Highlights
-
•
Laboratory diagnostic parameters in symptomatic Covid19 disease patients.
-
•
Monocyte distribution width and monocyte modification in infection disease and sepsis.
-
•
SARS COV2 infection, prognosis in severe respiratory failure with multiple organ failure, clinical and laboratory finding.
Keywords: Monocyte distribution width, COVID-19, SARS-CoV-2
Abstract
Introduction
Interesting results regarding the contribution of MDW (Monocyte Distribution Width) in the Infectious Disease Unit have been reported. An observational study is ongoing at San Donato Hospital with the aim to evaluate the contribution of MDW in the diagnostic pathway in adult patients entering in the ED setting and tested for SARS-CoV-2.
Material and method
COVID-19 symptomatic and paucisymptomatic patients presenting to ED (Emergency Department), have been enrolled consecutively. Whole blood venous samples have been collected on K2 EDTA for MDW determination, at the same time a nasopharyngeal swab for SARS-CoV-2 RNA detection have been collected.
Results
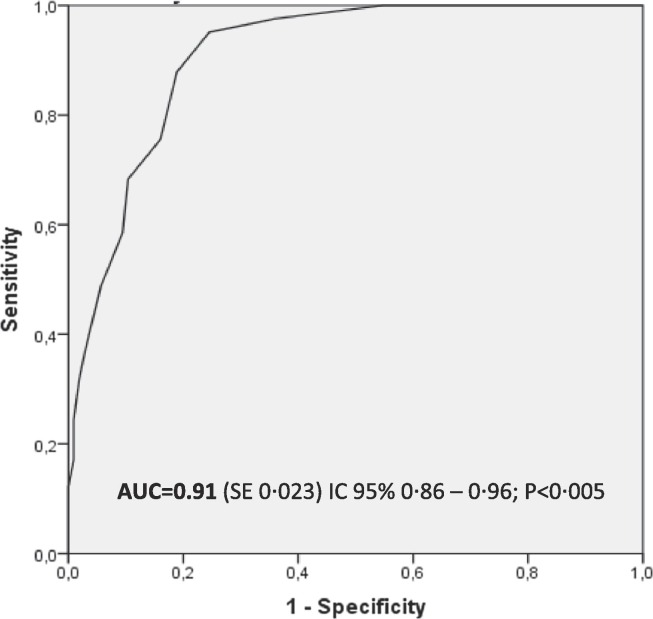
One hundred six patients were negative for SARS-CoV-2 with MDW mean value of 20.3 ± 3.3, while forty-one were positive for SARS-CoV-2 with higher MDW mean value of 27.3 ± 4.9 (P < 0.005). The ROC curve analysis has been evaluated showing MDW AUC of 0.91. Finally twenty-three patients hospitalized in high-intensity care unit showed an MDW value higher than the eighteen patients presenting few symptoms [28.8 ± 5.3 vs 25.4 ± 3.6 respectively, P < 0.05].
Discussion
Monocytic population, in Covid19 disease, are the first elements of innate immunity to be involved, these changes are the basis of the modification of the MDW, with evident efficacy in term of sensitivity, particularly in the studied Covid19 patients. Moreover the patients hospitalized in high-intensity care unit showed significantly elevated MDW respects to middle or low symptomatic one, suggest including this parameter as prognostic marker or of therapy efficacy, integrated with other laboratory findings.
1. Introduction
Due to the COVID-19 outbreak, Tuscany Region adopted countermeasures to cope with this emergency.
Among these countermeasures, separate pathways for patients suspected of COVID infection have been activated at every Emergency Departments and a Coronavirus test has been recommended at the onset of symptoms. To ensure the safest and the appropriate location, all the hospitalized patients have been tested for the virus [1]. As the WHO also pointed out, testing is a crucial phase for preventing virus transmission at community level [2]. The Arezzo hospital in Tuscany (San Donato Hospital) moved accordingly and nasopharyngeal swab for viral RNA detection has been collected.
Monocyte Distribution Width (MDW) is an in vitro diagnostic parameter automatically reported in the Complete Blood Count with differential (CBC-Diff) and routinely requested at the Emergency Department. It has been demonstrated that an elevated MDW value is effective for early detection of sepsis in adult patients presenting at the Emergency Department (ED) [3], [4].
Interesting results regarding the contribution of MDW in the Infectious Disease Unit have been reported, with MDW showing a Receiver Operating Characteristic (ROC) curve Area Under the Curve (AUC) nearly overlapping the PCT one [MDW AUC 0.87; PCT AUC 0.88] [5].
