Abstract
Objectives
This review aimed to identify which dental procedures generate droplets and aerosols with subsequent contamination, and for these, characterise their pattern, spread and settle.
Data resources
Medline(OVID), Embase(OVID), Cochrane Central Register of Controlled Trials, Scopus, Web of Science and LILACS databases were searched for eligible studies from each database’s inception to May 2020 (search updated 11/08/20). Studies investigating clinical dental activities that generate aerosol using duplicate independent screening. Data extraction by one reviewer and verified by another. Risk of bias assessed through contamination measurement tool sensitivity assessment.
Study selection
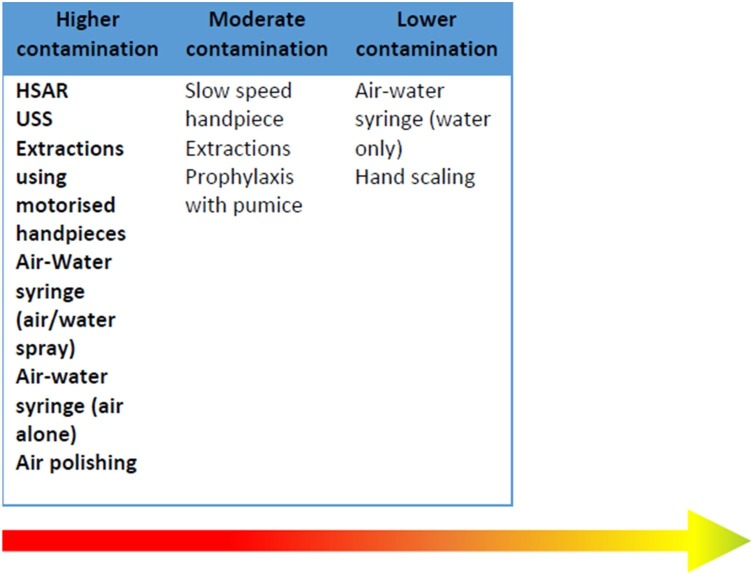
A total eighty-three studies met the inclusion criteria and covered: ultrasonic scaling (USS, n = 44), highspeed air-rotor (HSAR, n = 31); oral surgery (n = 11), slow-speed handpiece (n = 4); air-water (triple) syringe (n = 4), air-polishing (n = 4), prophylaxis (n = 2) and hand-scaling (n = 2). Although no studies investigated respiratory viruses, those on bacteria, blood-splatter and aerosol showed activities using powered devices produced greatest contamination. Contamination was found for all activities, and at the furthest points studied. The operator’s torso, operator’s arm and patient’s body were especially affected. Heterogeneity precluded inter-study comparisons but intra-study comparisons allowed construction of a proposed hierarchy of procedure contamination risk: higher (USS, HSAR, air-water syringe, air polishing, extractions using motorised handpieces); moderate (slow-speed handpieces, prophylaxis, extractions) and lower (air-water syringe [water only] and hand scaling).
Conclusion
Gaps in evidence, low sensitivity of measures and variable quality limit conclusions around contamination for procedures. A hierarchy of contamination from procedures is proposed for challenge/verification by future research which should consider standardised methodologies to facilitate research synthesis.
Clinical significance
This manuscript addresses uncertainty around aerosol generating procedures (AGPs) in dentistry. Findings indicate a continuum of procedure-related aerosol generation rather than the common binary AGP or non-AGP perspective. The findings inform discussion around AGPs and direct future research to support knowledge and decision making around COVID-19 and dental procedures.
Keywords: Aerosol generating procedures, Evidence-based dentistry, Systematic reviews, Infection control, COVID-19, Aerosols
1. Background
SARS-CoV-2 is the highly infectious coronavirus which causes COVID-19 [1]. Transmission is thought to be primarily via respiratory droplets (similar to other coronaviruses and circumstantial evidence from outbreaks during the current pandemic) with the highest viral load detected in the respiratory tract, just before symptoms are apparent and for the next 5 days [2,3]. Individuals are therefore most infectious when they are pre-symptomatic or have mild, often non-specific symptoms, although even the relative importance of this in transmission is unclear [4]. Laboratory studies have been criticised for not being representative of real-life situations and there is ongoing controversy around the extent to which direct contact and fomite routes, and airborne transmission [5,6] are responsible for viral spread [7,8].
Dental care involves close patient contact for prolonged periods leading to concern over transmission through aerosol generation during dental procedures [9,10], with the detection of SARS-CoV-2 in saliva [11] and the high viral load during the pre- and early symptomatic periods. In dentistry, universal precautions have been standard practice, based on evidence-informed infection control. These evolve as evidence emerges, particularly in response to blood and water borne infections and prion transmission [[12], [13], [14]].
The term Aerosol Generating Procedure (AGP) has been described as, “any procedure on a patient that can induce the production of aerosols of various sizes” although there is currently no agreed definition and a confusing lack of consistency in terminology. The UK National Emerging Respiratory Virus Threats Advisory Group has described “dental procedures (using highspeed devices such as Ultrasonic scalers and highspeed drills)” [15] as posing an increased risk of respiratory infection transmission. New terms such as aerosol generating exposure (AGE) have been suggested [16]. Policy documents have focused on ultrasonic scalers (USS), high-speed air-rotors (HSAR), air-water syringes (also known as triple or 3-in-1), and air polishers as sources of aerosols, with rubber dam and high-volume suction as mitigating measures [[17], [18], [19]].
To manage transmission risk of SARS-CoV-2, the extent and contamination of droplets and aerosols involved in dental procedures need to be identified. Similarly, the pattern, and timing, associated with spread and settle of droplets and aerosols in the context of clinical dentistry need to be understood to inform policy on surgery fallow times between patients. Globally specified patient spacing times for AGPs vary from none to 120 min [20].
An aerosol is defined as a suspension of liquid or solid in air [21,22]. When an aerosol is created with a liquid, a wide range of droplet sizes are produced. Particle size is a continuum, from larger heavier droplets, > 5 μm in diameter that fall rapidly to the ground, typically within 1 m of the source as splatter. Aerosols are composed of droplet nuclei ≤ 5 μm in diameter and can remain suspended in air for many hours and be moved by air currents. At present, dental procedures are categorised dichotomously as either aerosol producing or non-aerosol producing. The former refers to procedures considered to produce smaller droplets of ≤ 5 μm and the latter referring to procedures that are considered to produce few or no smaller droplets but may still produce larger droplets (> 5 μm). For the purposes of this review, aerosol will refer to suspensions of particles ≤ 5 μm in diameter.
This review aims to critically assess existing knowledge and reduce uncertainty around dental procedures that generate droplets and aerosol, supporting policy making and local IPC protocols.
1.1. Research question
When performing a specific dental procedure within a dental setting, what is known or still unknown regarding the level and spread of aerosol/droplet contaminants for that procedure and the outcomes/ outcome measures used?
Objectives:
-
1
To identify and catalogue activities within clinical dentistry and the dental surgery that generate aerosols and droplets
-
2For these activities, to:
-
aCharacterise the pattern of droplet and aerosol spread and settle relevant to the dental surgery and dental laboratories
-
bIdentify whether there is evidence of an association with exposure, infection and transmission of pathogenic micro-organisms
-
cList micro-organisms that have been studied
-
dRecord outcomes and outcome measures
-
a
-
3
To identify gaps in the evidence related to aerosols and droplets relevant to clinical dentistry
2. Methods
2.1. Protocol and registration
This review has been conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)(Appendix 2) [23], registered under the International Prospective Register of Systematic Reviews ID number CRD42020193058 and Appendix 1 gives full details.
2.2. Eligibility criteria for study selection
2.2.1. Inclusion criteria
-
•
Study methodology – including but not limited to; trials, observational, experimental (including those using manikins, modelling studies, etc.), qualitative studies, non-clinical reports and other relevant studies;
-
•
Topic of study - investigate activities that generate aerosols etc. relevant to clinical dentistry;
-
•
Where there is a measure of aerosols and droplets;
-
•
Types of settings: dental practices and hospital settings, including simulated environments where they are relevant to the conduct of dental procedures and investigations; and
-
•
English language and also literature written in Chinese if indexed in the searched database platforms.
