During the pandemic it has become clear that severe acure respiratory syndrome coronavirus (SARS-CoV-2) causes not just respiratory disease, but can affect multiple organs and tissues. Of note is the involvement of the CNS and PNS, and the fact that this involvement is independent from the severity of the respiratory disease. Acute and subacute neurological complications of SARS-CoV-2 infections are reported in up to 85% of patients, including those with severe COVID-19, but also in otherwise minimally symptomatic or asymptomatic people. As many as 65% of people with COVID-19 present with hyposmia, which is also a common premotor symptom in Parkinson's disease. This symptom, added to the fact that parkinsonism has been reported following COVID-19, has drawn the attention of the medical community to the hypothetical link between SARS-CoV-2 infection and Parkinson's disease.
So far, three cases of parkinsonism have been reported after SARS-CoV-2 infection. Their clinical details are important for evaluating whether or not parkinsonism is causally related with COVID-19. The three patients are relatively young (two men aged 45 and 58 years, and a woman aged 35 years). The men had hypertension and were on angiotensin-converting enzyme (ACE) inhibitors, and the younger man also had asthma, whereas the woman was healthy before the infection. In two cases (a male and the female), the sense of smell was affected. None of the male patients had a monogenic cause or known genetic predisposition for Parkinson's disease, while genetic tests were not done for the female patient. Onset was acute in the three cases (10–32 days after COVID-19 diagnosis); one patient (the 58 year old male) developed akinetic rigid syndrome in the context of a complex neurological presentation compatible with encephalopathy, including myoclonus and opsoclonus, while the other two patients had pure asymmetric akinetic-rigid features, with tremor, and mild respiratory disease. Spontaneous improvement was reported in this patient, with no response to an acute challenge with apomorphine; the female patient responded to short-term levodopa treatment, while the younger male patient had some improvement after treatment with a dopamine agonist and anticholinergics. Functional nigrostriatal neuroimaging was abnormal in all three cases, which implies dopaminergic nigrostriatal impairment, but is not diagnostic of Parkinson's disease.
The uncertainty about the neurological status of these patients pre-infection is a crucial issue regarding the possibility that their cases would reveal the unmasking of underlying preclinical Parkinson's disease. In the reports of two of the cases (the male patients), the authors explicitly state that they had no history of rapid eye movement (REM) sleep behaviour disorder or hyposmia prior to the infection. The acute onset and the association with SARS-CoV-2 infection raise the possibility that these cases might represent a post or para-infectious parkinsonian syndrome, as previously reported after other viral infections. Therefore, the evidence from these three cases is too limited to link the SARS-CoV-2 infection with the development of Parkinson's disease.
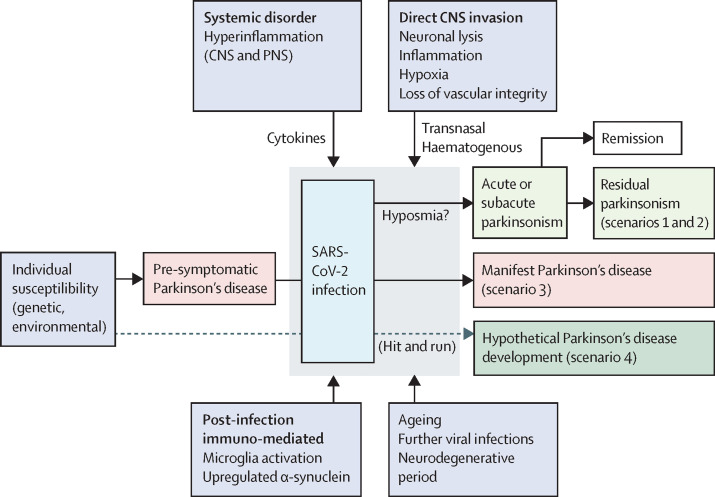
The occurrence of transient or permanent parkinsonism following a viral infection is well known. In these cases, parkinsonism might occur through different mechanisms: 1) structural and functional basal ganglia damage mainly involving the substantia nigra pars compacta and nigrostriatal dopaminergic projection; 2) extensive inflammation or even hypoxic brain injury within the context of an encephalopathy; 3) unmasking of underlying but still non-symptomatic Parkinson's disease; or 4) the hypothetical possibility that a viral infection might trigger a series of processes that result in the development of Parkinson's disease over the long term in individuals with genetic susceptibility. In each of these instances, there are fundamental clinical and anatomopathological differences (figure ).
Figure.
The relationship between SARS-CoV-2 infection and parkinsonism
Acute or subacute parkinsonism can develop as the direct consequence of viral CNS invasion or as a post-infectious inmune-mediated process (scenarios 1 and 2). Pre-symptomatic Parkinson's disease might manifest after a systemic disorder (scenario 3) and, although highly hypothetical, microglia activation and upregulation of α-synuclein triggered by the viral infection could act as the “initial hit” in the multifactorial process that would eventually end in the development of Parkinson's disease (scenario 4). Whether hyposmia is a marker of CNS invasion or of increased risk of parkinsonism is hypothetical.