An observational study is ongoing at San Donato Hospital intending to evaluate the contribution of MDW in the diagnostic pathway of adult patients suspected of sepsis in the ED setting.
Based on the suggestion about patients with severe COVID-19 having cytokine storm syndromes [6] and considering the role of monocytes in producing inflammatory cytokines and mediators [7], [8], we decided to observe MDW in adult patients presenting at the ED and tested for SARS-CoV-2.
2. Methods
One hundred forty-seven patients suspected of COVID-19 have been consecutively enrolled in the study. The subjects, presenting to the ED (Emergency Department), show unhealthy conditions and the majority of them has respiratory symptoms flu-specific or they are paucisymptomatic. Whole blood venous samples have been collected on K2 EDTA and a CBC-Diff analysis has been performed on UniCel DxH 900 analyzer (Beckman Coulter Inc.) including the parameter Monocyte Distribution Width (MDW). MDW is a volumetric parameter reported by the analyzer UniCel DxH 900 which characterizes the monocyte population using three measurements: Volume (V), Conductivity (C) and 5 angles Light Scatter (S). The Volume measurements correlate to cell size. The MDW is calculated as the Standard Deviation (SD) of a set of monocyte cell volume values. At the same time, a nasopharyngeal swab for SARS-CoV-2 RNA detection has been collected using Allplex™ 2019n-CoV Assay (Seegene), one of the most accurate methods available on the market reported to have a sensitivity greater than 90% [9]. Data were expressed as mean ± standard deviation [SD] and the Independent Samples t-Test has been used to compare the means. Data analysis was performed by IBM SPSS statistics version 20.0 (SPSS, Chicago, IL).
3. Results
Results from one hundred forty-seven patients have been collected. The patient’s characteristics are reported in Table 1 .
Table 1.
Patients characteristics, data expressed as mean [±SD].
| Variables | Male | Female | RNA detected | RNA not detected | Total |
|---|---|---|---|---|---|
| N | 76 | 71 | 41 | 106 | 147 |
| Age [±SD] | 55.4 [±20.2] | 55.5 [±19.6] | 61.1 [±16.8] | 53.5 [±20.5] | 55.4 [±19.8] |
| MDW | *28.5 ± 5.4 | *25.1 ± 2.8 | 27.3 ± 4.9 | 20.3 ± 3.3 | p < 0.005 |
| §20.3 ± 3.8 | §20.3 ± 2.8 |
RNA detected.
RNA not detected.
One hundred six patients were negative for SARS-CoV-2 with MDW mean value of 20.3 ± 3.3, while forty-one were positive for SARS-CoV-2 with a higher MDW mean value of 27.3 ± 4.9 (P < 0.005).
The ROC curve has been evaluated (Fig. 1 ) showing MDW AUC of 0.91. MDW demonstrated a sensitivity of 98% and a specificity of 65% at cut off = 20, with a PPV (Positive Predictive Value) and NPV (Negative Predictive Value) respectively of 51.9% and 98.6%, Table 2 showed SARS COV2 negative subjects with MDW high than cutoff.
Fig. 1.
ROC curve analysis for MDW performance in differentiating SARS-CoV-2 positive patients from negative ones. MDW demonstrated sensitivity of 98% and specificity of 65% at cut off = 20.
Table 2.
Characteristics of the studied patients.
| #Patients | Clinical features | MDW – Median (range) | |
|---|---|---|---|
| SARS COV2 Negative | |||
| 68 | Flu symptoms – ER discharge | 18,3 (range 15–20) | |
| 34 | Flu symptoms – ER discharge | 22,9 (range 21–27) | |
| 4 | Bacterial or viral infection with comorbidity | 29,5 (range 26–34) | |
| SARS COV2 Positive | |||
| 18 | Flu symptoms – Home quarantine | 24,5 (range 20–34) | |
| 23 | Hospitalized in high-intensity care | 27,0 (range 21–42) | |
We further investigated the forty-one hospitalized SARS-CoV-2 positive patients and a difference in MDW values has been observed: twenty-three patients hospitalized in high-intensity care unit showed an MDW value higher than the eighteen patients presenting few symptoms [28.8 ± 5.3 vs 25.4 ± 3.6 respectively, P < 0.05].
4. Discussion
Patients infected with SARS-CoV-2 show symptoms ranging from minimal symptoms to severe respiratory failure with multiple organ failure. Epithelial cells, macrophages and dendritic cells are the main components of innate immunity in the airways [10] against respiratory infections. Macrophages are located at the apical side of the epithelium; dendritic cells and macrophages serve as innate immune cells to fight against viruses until adaptive immunity is involved.
