Abstract
The Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-2) global pandemic significantly impacted CF clinical research within the Cystic Fibrosis Foundation Therapeutics Development Network (CFF TDN). A Research Electronic Data Capture (REDCap) survey was developed and sent to network sites to monitor and understand the impact on research teams, ongoing and anticipated clinical research, and specific clinical and research procedures. Key findings indicated an early impact on participant enrollment, research team stability, and procedures such as spirometry and sputum induction. These trends steadily improved over the months as research activities began to recover across the TDN. While SARS-CoV-2 created a significant challenge it also highlights new opportunities to expand CF research with greater focus on data collection outside of research centers and increased access for remote participation.
Keywords: Cystic fibrosis, Clinical research, Coronavirus, SARS-CoV-2
The Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-2) global pandemic significantly impacted the conduct of CF clinical research worldwide [1], [2], [3], in addition to disrupting many other aspects of daily life [4]. Underlying pulmonary diseases like cystic fibrosis (CF) are seen as risk factors for severe illness from SARS-CoV-2, resulting in heightened awareness of a need to minimize exposure to this virus [5]. Similarly, most CF clinical trials involve aerosol generating procedures (e.g. spirometry), which have been particularly scrutinized due to potential risk of aerosolization and transmission to research staff [6] and other subjects.
In an effort to accurately monitor the evolving and transformative impact of SARS-CoV-2 on the Cystic Fibrosis Foundation Therapeutics Development Network (CFF TDN), a US-based CF clinical trial network comprised of 91 clinical research sites, the CFF TDN Coordinating Center (TDNCC) launched a series of surveys between April 2020 and July 2020 (n = 6 surveys). The survey was drafted by CF Foundation and TDNCC Leadership with input from the TDN Research Coordinator Advisory Group. The survey was tested in Research Electronic Data Capture (REDCap) by senior Research Coordinators (RCs) and their input was incorporated. The survey, administered using REDCap, was sent bi-weekly to the Primary Research Coordinator contact at all TDN sites to understand the local impact on research team stability, ongoing and anticipated clinical research, and specific clinical and research procedures. Sites were asked to respond within 3 days and a reminder was sent the morning of the deadline.
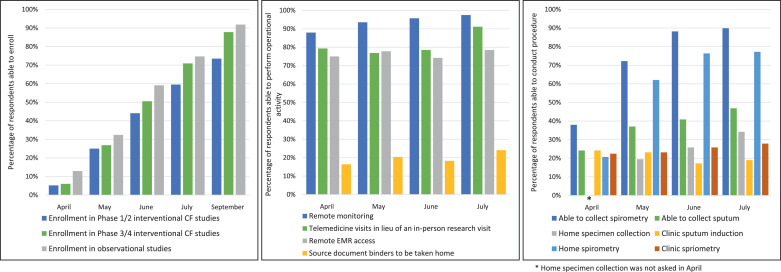
Key findings are presented in Fig. 1 and summarized below. Results are presented in percentages as the number of responses to each survey varied over the months (April n = 116, 100% response rate (91/91 sites), May n = 108, 99% response rate (90/91 sites), June n = 108, 98% response rate (89/91 sites), July n = 102, 95% response rate (86/91 sites)). Sites were again asked about enrollment capabilities in September (n = 98, 90% response rate (82/91 sites)). Some sites reported separate responses from their pediatric and adult institutions.
Fig. 1.
SARS-CoV-2 Impact on CF Therapeutic Development Network.
Left: Significant impact on participant enrollment reported in the initial months with steady improvement seen through September. Center: Operational response to SARS-CoV-2 to minimize risk of missing participant data and reduced safety monitoring. Right: Ability of sites to perform spirometry and sputum collection either in clinic or at home. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
1. Research team stability
Survey results indicate that the work of RCs and Regulatory Coordinators (RegCs) was heavily affected by SARS-CoV-2. Roughly 75% were required to work-from-home in the months of April and May and nearly 20% were laid off or were working reduced hours because of study conduct interruption in these first months. These percentages improved in the following months, with only 45% RCs and RegCs restricted to home by July and 8% laid off or working reduced hours, as clinical research activities began to recover across the Network.