2.2.2. Exclusion criteria
-
•
Studies that measure bioaerosol generation but where these are not related to single procedures and are carried out at an environmental or broader level (i.e. measure bacterial counts over a day in a surgery)
-
•
Non-English language articles, apart from Chinese journal articles (insufficient resources)
-
•
Aspects of the dental environment which may increase risk of infection and transmission e.g. waiting rooms, high throughput, reception areas, bathrooms (these are generic issues which may be covered elsewhere)
-
•
Grey literature
2.3. Information sources
Medline (OVID), Embase (OVID), Cochrane Central Register of Controlled Trials, Scopus, Web of Science and LILACS databases were searched for studies meeting the inclusion criteria and ClinicalTrials.gov was searched for recently completed, ongoing, or recruiting trials from the start of the databases to May 2020. The search was updated on 11 August 2020 to identify new studies published since the original search was conducted.
2.4. Search
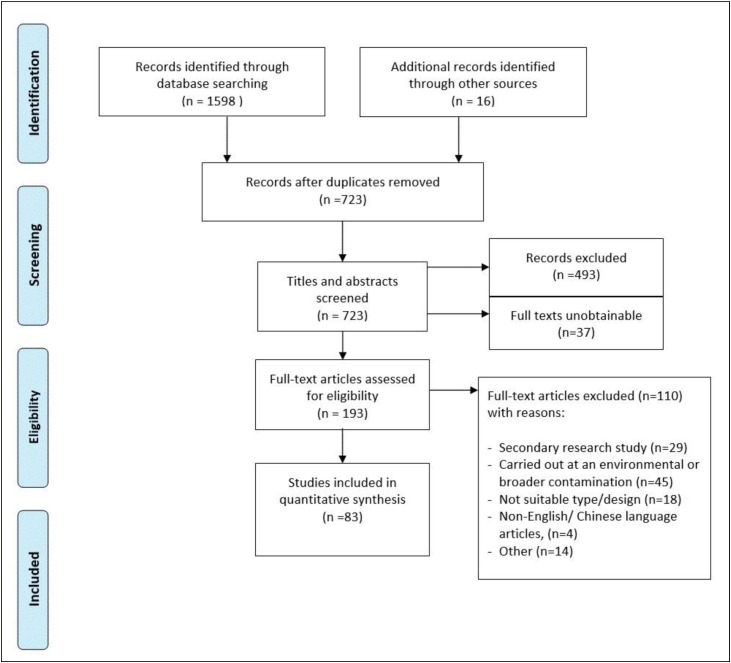
The search strategy (Fig. 1 ) comprised controlled vocabulary and keywords. The references of all reviews, policy documents and included studies were screened for eligible studies.
Fig. 1.
Outline of the search strategy, adapted for each database.
2.5. Screening and selection of studies
Titles and abstracts were deduplicated and screened in Rayyan [24] independently and in duplicate by two reviewers. Where either reviewer considered a paper potentially eligible for inclusion, the full text was sought. Full texts of potential articles were retrieved and assessed independently and in duplicate. It was prearranged that screening of any Chinese language literature would be carried out in consultation with an external researcher who speaks Chinese.
Full texts were exported into Endnote and a database created in Excel. Differences were resolved by consensus involving at least one other research group member.
2.6. Data extraction
A standardised data extraction form was developed a priori and refined based on repeat pilot testing with a minimum of five publications and three data extractors. Eight reviewers were trained in data extraction form completion. Reviewers extracted data into an excel spreadsheet singly but consulted another reviewer where data reporting was unclear. Key missing data items were managed by contacting study investigators where possible. For studies where an intervention was measured for its ability to alter droplet and aerosol spread, only data relating to the baseline or control (i.e. without the intervention effect) was extracted.
2.7. Data items
The items of data extracted included: study demographics; dental procedures investigated; methodology; findings – (related to the reviews’ outcomes). Detection methods for contamination were categorised as microbial, blood and other (non-microbial/non-blood) methodologies.
2.8. Study quality assessments/ Risk of bias
The quality of the papers/ risk of bias was assessed. There were no standard quality tools for methodologies used in these papers or for assessing the quality of their reporting. We therefore took a pragmatic approach and assessed quality measures we considered important that are commonly measured in other study types (industry funding; conflict of interest; relevance, adequate description of equipment / procedure; sample size; controls; confounders; outcome reporting) (Table 1 ). Rather than assign an arbitrary numerical value to these which may be misleadingly summated, we used a traffic light system to show a pictorial representation of the quality of key aspects for each study, allowing the overall quality for items to be seen as well as the quality for each study. For each item we assigned red where the study does not meet standard and green for meeting standard. For standards where we considered it possible for them to be partially met, an amber colour was assigned. The study protocol in Appendix 1 has further detail.
Table 1.
Description of criteria used to assess the methodology/reporting quality of included studies.
| Red (low quality) | Amber (moderate quality/ no mention) | Green (high quality) | |
|---|---|---|---|
| Was the study industry funded (related to the study materials being investigated)? | Yes, industry funded | Not mentioned | Statement that not industry funded |
| Was there a conflict of interest? | Conflict of interest declared (related to the topic or study materials being investigated) | Not mentioned | Clearly states not industry funded or no conflict of interest statement |
| Relevance to routine clinical dentistry | Low – mannikin or simulation study not involving human participants | Medium – human participant study but involving procedures e.g. closed chambers which are very unlike usual dentistry | High – undertaken in dental operatories with human participants |
| Procedure description | Inadequately described | Adequately described to be able to understand what was done but could not be reproduced and could be reproduced | Described in detail and could be reproduced |
| Equipment used in Procedure | Not mentioned | Mentioned but not adequately described (type of item e.g. air rotor)(but no further detail | Adequately described in detail and could be reproduced |
| Sample size | Not mentioned | Mentioned but not described in enough detail to reproduce | Adequately described in detail and could be reproduced |
| Controls (for microbial studies) | No control measures described | Control measures described for example leaving a plate out for an hour before the procedure | Not applicable |
| Sensitivity of measurement for contamination measure (separate for microbiological, blood and visual for spatter)(Further details found on Table 2) | Low sensitivity | Medium sensitivity | High sensitivity |
| Outcome | Outcome reporting do not meet standard i.e. not expressed or statistical tests were not appropriate, not reported | Outcome reporting partially meets standard | Outcomes clearly stated with appropriate descriptive statistics to express contamination for areas as point estimates and include measures of distribution (e.g. standard deviation, standard error and range) and if statistical tests are used to analyse associations, these are appropriate, and include confidence intervals and the probability levels (p value) |
2.9. Detection sensitivity of contamination assessment tool
The sensitivity of the detection methods used to assess contamination were evaluated using a schema tailored to the individual methodologies: microbial measures; blood measures; other [non-microbial/non-blood measures) (Table 2 ). These are presented overall for all studies and grouped by procedure to allow a picture of the detection sensitivity of the methods used, and therefore accuracy of the results in reflecting actual contamination. This allowed a judgement on the likelihood of under- or over-reporting of contamination for each study, and by procedure.
Table 2.