COVID-19 patients with severe respiratory diseases have been reported to have increased plasma concentrations of pro-inflammatory cytokines, including IL-6, IL-10, granulocyte-colony stimulating factor (G-CSF), monocyte chemo-attractant protein 1 (MCP1), macrophage inflammatory protein (MIP)1α, and tumor necrosis factor (TNF)-α [11], [12], [13]. The cytokine-producing monocyte population is an element of the innate immunity undergoing transformation not only in terms of functionality but also in their morphology [14]. These changes in response to infection are at the basis of the modification of the MDW parameter observed in different types of infection [3], [4], [5].
As is well known, the CBC-Diff is routinely requested at the Emergency Department. Since MDW is included in the CBC-Diff, Monocyte Distribution Width is automatically reported, resulting easily and quickly available for physicians.
The performance of MDW in differentiating SARS-CoV-2 positive patients from negative ones showed an evident efficacy in terms of sensitivity and NPV. As SARS-CoV-2 is not the only pathogen responsible for the disease as showed in table two, the low specificity of MDW described here could be then explained.
Hospitalized patients in high-intensity care unit showed significantly higher MDW respect to paucisymptomatic ones suggesting to consider MDW as a prognostic marker, together with other laboratory findings. Overall, these first and important results encourage us to consider MDW as a useful, rapid and easy to obtain parameter for COVID-19 patients and to study in deep its clinical value.
CRediT authorship contribution statement
Agostino Ognibene: Writing - review & editing, Conceptualization. Maria Lorubbio: Data curation, Project administration, Investigation. Pasqualino Magliocca: Data curation. Emanuela Tripodo: Investigation, Writing - review & editing. Guendalina Vaggelli: Investigation, Writing - review & editing. Giovanni Iannelli: Investigation, Writing - review & editing. Marco Feri: Investigation, Writing - review & editing. Raffaele Scala: Investigation, Writing - review & editing. Alessandro Polcini Tartaglia: Investigation, Writing - review & editing. Angelo Galano: Investigation, Writing - review & editing. Alessandro Pancrazzi: Investigation, Writing - review & editing. Danilo Tacconi: Investigation, Writing - review & editing.
Acknowledgments
The authors would like to thank Dr. Danila Crobu PhD for her correction of the English and collaboration.
References
- 1.Organizzazione per gestione pandemia COVID-19.pdf; https://www.regione.toscana.it/-/coronavirus.
- 2.https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- 3.Crouser E.D., Parrillo J.E., Seymour C.W. Monocyte distribution width: a novel indicator of sepsis-2 and sepsis-3 in high risk emergency department patients. Critical Care Med. 2019:1018–1025. doi: 10.1097/CCM.0000000000003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crouser E.D., Parrillo J.E., Seymour C.W. Improved early detection of sepsis in the ED with a novel monocyte distribution width biomarker. CHEST. 2017;152:518–526. doi: 10.1016/j.chest.2017.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polilli E., Sozio F., Frattari A., Persichitti L., Sensi M., Posata R. Comparison of Monocyte Distribution Width (MDW) and Procalcitonin for early recognition of sepsis. PLoS One. 2020;10:15. doi: 10.1371/journal.pone.0227300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta Puja. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devi Ramnath Raina. Inflammatory mediator in sepsis, Cytokines, chemokines, adhesion molecules and gases. J. Organ Dysfunct. 2006:80–92. [Google Scholar]
- 8.Chousterman B.G. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39(5):517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 9.Puck B. van Kasterena, Basvander Veera, Sharon van den Brinka, Lisa Wijsmana, Jørgende Jongea, Anne marievan den Brandta, Richard Molenkampb, Chantal B.E.M. Reuskena, Adam Meijera. Comparison of seven commercial RT-PCR diagnostic kits for COVID-19, J. Clin Virol. 128 (2020) 104412. [DOI] [PMC free article] [PubMed]
- 10.Yoshikawa T., Hill T., Li K., Peters C.J., Tseng C.T. Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J Virol. 2009;83(7):3039–3048. doi: 10.1128/JVI.01792-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y., Fu B., Zheng X., Wnag D., Zhao C., Qi Y., Sun R., Tian Z., Xu X., Wei H. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Nat. Sci. Rev. 2020 doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020;12:248. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernward Passlick, Dimitri Flieger H.W. Loems Ziegler-Heitbrock, Identification and Characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74(7):2527–2534. [PubMed] [Google Scholar]