. [published online ahead of print, 2020 Mar 16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [published correction appears in Lancet Respir Med. 2020 Feb 25;:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Combes A., Hajage D., Capellier G. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 57.Munshi L., Walkey A., Goligher E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med. 2019;7(2):163–172. doi: 10.1016/S2213-2600(18)30452-1. [DOI] [PubMed] [Google Scholar]
- 58.Pham D.T., Toeg H., De Paulis R. Establishment and Management of Mechanical Circulatory Support During the COVID-19 Pandemic. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047415. [published online ahead of print, 2020 May 4] [DOI] [PubMed] [Google Scholar]
- 59.Bangalore S., Sharma A., Slotwiner A. ST-segment elevation in patients with Covid-19—a case series. N Engl J Med. 2020 doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu C.M., Wong R.S., Wu E.B. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J. 2006;82(964):140–144. doi: 10.1136/pgmj.2005.037515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gautret P., Lagier J.C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;105949 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Molina J.M. Goff JL; Mela-Lima B, Ponscarme D, Goldwirt L, et al. Med Mal Infect: 2020. Delaugerre C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ray W.A., Murray K.T., Hall K. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366(20):1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mercuro N.J., Yen C.F., Shim D.J. Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1834. [published online ahead of print, 2020 May 1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geleris J., Sun Y., Platt J. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020;382(25):2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosenberg E.S., Dufort E.M., Udo T. Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA. 2020 doi: 10.1001/jama.2020.8630. [published online ahead of print, 2020 May 11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brugada J., Campuzano O., Arbelo E. Present status of brugada syndrome: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(9):1046–1059. doi: 10.1016/j.jacc.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 68.Amin A.S., Meregalli P.G., Bardai A. Fever increases the risk for cardiac arrest in the Brugada syndrome. Ann Intern Med. 2008;149(3):216–218. doi: 10.7326/0003-4819-149-3-200808050-00020. [DOI] [PubMed] [Google Scholar]
- 69.Bangalore S., Sharma A., Slotwiner A. ST-Segment Elevation in Patients with Covid-19 - A Case Series. N Engl J Med. 2020;382(25):2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.South A.M., Tomlinson L., Edmonston D. Controversies of renin-angiotensin system inhibition during the COVID-19 pandemic. Nat Rev Nephrol. 2020;16(6):305–307. doi: 10.1038/s41581-020-0279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vaduganathan M., Vardeny O., Michel T. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mancia G., Rea F., Ludergnani M. Renin-Angiotensin-Aldosterone System Blockers and the Risk of Covid-19. N Engl J Med. 2020;382(25):2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Y, Huang F, Xu J, Yang P, Qin Y, Cao M, et al. Anti-hypertensive angiotensin II receptor blockers associated to mitigation of disease severity in elderly COVID-19 patients. medRxiv. 2020:2020.03.20.20039586.
- 74.Li J., Wang X., Chen J. China; JAMA Cardiol: 2020. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsin D.H., Macer D.R. Heroes of SARS: professional roles and ethics of health care workers. J Infect. 2004;49(3):210–215. doi: 10.1016/j.jinf.2004.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ran L, Chen X, Wang Y, Wu W, Zhang L, Tan X. Risk factors of healthcare workers with corona virus disease 2019: a retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed]
- 77.Edelson DP, Sasson C, Chan PS, Atkins DL, Aziz K, Becker LB, et al. Interim guidance for basic and advanced life support in adults, children, and neonates with suspected or confirmed covid-19: from the Emergency Cardiovascular Care Committee and Get With the Guidelines®Resuscitation Adult and Pediatric Task Forces of the American Heart Association in collaboration with the American Academy of Pediatrics, American Association for Respiratory Care, American College of Emergency Physicians, The Society of Critical Care Anesthesiologists, and American Society of Anesthesiologists: supporting organizations: American Association of Critical Care Nurses and National EMS Physicians. Circulation;0(0).
- 78.Szerlip M., Anwaruddin S., Aronow H.D. Considerations for cardiac catheterization laboratory procedures during the COVID‐19 pandemic perspectives from the Society for Cardiovascular Angiography and Interventions Emerging Leader Mentorship (SCAI ELM ) Members and Graduates. Catheter Cardiovasc Interv. 2020:1–12. doi: 10.1002/ccd.28887. [DOI] [PubMed] [Google Scholar]
- 79.Chieffo A, Chair, Stefanini GG, Price S, Barbato E, Tarantini G, Karam N, et al. EAPCI Position statement on invasive management of acute coronary syndromes during the COVID-19 pandemic. European Heart Journal. 2020. [DOI] [PMC free article] [PubMed]
- 80.Guo L, Ren L, Yang S, Xiao M, Chang, Yang F, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed]
- 81.Jing Z.C., Zhu H.D., Yan X.W. Recommendations from the Peking Union Medical College Hospital for the management of acute myocardial infarction during the COVID-19 outbreak. Eur Heart J. 2020;41(19):1791–1794. doi: 10.1093/eurheartj/ehaa258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mahmud E., Dauerman H.L., Welt F.G. Management of Acute Myocardial Infarction During the COVID-19 Pandemic. J Am Coll Cardiol. 2020;S0735-1097(20):35026–35029. doi: 10.1016/j.jacc.2020.04.039. [published online ahead of print, 2020 Apr 21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abdelaziz H.K., Patel B., Chalil S. COVID-19 Pandemic and Acute Myocardial Infarction: Management Protocol From a British Cardiac Centre. Crit Pathw Cardiol. 2020;19(2):55–57. doi: 10.1097/HPC.0000000000000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.2020;https://www.asecho.org/ase-statement-covid-19/ on 4/5/2020 2020.