Sensitivity of measurement for contamination measure (separate for microbiological, blood and visual for spatter).
| Blood agar used? | Incubation environment | Incubation duration (days) | |
|---|---|---|---|
| Measurement of microbial contamination | |||
| Low | The study did not use blood agar as growth media. | Aerobic environment was used. | Incubation time (1–3 days) was unsatisfactory for cultivating a wide range of bacteria with different replication rate. |
| Not stated. | Not stated | Not stated. | |
| Moderate | The study used blood agar as growth media. | Aerobic or anaerobic (in consideration to other parameters). | The study used a moderate incubation time for cultivating a moderate range of bacteria with different replication rate. |
| High | The study used blood agar as growth media. | Anaerobic environment was adopted that allowed. | Incubation time (7 days or more) was satisfactory for cultivating a wide range of bacteria with different replication rate. |
| Measurement of blood contamination | |||
| Low | Visible detection with no other equipment used. | ||
| Moderate | Visible detection with the use of visibility of enhancers (e.g. fluorescent dye). | ||
| High | Sophisticated method used for blood detection such as DNA detection with PCR. | ||
| Measurement of non-microbial and non-blood contamination | |||
| Low | Visible detection with no other equipment used. | ||
| Used test with no consideration of dilution effect of blood in interpretation (false negatives). | |||
| Used test with no consideration of impact of hypochlorite in interpretation of surfaces in dental settings (false positives at higher dilutions which is relevant for surfaces rather than gowns/masks/drapes) | |||
| Moderate | Visible detection with the use of visibility of enhancers (e.g. fluorescent dye). | ||
| High | Direct testing; Used agents appropriately, these agents include:
|
||
2.10. Relative contamination of procedures
Where methodology was similar enough to compare contamination levels or studies included multiple procedures, the relative contamination levels between procedures were examined and a visual map represented by a network diagram was constructed to clarify where there were comparisons between different procedures and how many, and to identify where there were gaps.
3. Results
There were 83 studies (Appendix 3) which met the inclusion criteria and for which we could obtain full manuscripts. All included studies were published in English (see PRISMA flow chart Fig. 2 .).
Fig. 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Moher et al. 2009) flow chart. A full description of study characteristics can be found in Appendix 4.
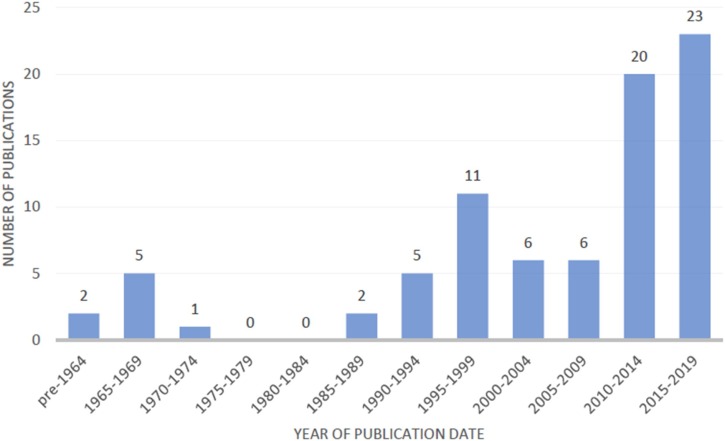
Studies originated from 24 countries, the majority conducted in the USA (n = 26) and India (n = 21). They were published between 1963–2020 with 43/83 (52 %) of the studies published in the last decade (Fig. 3 .)
Fig. 3.
Publications by date (n = 81; 2 publications from 2020 were not included).
A full description of the studies’ characteristics and the data extracted can be found in Appendix 4.
The extracted data were heterogeneous across key characteristics, including; aims, methodology and outcomes. A narrative summary was undertaken and within study comparisons of relative contamination made. Whilst there were no studies showing a direct association between dental procedures with exposure, infection and transmission of pathogenic micro-organisms (outcome 2b), there was evidence of contamination of persons in the dental surgery and the surgery environment (surfaces, equipment etc.) and air from all procedures investigated although the levels of contamination varied (Appendix 4).
Approaches to the investigation varied: some studied procedures, some instruments and some both. Data were separated into the following categories: USS (n = 44 studies), HSAR (n = 31); oral surgery (n = 11), slow-speed handpiece (n = 4); air-water syringe (n = 4), air polishing (n = 4), prophylaxis with cup and pumice (n = 2) and hand scaling (n = 2.
Settle plates were used in 48 studies, 12 used visual inspection and 23 used air samplers (specific for aerosol). The main findings for each of the instruments/ procedure categories is summarised below (n = number of studies), with further detail provided in Table 3 and Appendix 4.
Table 3.
Main characteristics of the included studies categorised by dental procedure/ instrument use (n = 83). Please note that the numbering in this table refers to the list of references (included studies) in Appendix 3 and are not the same numbering system as found in the main text.
| Procedure category (no of studies) | Procedures investigated (e.g. cavity preparation) and type of investigation (clinical/ laboratory, patient/ mannequin) | Investigated contamination of operator, assistant, patient | Area within dental surgery investigated* | Different timepoints sampled (hours, minutes, seconds) | Detection sensitivity scores for outcome methodologies |
|---|---|---|---|---|---|
| USS (n=44)a | Ultrasonic scaling (n=44) | Operator (n=18) 5,8,9,10,16,21,31,44,45,55,59,60,63, 65,70,72,83,85 | Environmental surfaces < 1m (n=15) 8,16,21,33,37,45,52,54,61,66,71,73,74,76,78) | During procedure (n=28) 8,9,22,23,24,25,32,48,90,52,54,59,60,61,62,64,65,66,69, 70,72, 73,74,76,78,80,85,87 | High detection sensitivity (n=5) |
| Simulation Mannequin (n=3)8,25,83 | Head/face (n=13)8,9,10,16,21,31,44,53,59,65,72,83,85 | >1m (n=3)25,44,64 | During and after combined (n=8) 10,16,21,31,41,44,45, 71 | Moderate detection sensitivity (n=3) | |
| Simulation Typodont Laboratory (n=3)33,34,66 | Body (n=8)5,8,10,31,60,70,83,85 | < 1m and > 1m (n=9) 10,41,48,69,90,70,80,83,87 | After procedure(n=3) | Low detection sensitivity | |
| Assistant (n=4) | Air Samples (n=7)6,22,23,24,29,32,87 | 0–30 min29,37,83 | (n=34) | ||
| Head (n=2)16,83 | 30 min-1 hr29,37,83 | Uncategorized detection sensitivity (n=2) | |||
| Body (n=3)31,70,83 | 1–2 hr29,83 | ||||
| Patient (n=14) | 2–4 hr29 | ||||
| Chest (n=14)5,8,16,21,31,44,60,61 62,65,70,71,73,85 | |||||
| HSAR (n= 31)b | Tooth/cavity preparation (clinic) (n= 24) | Operator (n=10) | Using settle plate within 1m of operating area | Within the first 30 minutes after the procedure (n=4)8, 29,51, 94 | High detection sensitivity (n=4) |
| Mannequin/ Typodont (clinic) (n=3)8,36,42 | Head/face (n=9)96,95, 20,8,81,59,63,55,3, Body (n=2)8,60 | (n=12)93,8,92,42,48,59,63,90,60,81,94,87 | Between (30 min -1 hr) after procedure (n=2)29,51 | Moderate detection sensitivity (n=6) | |
| Mannequin/ typodont simulation (laboratory) (n=4)13,30,51,89 | Assistant (n=3) | Using settle plate 1m or more from operating area (n=9)93,48,90,56,63,94, 60,63, 81 | 1-2 hr (n=2)29,51, 2-4 hr (n=2)29,51, > 4 hr (n=1)51 | Low detection sensitivity (n=21) | |
| Head/face (n=3)8,81,63, Body (n=1)8 | |||||
| Patient (n=6) | |||||
| Intra-oral (n=1)20, Head/face (n=1)95, Body (n=4)92, 20, 8, 94 | |||||
| Oral surgery (including extractions) (n=11)c | Surgical removal of teeth (clinic) (n=8) 4, 17, 38,39,84, 43, 87, 98 | Operator (n=5) | Environment and surfaces within 1m from operating area (n=4) 17,32, 39, 46, 87 | Up to 30 min during procedure (n=2)17,43 | High detection sensitivity (n=3) |
| Head/ face/neck (n=4) 4, 38, 40, 98 | |||||
| Implants (clinic) (n= 1) 98 | Arms/ Cuffs/ hands | Environment and surfaces 1m or more from | During treatment with agar plates replaced every 10 min (n=1)32 | Moderate detection sensitivity (n=2) | |