2. Ongoing and upcoming clinical research
As expected, enrollment was significantly affected in the early months of SARS-CoV-2 with only 15% of sites able to enroll in observational studies, 5% in early phase interventional trials (i.e. phase 1 or 2), and 6% in later phase 3/4 studies. This steadily improved over the summer months with enrollment capability in observational studies increasing to 75%, phase 1/2 trials to 60%, and phase 3/4 to 70%. Despite the significant impacts, institutions and site teams adapted by allowing remote monitoring (97% of sites by July), remote electronic medical record (EMR) access (78%), telemedicine in lieu of in-person visits (91%), and source documents to be taken home (24%) in accordance with data protection policy. This flexibility in standard practices assuredly reduced missing data for participants actively enrolled in studies while likely improving safety monitoring.
3. Clinical and research procedures
Potentially aerosol-generating procedures like spirometry and sputum induction were the most restricted by limitations to research conduct at study sites. Only 27% of sites were able to conduct spirometry in July 2020. However, this paralleled a dramatic increase in the number of sites able to conduct home spirometry for clinical care (20% in April and 75% in July). Some study sponsors provided home spirometers to participating sites and the CF Foundation purchased and distributed several thousand devices to CFF Accredited Care Centers in support of both clinical care and ongoing academic research trials.
Sites also faced restrictions in performing sputum induction with only 19% of sites reporting the ability to perform the procedure by July. Similar to the nimble pivot to home spirometry, sites implemented collection of sputum specimens at home with shipment to local or centralized laboratories. Home sputum collection was available at 34% sites in July, an increase from just 14% in April.
4. Next steps
To continue monitoring the dynamic impact of SARS-CoV-2 on the TDN, a streamlined Smartsheet form was implemented in August 2020 to continue to collect a limited number of key data, which will allow us to monitor both the heterogeneity and commonality of limitations to research conduct over the coming months. As of September, enrollment was resuming at most sites with 90% able to enroll in observational and phase 3/4 studies and 75% in phase 1/2 studies. Additionally, 90% of sites were able to perform in-person spirometry and 50% could perform sputum induction. Combined with the adaptations described above, this indicates that research capacity and activity were returning toward a modified pre-pandemic state, although this remains heterogeneous depending on geographic and institutional considerations. As we transition into the winter months marked with a significant rise of SARS-CoV-2 in many parts of the country, it remains imperative that we continue collecting survey data. This effort paired with others across the globe [7] with allow us to monitor and report out on the impact of SARS-CoV-2 on clinical trials networks as well as the health and well-being of people with CF.
The longitudinal survey results reported here proved essential in the TDN Coordinating Center's ability to monitor and support sites over the first several months of the SARS-CoV-2 pandemic. Additionally, a subset of results was shared with industry sponsors to better inform their decisions for ongoing and upcoming trials. Overall, while SARS-CoV-2 created a significant barrier to clinical trial activity in the TDN, it also fostered resilience and innovation. Study sites and sponsors rose to the challenge to keep research going in new and creative ways and to ensure that people with CF enrolled in clinical trials remained safe with minimal interruptions to study drug access or key safety monitoring.
The pandemic also highlights new opportunities to expand CF research with greater focus on data collection outside of research centers and increased access for remote participation. Technologies (e.g. home spirometry, electronic patient-reported outcomes, ePROs) and logistical operations (e.g. temperature-controlled shipping, remote consenting, virtual study visits) that have been appreciated but little used are now commonplace across many TDN sites. In the near term there is a need to better understand and optimize such procedures. Focus areas include a robust evaluation of the accuracy and precision of home spirometry, feasibility and quality of home sputum collection, and how best to decentralize collection of laboratory studies. Work like this will better determine when and how in-person research visits can be circumvented and where these tools can expand or improve clinical trials even when in-person visits or procedures are not as challenging. The TDN looks to partner with invested parties around the world to use what is learned from this experience in support of better health outcomes for those with CF.