- 85.Douglas PS, Khandheria B, Stainback RF, Weissman NJ, Peterson ED, Hendel RC, et al. ACCF/ASE/ACEP/AHA/ASNC/SCAI/SCCT/SCMR 2008 appropriateness criteria for stress echocardiography: a report of the American College of Cardiology Foundation Appropriateness Criteria Task Force, American Society of Echocardiography, American College of Emergency Physicians, American Heart Association, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance endorsed by the Heart Rhythm Society and the Society of Critical Care Medicine. J Am Coll Cardiol. 2008;51(11):1127–47. [DOI] [PubMed]
- 86.Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P, Dehmer GJ, et al. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2019 appropriate use criteria for multimodality imaging in the assessment of cardiac structure and function in nonvalvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2019;73(4):488–516. [DOI] [PubMed]
- 87.Bernheim A., Mei X., Huang M. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;200463 doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hani C., Trieu N.H., Saab I. COVID-19 pneumonia: a review of typical CT findings and differential diagnosis. Diagn Interv Imaging. 2020;101(5):263–268. doi: 10.1016/j.diii.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu X., Yu C., Qu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47(5):1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Choi AD, Abbara S, Branch KR, Feuchtner GM, Ghoshhajra B, Nieman K, et al. Society of Cardiovascular Computed Tomography guidance for use of cardiac computed tomography amidst the COVID-19 pandemic. J Cardiovasc Comput Tomogr [DOI] [PMC free article] [PubMed]
- 91.Alhazzani W., Møller M.H., Arabi Y.M. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19) Crit Care Med. 2020;48(6):e440–e469. doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Resuscitation Council UK statement on COVID-19 in relation to CPR and resusctiation in healthcare settings. https://www.resus.org.uk/media/statements/resuscitation-council-uk-statements-on-covid-19-coronavirus-cpr-and-resuscitation/covid-healthcare/ 4/5/2020 2020.
- 93.2020 https://professional.heart.org/idc/groups/ahamah-public/@wcm/@sop/@smd/documents/downloadable/ucm_505872.pdf on 4/5/2020 2020.
- 94.Romaguera R., Cruz-González I., Jurado-Román A. Impact of the COVID-19 pandemic on interventional cardiology activity in Spain. REC Interv Cardiol. 2020;2:82–89. doi: 10.24875/RECICE.M20000123. [DOI] [Google Scholar]
- 95.Garcia S., Albaghdadi M.S., Meraj P.M. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;27259 doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Filippo O., D'Ascenzo F., Angelini F. Reduced Rate of Hospital Admissions for ACS during Covid-19 Outbreak in Northern Italy. N Engl J Med. 2020 doi: 10.1056/NEJMc2009166. published online ahead of print, 2020 Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wood D.A., Mahmud E., Thourani V.H. Safe Reintroduction of Cardiovascular Services during the COVID-19 Pandemic: Guidance from North American Society Leadership. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.063. published online ahead of print, 2020 Apr 30. S0735-1097(20)35165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang P., Zhu L., Cai J. Association of Inpatient Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers With Mortality Among Patients With Hypertension Hospitalized With COVID-19. Circ Res. 2020;126(12):1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bhatt A.S., Moscone A., McElrath E.E. Declines in Hospitalizations for Acute Cardiovascular Conditions During the COVID-19 Pandemic: A Multicenter Tertiary Care Experience. J Am Coll Cardiol. 2020;S0735-1097(20):35393–35396. doi: 10.1016/j.jacc.2020.05.038. published online ahead of print, 2020 May 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Solomon M.D., McNulty E.J., Rana J.S. The Covid-19 Pandemic and the Incidence of Acute Myocardial Infarction. N Engl J Med. 2020 doi: 10.1056/NEJMc2015630. published online ahead of print, 2020 May 19. [DOI] [PubMed] [Google Scholar]
- 102.Roffi M., Capodanno D., Windecker S. Impact of the COVID-19 pandemic on interventional cardiology practice: results of the EAPCI survey. EuroIntervention. 2020 doi: 10.4244/EIJ-D-20-00528. [published online ahead of print, 2020 Jun 3] EIJ-D-20-00528. [DOI] [PubMed] [Google Scholar]
- 103.Fosbøl EL, Butt JH, Østergaard L, et al. Association of Angiotensin-Converting Enzyme Inhibitor or Angiotensin Receptor Blocker Use With COVID-19 Diagnosis and Mortality. JAMA (Published online June 19, 2020) doi: 10.1001/jama.2020.11301 [DOI] [PMC free article] [PubMed]