| Extraction (clinic) (n=2) 32,98 | (n= 3) 4, 38, 40 | operating area (n=0) | Low detection sensitivity (n=6) | ||
| Other: restorative and periodontal procedures (clinic) (n=1) 87 | Abdomen/ shoes (n= 3) 4, 38, 4 | Sampled at mouth (n=0) | |||
| Nearby operator (n=1) 43 | |||||
| Assistant (n=3) | |||||
| Head/ face/neck (n=2) 4, 98 | |||||
| Not verified/ unclear (clinic) (n=2) 40, 46 | Arms/ Cuffs/ hands, (n=1) 4 | ||||
| Other body parts (n=1) 4 | |||||
| Nearby assistant (n=1) 43 | |||||
| Patient: (n=3) | |||||
| Head/ face, (n=1)4 | |||||
| Chest (n=2)4, 17, 43 | |||||
| Slow-speed handpiece (n=5)d | Removal of excess material following fixed orthodontic appliance removal (n=3)14,15,97 | Operator (n=1)2 (thorax and abdomen area of technician) | Air sampler 30cm from patient’s mouth (n=2)14,15 | Not specified (n=5) | High detection sensitivity (n=3) |
| Air sampler 10cm from patient’s mouth (n=1)97 | Moderate detection sensitivity (n=0) | ||||
| Polishing and trimming of denture (n=2)2,47 | Settle plates at 1ft, 2ft and 3ft from the motor n=147 | Low detection sensitivity (n=2) | |||
| Air-water syringe (n=4)e | Air-water syringe with air and water used together (n=3)89,90,95 | N/A | Experimental Simulation-Air Samples Human aerosol test chamber89 | During procedure (n=3)89,90,93 | High detection sensitivity (n=0) |
| After procedure (n=1)51: at 2 min, 35 min, 2 hr, 4 hr and 6 hr. | |||||
| Air-water syringe with air alone (n=4)51,89, 90,93 | Quartz crystal microbalance cascade impactor (QCMCI)95 | Moderate detection sensitivity (n=1) | |||
| Air-water syringe with water alone (n=4)51,89, 90,93 | Clinical (splatter plate) 90,91 | Low detection sensitivity (n=3) | |||
| 0–1m90,91 | |||||
| 1–2m90,91 | |||||
| Air polishing (n=4)f | Air polishing (clinic) (n=3)18,49,53 | Operator | Air sampled 12 inches from patients’ mouth at 50o angle53 | 30 min including procedure of 2 min49,53 | High detection sensitivity (n=0) |
| Head/face (n=3)18,49,53 | |||||
| Mannequin/ typodont simulation (laboratory) (n=1)35 | Patient | Air sampled < 1m of operating area35 | During procedure (n=2)18,35 | Moderate detection sensitivity (n=1) | |
| Body (n=1)18 | Surface < 1m operating area (n=1)49 | Low detection sensitivity (n=3) | |||
| Surface > 1 m (n=1)49 | |||||
| Prophy: cup and pumice (n=2)g | Prophy on patient’s head in closed experimental test chamber with side glove ports (clinic) (n=1)89 | N/A | Air sampling of the closed chamber around the patient’s head89 | During the procedure (10–120 sec) with 4 min clearance period of quiet breathing before and after89 | High detection sensitivity (n=0) |
| Prophy (clinic) (n=1)90 | Air sampled 3 ft from floor and 1 ft from patients’ mouth (in front) to the sides and end of the surgery90 | During procedure (30 sec)90 | Moderate detection sensitivity (n=0) | ||
| Low detection sensitivity (n=2) | |||||
| Hand scaling (n=2)h | Hand scaling patient head in closed test chamber with side glove ports (clinic) (n=1)89 | N/A | Air sampling of the closed chamber around the patient’s head89 | During procedure (10–120 sec) with 4 mins clearance period of quiet breathing before and afterwards89 | High detection sensitivity (n=0) |
| Moderate detection sensitivity (n=0) | |||||
| Mannequin/typodont simulation (laboratory) (n=1)34 | Air sampled around mouth distance not specified34 | During procedure (2 sec)34 | Low detection sensitivity sampling/measuring approach (n=3) |
Unique study identification numbers.
Dos Santos 201418, Harrel 199935, Logothetis 199549, Muzzin 199953.
m = metre(s); ft – foot/feet; in = inch(es); cm = centimetre(s); hr = hour(s); min = minute(s); sec = second(s).
Balcos 20195, Barnes 19986, Bentley 19948, Choi 20189, Chuang 201410, Devker 201216, Feres 201021, Fine 199222, Fine 1993 a23, Fine 1993b24, Graetz 201425, Grenier 199529, Gupta 201431, Hallier 201032, Harrel 199834, Harrel 199633, Holloman 201537, Jawade 201641, Kaur 201444, King 199745, Labaf 201148, Miller 197190, Mohan 201652, Narayana 201654, Nejatidanesh 201355, Prospero 200359, Purohit 200960, Ramesh 201561, Rao 201562, Reddy 201264, Retamal-Valdes 201765, Rivera-Hidalho 199966, Sadun 202069, Saini 201570, Sawhney 201571, Serban 201372, Sethi 201973, Shetty 201374, Singh 201676, Swaminathan 201478, Timmerman 200480, Veena 201583, Watanabe 201385, Yamada 201187.
Al-Amad 20173, Belting 196393, Bentley 19948, Cochrane 198992, Dahlke 201213, Day 200815, Earnest20, Greco 200828, Grenier 199529, Grundy 196730, Hallier 201032, Hausler 196636, Junevičius 200542, Labaf 201148, Larato 196691, Manarte-Monteiro 201350, Micik 196989, Miller 197190, Miller 199551, Neiatidanesh 201355, Oliveira 201856, Prospero 200359, Purohit 200960, Rautemaa 200663, Samaranayake 198988, Stevens 196396, Tag El-Din 199794, Toroğlu 200181, Toroğlu 20038, Travaglini95, Yamada 201187.
Al-Eid 20184, Divya 201917, Hallier 201032, Ishiharma 200838, Ishiharma 200939, Janani 201840, Jimson 201543, Kobza 201846, Wada 201084, Yamada 201187, Aguilar-Duran 202098.
Agostinho 20042, Dawson 201614, Day 200815, Ireland 200397, Kritivasan 201947.
Belting 196393,, Micik 196989, Miller 197190, Miller 199551.
Micik 196989, Miller 19719.
Harrel 199834, Micik 196989.
3.1. USS (n = 44 studies)
All 37 studies with measures for droplet contamination (splatter) and where samples were collected from the air (n = 7) had positive findings including those that used high volume and standard suction. There was greater droplet contamination within 1 m distance of the patient (n = 8) [[25], [26], [27], [28], [29], [30], [31], [32]]. The operator’s face (mask and visor area) were heavily contaminated (n = 10) as were areas closer to the patient (operator’s nearest arm) (n = 2) [31,33]. When assessed, contamination was identified on the assistant’s face and arm (n = 4). The patient’s body was heavily contaminated and their chest one of the most heavily contaminated areas. Contamination levels reduced with increasing distance from the mouth. At the maximum measured distance of 3 m, contamination was identified. Air sampling (n = 3) found contaminated aerosol was generated during treatment [31,34,35] with a small proportion detectable at four feet before returning to baseline two hours post-treatment [34].
3.2. HSAR (31 studies)
There was wide variability in the procedures investigated: Restorative (n = 27); cavity preparation, gaining endodontic access, fixed prosthesis tooth preparation; and Orthodontic (n = 4); cement removal [following fixed appliances]; and procedural time (10 s to four hours). Contamination was detected on settle plates on surfaces across all (n = 15) areas measured up to three meters from patient mouth [27,36]. Contamination levels were highest in front of the patient and reduced with increasing distance from the mouth [25,36,37] and the lowest areas of contamination levels were behind the patient [37]. One study found approximately 80 % of aerosol settled immediately following the procedure and reached baseline levels after two hours [34]. The operator, assistant and patient were consistently contaminated, most heavily on the operator’s head and patient’s chest. One study directly compared both single and multi-surgeries and found that contamination was detected over a larger distance when there were multiple dental chairs in an area [34].
