Abstract
The COVID-19 pandemic placed public health measures against infectious diseases at the core of global health challenges, especially in cities where more than half of the global population lives. SARS-CoV-2 is an exposure agent recently added to the network of exposures that comprise the human exposome, i.e. the totality of all environmental exposures throughout one’s lifetime. At the same time, the application of measures to tackle SARS-CoV-2 transmission leads to changes in the exposome components and in characteristics of urban environments that define the urban exposome, a complementary concept to the human exposome, which focuses on monitoring urban health. This work highlights the use of a comprehensive systems-based approach of the exposome for better capturing the population-wide and individual-level variability in SARS-CoV-2 spread and its associated urban and individual exposures towards improved guidance and response. Population characteristics, the built environment and spatiotemporal features of city infrastructure, as well as individual characteristics/parameters, socioeconomic status, occupation and biological susceptibility need to be simultaneously considered when deploying non-pharmacological public health measures. Integrating individual and population characteristics, as well as urban-specific parameters is the prerequisite in urban exposome studies. Applications of the exposome approach in cities/towns could facilitate assessment of health disparities and better identification of vulnerable populations, as framed by multiple environmental, urban design and planning co-exposures. Exposome-based applications in epidemics control and response include the implementation of exposomic tools that have been quite mature in non-communicable disease research, ranging from biomonitoring and surveillance to sensors and modeling. Therefore, the exposome can be a novel tool in risk assessment and management during epidemics and other major public health events. This is a unique opportunity for the research community to exploit the exposome concept and its tools in upgrading and further developing site-specific public health measures in cities.
Keywords: Exposome, COVID-19, Interventions, Systems-based approach
1. Introduction
The COVID-19 pandemic is an unprecedented global health emergency that clearly demonstrates the importance of timely and effective interventions in tackling public and global health challenges. Improving health in cities is inevitably one of the main targets of the 21st century’s public health policy agenda as they currently host more than half of the global population and generate over 80% of the global gross domestic product (Urban Development Overview, n.d., Zhang, 2011). Large cities, such as New York, Milan, Madrid, and Paris have been significantly affected by the COVID-19 pandemic (Bouffanais and Lim, 2020, CDC COVID-19 Response Team, 2020, Riccardo et al., 2020, Salje et al., 2020;), suggesting that public health measures tailored to city needs must to be considered. In the first few months of the pandemic, a suite of public health measures notably “blanket”/population-wide non-pharmacological measures were deployed to contain and mitigate the spread of the severe respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes coronavirus disease 2019 (COVID-19), including wide governmental lockdowns (Chu et al., 2020). This pandemic response sparked numerous discussions about the timing, the extent and proportionality of public health measures, as well as ethical debates, while guidelines and advice were (and still are) continuously adjusted, as further evidence becomes available.
The deployment of public health interventions, especially in urban settings, is challenging as cities include increasingly large, multidimensional and complex networks and systems of human interactions with distinct spatial, temporal and population characteristics. The pandemic offers a unique opportunity for cities and urban settlements to recognize that preparedness and response to epidemics should be informed by the specific complexities and challenges that urban settings face, while accounting for the pandemic unknowns/uncertainties (WHO, 2020).
The characteristics of urban structural features, such as location of commercial shopping zones, academic facilities, residential areas, industrial zones, green/blue space, (public) transportation, etc. and the within-city socioeconomic gradient have properties that contribute and shape the human exposome, which has been defined as the totality of exposures encountered throughout one’s lifetime (Wild, 2005). The characteristics of the urban environment also shape the urban exposome, a complementary concept to the human exposome, which views the city as the unit of measurement (Andrianou and Makris, 2018). The urban exposome has been defined as the qualitative and quantitative assessment of environment and health indicators that describe the framing and evolution of urban health and its interactions with urban infrastructure, climate, and small area (neighborhood) features (Andrianou and Makris, 2018). During the pandemic, another parameter has been added to the network of environmental components that comprise the human exposome and that is the pathogen itself (SARS-CoV-2), increasing both individual and population disease risk in the urban setting. At the same time, non-pharmacological interventions (NPI), such as lockdowns have been widely implemented to tackle SARS-CoV-2 transmission. Such NPI may both indirectly or directly impact and modify the exposome profile of individuals, life in the city and population exposures. Therefore, there is a combined effect of the pandemic on individual, population and urban exposomes.
The description and assessment of the impact of the pandemic, and the (in)direct impacts of public health measures taken in response to the pandemic in each city, could likely provide the basis for comprehensive and site-specific disease prevention and response approaches. These site-specific approaches are needed to apply measures in a proportionate way while allowing for quick identification of COVID-19 cases and for limiting the risk of transmission. The choice of the most appropriate response approach is critical, since parameters that contribute to increases in cases or new outbreaks in different geographic areas may vary, exhibiting differential impact in areas of the same urban center. The complexities of deploying public health measures in urban settings during the pandemic highlights and reinforces the need to reach across borders of scientific disciplines that are traditionally considered to be separate in research efforts. Cross- and trans-disciplinary approaches allow the scientific community to integrate knowledge, methods, and tools towards evaluating the complexity of the urban environment and the societies from different disciplinary perspectives, thus allowing public health threats to be addressed more comprehensively.