5. Author contributions
All authors drafted the manuscript or revised it critically for important intellectual content. All authors approved the final version of the manuscript.
6. Funding source
This work was supported by the Cystic Fibrosis Foundation (CFF). NMH, CHG, and DPN were also supported by National Institutes of Health (NIH) grants P30 DK 089507. CHG is also supported by the following federal awards: UM1HL119073, UL1TR000423, R01FD003704 and R01FD006848.
Declaration of Competing Interest
NMH has received grant funding from the Cystic Fibrosis Foundation (CFF) and National Institutes of Health (NIH). CHG reports grant funding from CFF, European Commission, National Heart, Lung, and Blood Institute, National Institute of Diabetes and Digestive and Kidney Diseases, National Center for Research Resources, Gilead Sciences, Novartis, NIH, Food and Drug Administration, Boehringer Ingelheim, Vertex Pharmaceuticals. None of the work presented in this short communication was influenced by the funding sources reported by CHG. KP, GZRB, JMVD, PB, DR., JPC, AH, and DPN have nothing to disclose related to this work.
Acknowledgements
We would like to thank Jing Xie, Statistical Clinical Data Management Programmer, at the TDN Coordinating Center for her expertise in programming the survey in REDCap. We also want to acknowledge the hard work and contributions of the Research Coordinators who filled out the bi-weekly surveys.
References
- 1.Upadhaya S., Yu J.X., Oliva C., Hooton M., Hodge J., Hubbard-Lucey V.M. Impact of COVID-19 on oncology clinical trials. Nat Rev Drug Discov. 2020;19:376–377. doi: 10.1038/d41573-020-00093-1. [DOI] [PubMed] [Google Scholar]
- 2.Desai S., Manjaly P., Lee K.J., Li S.J., Manjaly C., Mostaghimi A. The impact of COVID-19 on dermatology clinical trials. J Invest Dermatol. 2020 doi: 10.1016/j.jid.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Koningsbruggen-Rietschel S., Dunlevy F., Bulteel V., Downey D.G., Dupont L. SARS-CoV-2 disrupts clinical research: the role of a rare disease-specific trial network. Eur Respir J. 2020:56. doi: 10.1183/13993003.02114-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosgriff R., Ahern S., Bell S.C., Brownlee K., Burgel P.R., Byrnes C., Corvol H., Cheng S.Y., Elbert A., Faro A., Goss C.H., Gulmans V., Marshall B.C., McKone E., Middleton P.G., Ruseckaite R., Stephenson A.L., Carr S.B. A multinational report to characterise SARS-CoV-2 infection in people with cystic fibrosis. J Cyst Fibros. 2020;19:355–358. doi: 10.1016/j.jcf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prevention CfDCa. People with Certain Medical Conditions . 2020. [Google Scholar]
- 6.Pasnick S., Carlos W.G., Dela Cruz C.S., Gross J.E., Garrison G., Jamil S. SARS-CoV-2 Transmission and the Risk of Aerosol Generating Procedures. 2020 Jun 30. Available from: https://www.thoracic.org/patients/patient-resources/resources/aerosol-generating-procedures-and-risk-of-covid-19-transmission.pdf. [DOI] [PubMed]
- 7.McClenaghan E., Cosgriff R., Brownlee K., Ahern S., Burgel P.R., Byrnes C.A., Colombo C., Corvol H., Cheng S.Y., Daneau G., Elbert A., Faro A., Goss C.H., Gulmans V., Gutierrez H., de Monestrol I., Jung A., Justus L.N., Kashirskaya N., Marshall B.C., McKone E., Middleton P.G., Mondejar-Lopez P., Pastor-Vivero M.D., Padoan R., Rizvi S., Ruseckaite R., Salvatore M., Stephenson A., Filho L., Melo J., Zampoli M., Carr S.B. The global impact of SARS-CoV-2 in 181 people with cystic fibrosis. J Cyst Fibros. 2020 doi: 10.1016/j.jcf.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]