3.3. Oral surgery (n = 11)
Oral surgery involving removal of teeth, generally third molars, used motorised handpieces of variable speeds (n = 10; 1 assumed), reporting the use of irrigation (n = 6), intra-oral suction/aspiration (n = 5) in single and multiple chair dental settings, found risk of contamination (mostly blood, with evidence of anaerobic bacteria). Contamination was present on the patient (chest and face), operator (face/head, arm/glove/cuff, chest, abdomen, leg) and assistant (face/head, arm/glove/cuff, body) as well as the dental operatory and air environment (< 1 m). Surgeons and assistants wore surgical PPE including gowns and the research was conducted in a range of settings, mainly dental hospital outpatient facilities (n = 10). Most evidence was of blood spatter (visible and imperceptible) whilst microbiological examination was limited to aerobic testing. Imperceptible blood splatter was significantly higher than visible stains. Very limited evidence on extractions (n = 2) suggests lower risk but not without risk of contamination. Risk increased with time, type of procedure and decreased with distance.
3.4. Slow-speed handpiece (n = 5)
Three studied removal of excess material following fixed orthodontic appliance treatment [[38], [39], [40]] with air sampling equipment (to detect aerosol). Findings varied but could be related to study design differences. Dawson et al. [40] and Day et al. [39] found a marked increase in bacterial load during debonding and enamel cleaning compared to baseline levels. Ireland et al. [34] detected particles (2 μm to >30 μm diameter) demonstrating facilitation of aerosol and droplets.
With denture polishing and trimming [41,42] microbiological contamination was highest 2 feet from the operatory position compared to 1 ft and 3 ft [41] and microorganisms such as yeasts and Gram-negative bacteria were in the aerosol generated [42].
3.5. Air-water syringe (n = 4)
All studies identified contamination following use [25,[43], [44], [45]]. However, the extent of contamination varied widely (n = 3). Air and water used together (spray) generated more than air alone and water alone the least [25,43,44]. Smaller particles remained in the air for more than 6 h [45]. Bacterial contamination from droplets was detectable at 4 feet from the patient [43] and 6 feet from patients [25]. These were the maximum points sampled
3.6. Air polishing (n = 4)
Air polishing demonstrated contamination of, in ascending order, the operator’s forehead, operator’s mouth and patients’ chest. Contamination from air polishing was found nine feet from the treatment area even in a surgery with 13 air changes/hour [46].
3.7. Prophylaxis (n = 2)
Prophylaxis with cup and pumice (n = 2), [25,44] produced less contamination than air-polishing (n = 4). It produced a higher rate of contamination than washing teeth with a water stream, but lower than drying teeth with an air spray or using a high turbine with water coolant, as measured using the same closed test chamber (n = 1).
3.8. Hand scaling (n = 3)
Hand scaling produced minimal contamination (two artificial environments; patients’ head in a closed experimental test chamber with side glove ports for the operator (n = 1), and a mannequin in a closed box (n = 1). Levels of air contamination in the test chamber were comparable to a clinical examination in the same experiment. When hand scaling with orthodontic treatment was compared to HSAR contamination was much lower when no powered instruments were used [37].
3.9. Relative contamination levels
Although there were 83 studies, the degree of heterogeneity in methodology meant it was not possible to compare data between them. The outcome of interest was contamination. It could be grouped as being microbial, blood and non-microbial/non-blood. However, within these, there were a large variety of outcome measures (Appendix 4). For example, even within those looking at colony forming units, the outcome measures encompassed; whole plate (CFU/mm2); (CFU/mm3); (CFU/cm2); (CFU/m3), volume of sampled air (CFU/cm2/min), Index of Microbial Air Contamination (CFU/m2/h) and rate of production (CFU/min).
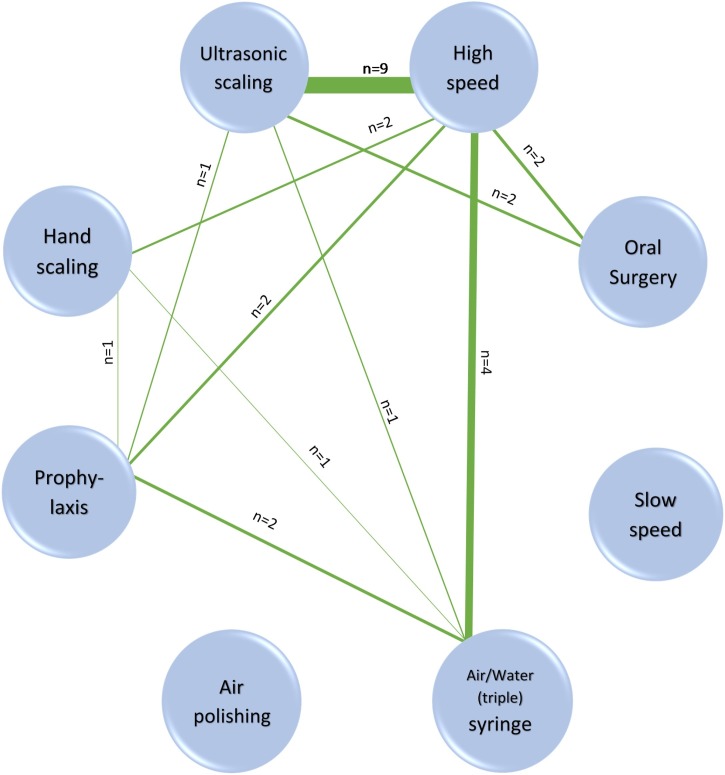
However, it was evident that there was a hierarchy of contamination levels with some procedures generating more contamination than others. A network diagram (Fig. 4 ) illustrates where intra-study comparisons exist. Data from these 13 studies were tabulated (Appendix 5, Table 1) and compared for relative contamination levels within studies (Appendix 5, Table 2).
Fig. 4.
Network diagram illustrating where studies included comparison between different procedures within them. (See also Appendix 5). The nodes represent the eight procedures and the lines between them show where a study compares them. The number of studies is shown by ‘n=’ and also the relative thickness of the lines. Where a node has no linkages, there are no studies comparing it with another procedure.
These studies and their data were then categorised as higher, moderate and lower relative contamination levels (Fig. 5 ). This is a proposed hierarchy model and it should be noted that positions denote relative positions along a spectrum rather than definitive cut-offs between the three levels.
Fig. 5.
Proposed levels of contamination associated with different procedures, drawn from Appendix 5 showing levels of contamination within studies to minimize dissimilarities in methodology, procedures and outcomes that might account for differences. Note that this must be interpreted with caution and will need to be modified as further evidence becomes available. *Indicates very low certainty.
3.10. Quality assessment/ risk of bias
The quality assessment (Table 4 ) for the studies showed a mixed picture for each of the seven domains; the majority of studies scored “high” quality for one domain (controls), “moderate” for four (study funding, conflict of interest, procedure description and outcome reporting) and “low” for two (equipment use and sample size).
Table 4.
Quality assessment of included studies (n = 83). Studies were ranked as low (red), moderate (amber) or high (green) for each parameter (see protocol for full description in Appendix 1). There is no summation across fields.
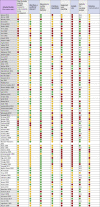 |
Risk of bias was assessed across the cumulative evidence. It was not possible to carry out a statistical assessment of publication bias but the spread of evidence was considered through publications dates’ analysis to look for increases in evidence production to see if these align to events such as infectious disease outbreaks.