Thus, patients in whom scenarios 1 and 2 apply will present with neurological manifestations generally incompatible with the diagnostic criteria for Parkinson's disease, such as acute onset, myoclonus, cerebellar or pyramidal signs, and scarce or no response to levodopa treatment. Individuals in whom an infection or other noxious event unravel an underlying Parkinson's disease are commonly seen in clinical practice, and are usually easy to recognise by the acute or sub-acute presentation of their motor features. The intriguing scenario that SARS-CoV-2 could lead to Parkinson's disease in the long term deserves further discussion. This possibility has also been suggested in the past for other viral infections, but is now of particular concern given the high infectivity of SARS-CoV-2 and population ageing.
Following the 1918 flu pandemic, as described by Von Economo, many patients developed parkinsonism acutely or after a delay of months or even years after encephalitic illness, accompanied or preceded by oculogyric crisis, pupillary disturbances, alteration of sleep cycles, psychiatric symptoms, and corticospinal tract signs. However, this association is not without controversy, as several cases had apparently been reported 1 or 2 years prior to the influenza pandemic. Furthermore, serum and CSF tests for 17 different arboviruses in cases of parkinsonism post-encephalitis lethargica did not differ from controls. Case reports in the late 1970s, similar to those initially reported by Von Economo, raised again the hypothesis of a causal relationship. The neuropathological assessment of some of these patients with parkinsonism post-encephalitis showed extensive bilateral loss of neurons in the substantia nigra and locus coeruleus. Lewy bodies were not observed, but glial scars were seen. Slight demyelination of the mid portion of the cerebellar peduncles was detected, and neurofibrillary tangles were observed in the brain stem and striatum in the absence of neuritic plaques. Importantly, these findings are not typical of Parkinson's disease pathology by today's standards. Hence, this classic example of a respiratory virus associated with damage of the substantia nigra triggered a condition clearly different from Parkinson's disease, both clinically and pathologically.
Some strains of influenza, such as H5N1 and H1N1, are neurotropic in mammals, and H1N1 preferentially targets the substantia nigra. H5N1-infected poultry showed abnormal postures and an inability to initiate movement. In mice, H5N1 spreads to the brain along olfactory routes or the trigeminal vagal and sympathetic nerves following intranasal instillation. In two case reports of people infected with H5N1, the authors reported seizures and rapid encephalopathy, followed by coma. In animal models, influenza viruses can also cause long-term behaviour disturbances and altered gene expression by a so-called hit and run mechanism, which suggests that multiple hits (ie, respiratory viral infections) might act as risk factors for Parkinson's disease development, adding to the risk attributed to other factors that can occur through life.
The neuroinvasivity of SARS-CoV-2 has been suspected on the grounds of the detection of viral RNA in the CSF of one patient with COVID-19-associated encephalopathy. Besides, in a neuropathological study of patients who died after COVID-19, SARS-CoV-2 was detected in the brains of 21 (53%) of these patients. However, the failure to detect SARS-CoV-2 in CSF in most patients with COVID-19-related encephalitis, despite evidence of brain inflammation, suggests immune-mediated mechanisms in the absence of direct virus invasion.
A disrupted blood–brain barrier due to the cytokine storm and virus-activated lymphocytes crossing the blood–brain barrier to access the CNS in a so called Trojan horse mechanism have been suggested as possible routes of viral CNS invasion. Translational models also suggest an olfactory route into the CNS, with further transport along axons and neuron-to-neuron propagation towards the brainstem. At the cellular level, ACE2 receptors have proved crucial for SARS-CoV-2 tropism, as they are used by the virus to infect the cell. ACE2 receptors are expressed in neurons, astrocytes, and oligodendrocytes in the substantia nigra and olfactory bulb, among other brain regions, which could explain the cases of hyposmia. However, unlike in Parkinson's disease, hyposmia is reversible in the majority of cases with COVID-19, and whether or not it reflects the ability of the virus to reach the nigrostriatal pathway remains speculative as there is no direct connection between the olfactory bulb and the substantia nigra in primates. Thus, we could perhaps conclude, on the basis of the limited evidence, that SARS-CoV-2 enters the CNS, but no data support a preferential tropism for the substantia nigra.
The causal association of SARS-CoV-2 infection with the development of Parkinson's disease is therefore not supported by robust evidence yet. Although the potential neurological sequelae of this novel coronavirus should not be underestimated, we are concerned about unjustified claims anticipating a future high incidence of Parkinson's disease, secondary to the SARS-CoV-2 pandemic. A coordinated international effort to investigate viral effects is essential, and should be based on well-designed prospective studies. Rather than speculation, the obtention of robust data is warranted.