3.11. Detection sensitivity
Across all studies, for detecting contamination, 59 were rated as low detection sensitivity, 11 were moderate and 11 as having a high detection sensitivity. Detection sensitivity gradings for each procedure and by study are detailed in Appendix 6.
4. Discussion
The 83 studies included in this review looked at eight different activities classified as; USSs, HSAR and slow-speed handpieces, oral surgery, air-water syringe, air polishing and hand scaling. There was heterogeneity between methodologies used to investigate contamination and a lack of consistency even when the same methodology was used by different studies. Few studies used a high detection sensitivity measure making under-reporting of levels of contamination a concern. Despite the variable methodology and broadly low detection sensitivity of the methods used, all activities and all studies identified contamination from droplets that had either settled (splatter) or droplet nuclei remaining in the air as aerosol and at the furthest points studied.
Contamination levels varied with some activities such as hand scaling generating contamination no greater than occurs during speaking [25]. The greatest levels of contamination were found with procedures involving powered devices and water (HSAR and USS). Devices that used air and water together also generated splatter and aerosol and highest nearest the patient. It was not possible to draw conclusions around the use of the slow-speed handpiece with any certainty because no study compared it with anything else for either use of carious tissue removal or orthodontic cement removal.
Although dental procedures are commonly categorised dichotomously as either aerosol producing or non-aerosol producing, this is an over-simplification, bearing in mind that while droplet nuclei ≤ 5 μm in diameter are categorised as aerosols, in reality droplet size lies on a continuum. Since SARS-CoV2 transmission has been reported up to 4 m from the source [47], aerosol transmission remains a possibility.
The majority of studies used a settle plate methodology which is limited to capturing droplets which can carry viruses. Settle plates can detect droplets that have fallen onto a surface. However, air turbulence caused by movement in the surgery may affect what is captured. Air samplers, most of which actively sample the air in the room, will therefore detect both aerosol and airborne droplets before they have fallen to ground (n = 23 studies). Many of the studies that stated they were detecting aerosols, did not use a methodology that investigated airborne transmission (i.e. droplets <5 μm) such as air samplers. Most studies’ findings related to droplet splatter detected on settle plates. However, both droplets and aerosols can carry viruses although the universal precautions currently in use will provide protection from droplet transmission.
Although we identified 83 studies investigating eight different procedures, it was difficult to draw definitive conclusions about relative droplet and aerosol generation between procedures. Firstly, because diverse methodologies had been used and secondly, because study quality was generally low, especially in relation to the sensitivity of measuring contamination. Low detection sensitivity could result in significant underestimates: for example, saliva cultured under highly sensitive conditions will yield c 108 CFU/ml but only 105 -106 using low detection sensitivity methods. Thirdly, all studies found contamination as far as they measured it and most, for as long as they measured it – which begs the question as to whether greater distances and times measured would have resulted in further positive findings. There was also insufficient data to explore differences according to environmental context such as ventilation or single/multi-chair surgeries (although where multi-surgery environments were studied this showed contamination over larger distances [34,48].
There has been growing research in this area with over half of the studies being published in the last decade. Most studies (n = 62) used microbes purported to be from the oral cavity to monitor contamination from procedures. All, apart from two looking at the bloodborne virus Hepatitis B [49,50] and three at fungi, investigated bacteria. None of the studies investigated contamination by respiratory viruses. This is despite several significant outbreaks of respiratory viruses where AGPs might have been a risk factor (Severe Acute Respiratory Syndrome (SARS) 2003; Swine flu 2009; Middle East Respiratory Syndrome in 2012). Viruses are difficult to culture, in comparison to bacteria, which may account for this. Most of the studies looked at easily cultured oral bacteria as surrogates of contamination from the droplets and aerosol generated from the procedure. However, viruses are small (typically between 20−300 nm in diameter) and can be carried in the same way as bacteria, therefore patterns of bacterial splatter and aerosol can provide some information to inform viral spread.
None of the studies directly explored exposure (for the dental team or patient) to potentially pathogenic micro-organisms. Studies did, however, identify significant contamination relating to the operator’s head and the patient’s body when powered devices (HSARs and USSs) were used. Visors, glasses and masks were often heavily contaminated, with a small number of ultrasonic studies finding contamination under the visor and mask. The body and operating arm of the operator were subject to significant contamination and studies of the assistant found less contamination (although this varied depending on the area of the mouth being worked on). Areas closest to the patient were most affected. This has implications for decisions about personal protective equipment and the coverage needed. The patient’s face and body were significantly contaminated as a result of powered devices (HSARs and USSs). These were often measured in the oral surgery procedures and were also found to be contaminated. There were two studies, both oral surgery ones, that investigated the lower part of the operator body. One indicated contamination of the abdomen and upper leg areas [51], and another showing no contamination of shoe covers used by surgeons and their assistants [52]. Although these are only two studies, they have implications for infection control measures to reduce the chances of cross contamination in dental settings.
Beyond time, distances and settings there were further obvious gaps in the data as a whole. These include a lack of negative controls and baseline measures, clarity of reporting over specific procedure times and time periods during and following them to see when there was no longer contamination from the procedure. However, the most concerning gap may be the general failure across the studies to report the limits of contamination for distance and very few reporting on time for settle. Studies on mitigating interventions (such as high-volume evacuators, HEPA filters, air changes etc.) may be able to clarify this area further. However, it is encouraging to see more research in the field being undertaken, with three papers published since this search was undertaken, fitting the inclusion criteria [[53], [54], [55]] and one further paper [56] awaiting full text assessment at the time of submission, hopefully these will add to the body of well conducted research informing risk and risk mitigation in relation to AGPs.
A limitation of the study is that, as a result of the diverse nature of the study designs included, the experimental approach and the type of data extracted from those studies, it was not appropriate to use a standardised quality assessment tool."
Since the COVID-19 pandemic began, much research effort has focussed on elucidating the modes of SARS-CoV-2 transmission to intervene and effectively reduce its spread. It is not disputed that COVID-19 is highly transmissible with the most common routes similar to other respiratory viruses: respiratory droplets and direct contact (although the role of fomites remains controversial) [4]. In addition, there is broad agreement, and evidence from outbreaks, that transmission via aerosols occurs [5]. In response to this evidence, dentists around the globe paused clinical practice and at the time of publishing this review, dental activity remains significantly reduced. However, the potential role that dental aerosols play in transmission of COVID-19 remains poorly understood. It is important to highlight that all included studies in this review were concerned with where aerosol/droplet spread reached in the dental setting, and/or the associated microbial load. None of these studies directly investigated infectious disease transmissibility from AGPs. Such studies focused on Covid-19 are needed but may be difficult to perform giving the challenges of working with live virus and the uncertain relevance of possible viral surrogates.
This review has focused on droplet and aerosol contamination relating to specific dental procedures, extending beyond previous work that looked at micro-organisms and hazards generated in dental practice [57]. This provides evidence which may be used to determine the baseline risks associated with dental procedures, helping dental professionals to identify clinical situations with increased aerosol transmission risks where mitigation would be strongly advisable.
5. Conclusion
Despite generally low detection sensitivity measures being used, there was evidence of contamination of surfaces around the surgery environment/ personnel or contamination in air from all procedures that were studied. There was evidence that this varied by procedure type. Most studies used microbial surrogate measures (mainly oral microbiota) and blood or colored water for detecting contamination following these procedures. None looked at respiratory viruses. The variability in methodology and variety of outcome measures thwarted attempts to synthesise across studies. By looking at comparisons of procedures within studies, blunt generalisations could be made over higher, moderate and lower risk procedures. There are significant gaps in the evidence and its quality that limit conclusions around all aspects of contamination for different procedures. These hamper evidence-based clinical recommendations and policy decision making, especially relevant for dentistry and COVID-19.
Funding
There are no external sources of funding for this research and it was supported by the authors’ institutions
Authors contributions
NI, IJ, RH, JE had oversight of the study planning and execution, and to the conception, design, data acquisition, synthesis, visualization and interpretation, drafted and critically revised the manuscript. WW conception, design, data synthesis and interpretation, drafted and critically revised the manuscript WA, MR data acquisition, synthesis, visualization and interpretation, drafted and critically revised the manuscript. SKC, RH data acquisition, synthesis and interpretation, drafted and critically revised the manuscript. SM design of the study, provision of and management of study literature resources and critically revised the manuscript.
All authors gave their final approval and agree to be accountable for all aspects of the work.
Declaration of Competing Interest
All of the authors confirm they have no conflict of interest with regard to this work, to report. There are no external sources of funding for this research and it was supported by the authors’ institutions
Acknowledgments
We would like to thank library team at the British Dental Journal, for their quick response and dedication to try to supply the full text of papers we required where there was limited access to hard copies in the face of challenges imposed by the COVID-19 lockdown. A special thanks to the American Journal of Dentistry, who provided us a copy of one of their articles at no charge. This work was not externally funded and authors were supported by their institutions.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jdent.2020.103556.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.World Health Organization . 2020. Naming the Coronavirus Disease (COVID-19) and the Virus That Causes It.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (Accessed 12 June 2020) [Google Scholar]
- 2.Cevik M., Kuppalli K., Kindrachuk J., Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ. 2020;371:m3862. doi: 10.1136/bmj.m3862. [DOI] [PubMed] [Google Scholar]
- 3.Qiu X., Nergiz A.I., Maraolo A.E., Bogoch I.I., Low N., Cevik M. medRxiv; 2020. Defining the Role of Asymptomatic and Pre-symptomatic SARS-CoV-2 Transmission – a Living Systematic Review. 2020.09.01.20135194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldman E. Exaggerated risk of transmission of COVID-19 by fomites. Lancet Infect. Dis. 2020;20(8):892–893. doi: 10.1016/S1473-3099(20)30561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . 2020. Coronavirus Disease (COVID-19): How Is It Transmitted?https://www.who.int/news-room/q-a-detail/q-a-how-is-covid-19-transmitted (Accessed 21 November 2020) [Google Scholar]
- 6.Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . 2020. Modes of Transmission of Virus Causing covid-19: Implications for Ipc Precaution Recommendations.https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations (Accessed 12 June 2020) [Google Scholar]
- 8.Lewis D. Is the coronavirus airborne? Experts can’t agree. Nature. 2020;580(7802):175. doi: 10.1038/d41586-020-00974-w. [DOI] [PubMed] [Google Scholar]
- 9.Meng L., Hua F., Bian Z. Coronavirus disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. J. Dent. Res. 2020;99(5):481–487. doi: 10.1177/0022034520914246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng X., Xu X., Li Y., Cheng L., Zhou X., Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020;12(1):9. doi: 10.1038/s41368-020-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.To K.K., Tsang O.T., Chik-Yan Yip C., Chan K.H., Wu T.C., Chan J.M.C., Leung W.S., Chik T.S., Choi C.Y., Kandamby D.H., Lung D.C., Tam A.R., Poon R.W., Fung A.Y., Hung I.F., Cheng V.C., Chan J.F., Yuen K.Y. Consistent detection of 2019 novel coronavirus in saliva. Clin. Infect. Dis. 2020;ciaa149 doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porter S. Dental treatment and risk of vCJD. Br. Dent. J. 2007;202:470–471. doi: 10.1038/bdj.2007.126. [DOI] [PubMed] [Google Scholar]
- 13.Johnson I., Gallagher J.E., Verbeek J.H., Innes N.P.T. Personal protective equipment: a commentary for the dental and oral health care team. Cochrane Oral Health. 2020 https://oralhealth.cochrane.org/news/personal-protective-equipment-commentary-dental-and-oral-health-care-team (Accessed 18 June 2020) [Google Scholar]
- 14.Verbeek J.H., Rajamaki B., Ijaz S., Sauni R., Toomey E., Blackwood B., Tikka C., Ruotsalainen J.H., Kilinc Balci F.S. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst. Rev. 2020;4(4) doi: 10.1002/14651858.CD011621.pub4. Cd011621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NHS National Services Scotland . 2020. Assessing the Evidence Base for Medical Procedures Which Create a Higher Risk of Respiratory Infection Transmission From Patient to Healthcare Worker.https://hpspubsrepo.blob.core.windows.net/hps-website/nss/3055/documents/1_agp-sbar.pdf (Accessed 12 June 2020) [Google Scholar]
- 16.Faculty of General Dental Practice . 2020. COVID-19: Updated Guidance and Resources As Lockdown Eases.https://www.fgdp.org.uk/news/covid-19-updated-guidance-and-resources-lockdown-eases (Accessed 18 June 2020) [Google Scholar]
- 17.American Dental Association . 2020. ADA Coronavirus (COVID-19) Center for Dentists.https://success.ada.org/en/practice-management/patients/infectious-diseases-2019-novel-coronavirus?utm_source=adaorg&utm_medium=covid-hubspot-lp&utm_content=cv-virus&utm_campaign=covid-19&_ga=2.258854413.600422100.1594463817-1866440118.1594463817 (Accessed 12 June 2020) [Google Scholar]
- 18.2020. Centers for Disease Control and Prevention, Guidance for Dental Settings. Interim Infection Prevention and Control Guidance for Dental Settings During the COVID-19 Response.https://www.cdc.gov/coronavirus/2019-ncov/hcp/dental-settings.html (Accessed 12 June 2020) [Google Scholar]
- 19.Faculty of General Dental Practice . 2020. Implications of COVID-19 for the Safe Management of General Dental Practice a Practical Guide.https://www.fgdp.org.uk/sites/fgdp.org.uk/files/editors/FGDP%20CGDent%20Implications%20of%20COVID19%20for%20the%20safe%20management%20of%20general%20dental%20practice%2016%20June%202020%20ed1.1.pdf (Accessed 18 June 2020) [Google Scholar]
- 20.Cochrane Oral Health . 2020. Recommendations for the Re-opening of Dental Services: a Rapid Review of International Sources, UK. [Google Scholar]
- 21.Tellier R. Aerosol transmission of influenza A virus: a review of new studies. J. R. Soc. Interface. 2009;6(Suppl 6):S783–90. doi: 10.1098/rsif.2009.0302.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Judson S.D., Munster V.J. Nosocomial transmission of emerging viruses via aerosol-generating medical procedures. Viruses. 2019;11(10) doi: 10.3390/v11100940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst. Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller R.L., Micik R.E., Abel C., Ryge G. Studies on dental aerobiology. II. Microbial splatter discharged from the oral cavity of dental patients. J. Dent. Res. 1971;50(3):621–625. doi: 10.1177/00220345710500031701. [DOI] [PubMed] [Google Scholar]
- 26.Timmerman M.F., Menso L., Steinfort J., van Winkelhoff A.J., van der Weijden G.A. Atmospheric contamination during ultrasonic scaling. J. Clin. Periodontol. 2004;31(6):458–462. doi: 10.1111/j.1600-051X.2004.00511.x. [DOI] [PubMed] [Google Scholar]
- 27.Labaf H., Owlia P., Taherian A., Haghgoo R. Quantitative analysis of changes in bacterial aerosols during endodontic, periodontic and prosthodontic treatments. Afr. J. Microbiol. Res. 2011;5(27):4946–4948. [Google Scholar]
- 28.Yamada H., Ishihama K., Yasuda K., Hasumi-Nakayama Y., Shimoji S., Furusawa K. Aerial dispersal of blood-contaminated aerosols during dental procedures. Quintessence Int. (Berlin, Germany: 1985) 2011;42(5):399–405. [PubMed] [Google Scholar]
- 29.Chuang C.Y., Cheng H.C., Yang S., Fang W., Hung P.C., Chuang S.Y. Investigation of the spreading characteristics of bacterial aerosol contamination during dental scaling treatment. J. Dent. Sci. 2014;9(3):294–296. [Google Scholar]
- 30.Saini R. Efficacy of preprocedural mouth rinse containing chlorine dioxide in reduction of viable bacterial count in dental aerosols during ultrasonic scaling: A double-blind, placebo-controlled clinical trial. Dent. Hypotheses. 2015;6(2):65–71. [Google Scholar]
- 31.Veena H.R., Mahantesha S., Joseph P.A., Patil S.R., Patil S.H. Dissemination of aerosol and splatter during ultrasonic scaling: a pilot study. J. Infect. Public Health. 2015;8(3):260–265. doi: 10.1016/j.jiph.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Jawade R., Bhandari V., Ugale G., Taru S., Khaparde S., Kulkarni A., Ardale M., Marde S. Comparative evaluation of two different ultrasonic liquid coolants on dental aerosols. J. Clin. Diagn. Res. 2016;10(7):ZC53–ZC57. doi: 10.7860/JCDR/2016/20017.8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe A., Tamaki N., Yokota K., Matsuyama M., Kokeguchi S. Use of ATP bioluminescence to survey the spread of aerosol and splatter during dental treatments. J. Hosp. Infect. 2018;99(3):303–305. doi: 10.1016/j.jhin.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Grenier D. Quantitative analysis of bacterial aerosols in two different dental clinic environments. Appl. Environ. Microbiol. 1995;61(8):3165–3168. doi: 10.1128/aem.61.8.3165-3168.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holloman J.L., Mauriello S.M., Pimenta L., Arnold R.R. Comparison of suction device with saliva ejector for aerosol and spatter reduction during ultrasonic scaling. J. Am. Dent. Assoc. 2015;146(1):27–33. doi: 10.1016/j.adaj.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Samaranayake L.P., Reid J., Evans D. The efficacy of rubber dam isolation in reducing atmospheric bacterial contamination. ASDC J. Dent. Child. 1989;56(6):442–444. [PubMed] [Google Scholar]
- 37.Rautemaa R., Nordberg A., Wuolijoki-Saaristoe K., Meurman J.H. Bacterial aerosols in dental practice - a potential hospital infection problem? J. Hosp. Infect. 2006;64(1):76–81. doi: 10.1016/j.jhin.2006.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ireland A.J., Moreno T., Price R. Airborne particles produced during enamel cleanup after removal of orthodontic appliances. Am. J. Orthod. Dentofacial Orthop. 2003;124(6):683–686. doi: 10.1016/s0889-5406(03)00623-1. [DOI] [PubMed] [Google Scholar]
- 39.Day C.J., Price R., Sandy J.R., Ireland A.J. Inhalation of aerosols produced during the removal of fixed orthodontic appliances: a comparison of 4 enamel cleanup methods. Am. J. Orthod. Dentofacial Orthop. 2008;133(1):11–17. doi: 10.1016/j.ajodo.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 40.Dawson M., Soro V., Dymock D., Price R., Griffiths H., Dudding T., Sandy J.R., Ireland A.J. Microbiological assessment of aerosol generated during debond of fixed orthodontic appliances. Am. J. Orthod. Dentofacial Orthop. 2016;150(5):831–838. doi: 10.1016/j.ajodo.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 41.Kritivasan S., Nazia Zareen I., Muralidharan N.P. Assessing the extent of aerosol spread in prosthetic dental lab. Int. J. Sci. Technol. Res. 2019;8(11):3190–3192. [Google Scholar]
- 42.Agostinho A.M., Miyoshi P.R., Gnoatto N., Paranhos Hd.F.O., Figueiredo L.Cd., Salvador S.L. Cross-contamination in the dental laboratory through the polishing procedure of complete dentures. Braz. Dent. J. 2004;15(2):138–143. doi: 10.1590/s0103-64402004000200010. [DOI] [PubMed] [Google Scholar]
- 43.Belting C.M., Haberfelde G.C., Juhl L.K. Spread of organisms from dental air rotor. J. Am. Dent. Assoc. 1964;68:648–651. doi: 10.14219/jada.archive.1964.0145. [DOI] [PubMed] [Google Scholar]
- 44.Micik R.E., Miller R.L., Mazzarella M.A., Ryge G. Studies on dental aerobiology. I. Bacterial aerosols generated during dental procedures. J. Dent. Res. 1969;48(1):49–56. doi: 10.1177/00220345690480012401. [DOI] [PubMed] [Google Scholar]
- 45.Miller R.L. Characteristics of blood-containing aerosols generated by common powered dental instruments. Am. Ind. Hyg. Assoc. J. 1995;56(7):670–676. doi: 10.1080/15428119591016683. [DOI] [PubMed] [Google Scholar]
- 46.Logothetis D.D., Martinez-Welles J.M. Reducing bacterial aerosol contamination with a chlorhexidine gluconate pre-rinse. J. Am. Dent. Assoc. 1995;126(12):1634–1639. doi: 10.14219/jada.archive.1995.0111. [DOI] [PubMed] [Google Scholar]
- 47.Bahl P., Doolan C., de Silva C., Chughtai A.A., Bourouiba L., MacIntyre C.R. Airborne or droplet precautions for health workers treating COVID-19? J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kobza J., Pastuszka J.S., Bragoszewska E. Do exposures of aerosols pose a risk of dental professionals? Occup. Med.-Oxf. 2018;68(7):454–458. doi: 10.1093/occmed/kqy095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toroglu M.S., Haytac M.C., Koksal F. Evaluation of aerosol contamination during debonding procedures. Angle Orthod. 2001;71(4):299–306. doi: 10.1043/0003-3219(2001)071<0299:EOACDD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 50.Toroglu M.S., Bayramoglu Z., Yarkin F., Tuli A. Possibility of blood and hepatitis B contamination through aerosols generated during debonding procedures. Angle Orthod. 2003;73(5):571–578. doi: 10.1043/0003-3219(2003)073<0571:POBAHB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 51.Ishihama K., Iida S., Koizumi H., Wada T., Adachi T., Isomura-Tanaka E., Yamanishi T., Enomoto A., Kogo M. High incidence of blood exposure due to imperceptible contaminated splatters during oral surgery. J. Oral Maxillofac. Surg. 2008;66(4):704–710. doi: 10.1016/j.joms.2007.06.663. [DOI] [PubMed] [Google Scholar]
- 52.Al-Eid R., Ramalingam S., Sundar C., Aldawsari M., Nooh N. Detection of visually imperceptible blood contamination in the oral surgical clinic using forensic luminol blood detection agent. J. Int. Soc. Prev. Community Dent. 2018;8(4):327–332. doi: 10.4103/jispcd.JISPCD_10_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teichert-Filho R., Baldasso C.N., Campos M.M., Gomes M.S. Protective device to reduce aerosol dispersion in dental care in times of COVID-19 pandemic. Int. Endod. J. 2020 doi: 10.1111/iej.13373. [published online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chanpong B., Tang M., Rosenczweig A., Lok P., Tang R. Aerosol-generating procedures and simulated cough in dental anesthesia. Anesth. Prog. 2020 doi: 10.2344/anpr-67-03-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ge Z.-Y., Yang L.-M., Xia J.-J., Fu X.-H., Zhang Y.-Z. Possible aerosol transmission of COVID-19 and special precautions in dentistry. J. Zhejiang Univ. Sci. B. 2020;21(5):361–368. doi: 10.1631/jzus.B2010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ravenel T.D., Kessler R., Comisi J.C., Kelly A., Renne W.G., Teich S.T. Evaluation of the spatter-reduction effectiveness and aerosol containment of eight dry-field isolation techniques. Quintessence Int. (Berlin, Germany: 1985) 2020;51(8):660–670. doi: 10.3290/j.qi.a44919. [DOI] [PubMed] [Google Scholar]
- 57.Zemouri C., De Soet H., Crielaard W., Laheij A. A scoping review on bio-Aerosols in healthcare & the dental environment. PLoS One. 2017;12(5) doi: 10.1371/journal.pone.0178007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.