Thus, the objective of this work was to demonstrate how the exposome concept and the urban exposome study framework (Wild, 2012, 2005; Andrianou and Makris, 2018) could act together under an interdisciplinary methodological umbrella for the development and testing of public health measures that may concomitantly lead to more effective NPI for infectious diseases management in urban settings.
2. Discussion
2.1. Human exposome and urban exposome characteristics in epidemics
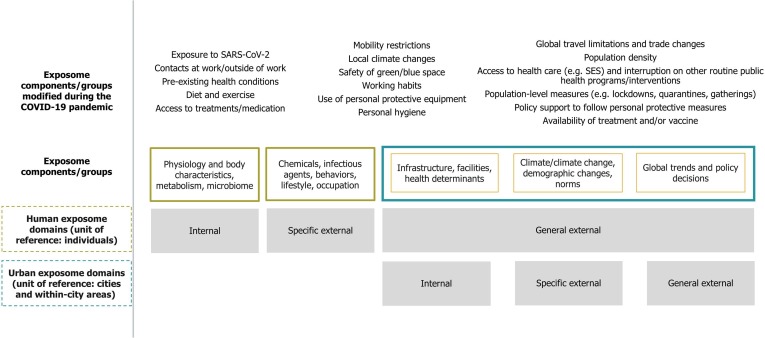
The human exposome aims to measure all exposures throughout lifetime by systematically assessing environmental parameters (including lifestyle, behavioral, biological, etc.) that are classified into the external (general and specific) and internal domains (i.e. categories of exposures) (Wild, 2012, Wild, 2005). The broad range of parameters that comprise the exposome have the individual as unit of reference and include infectious diseases (initially placed in the specific external exposome domain) (Wild, 2012). In parallel with humans, urban exposures influence and are influenced by various human exposures and they can be categorized into external (general and specific) and internal exposome domains with reference to the city and its neighborhoods. In the urban exposome, infectious diseases transmission influences and is influenced by all aspects of city life and they are a parameter that is internal to the city, while policies or public health measures/interventions can be internal or external to the city (Fig. 1 ).
Fig. 1.
Schematic of urban exposome and human exposome domains and examples of relevant exposome components and their groups that were modified due to COVID-19 pandemic.
To map the extent to which exposome studies have addressed issues of infectious diseases, we conducted a scoping review of peer-reviewed literature published in PubMed. We searched for records with in-text mentions of the terms “exposome” and “infectious diseases” or “communicable”, or combinations of different keywords related to specific infectious diseases (e.g. tuberculosis, measles, antimicrobial, food-, water- or vector-borne) published until September 13, 2020. The majority (40 out of 61) of studies were reviews, commentaries and editorials. Among them, only 18 were original research studies (either observational or experimental) and three of them were protocols or descriptions of cohorts discussing infectious diseases in the scope of addressing other objectives. Although this was not a systematic review of the literature on the topic, it illustrated the level of attention infectious diseases have received among exposome studies. Notwithstanding, there were two studies that included measurements of pathogens as exposures in personal exposure monitoring schemes (Cissé et al., 2020, Jiang et al., 2018). Moreover, infectious diseases have been described as components of the mixture of exposures in association with physiological aspects of infection to SARS-CoV-2 in a commentary and a review article (Land, 2020, Naughton et al., 2020). So far, the exposome concept has predominantly found use in exposure science and environmental health. In this context, the exposome and its urban exposome “extension” provide us with a theoretical framework, where infectious diseases can be integrated within a broader urban health and environmental health research niche.
The transmission of SARS-CoV-2, the progress and outcome of COVID-19 for each individual depends on the virus or infection characteristics (e.g. viral load), existing co-morbidities and the multitude of exposures in various settings (e.g. at school, home, work) that belong to the specific external and internal human exposome domains (Jang et al., 2020, Richardson et al., 2020, Wild, 2012). The public health measures that were taken to restrict mobility and the number and duration of contacts at home, school and work or in other social events towards decreasing virus transmission modified also a suite of other individual and urban exposures (general and specific external exposome domains). For example, restrictions in public transportation and/or travel led to less people commuting, thus affecting their exposure to outdoor air pollution. In addition, closure of workplaces (e.g. construction) led to workers being less exposed to potentially hazardous substances typically experienced in the industry. Mandatory lifestyle changes, such as having to work or study at home coupled with fewer on average contacts might have also affected mental health outcomes, while staying more at home (increasing exposures to indoor air), or using more cleaning/disinfection products might have led to increased chemical exposures e.g. to disinfection by-products (Kim et al., 2020, Li et al., 2020). Upon school closures, education for many moved to online platforms, thereby adversely impacting learning opportunities for students with limited access to such technologies. At the same time, the routine implementation of public health activities, such as vector control or immunization programs might have faced interruptions, leading to possible increase in vector-borne diseases or decreased vaccination uptake, respectively. In cities/towns, most, if not all facilities, infrastructures, transportation systems and networks had to adapt to the new reality of the pandemic. Together, the above examples highlight that the introduction of SARS-CoV-2 and response measures to counteract viral transmission have invoked a complex feedback loop in the system of exposures that comprise the exposome framework (Fig. 1).
The experience of the COVID-19 pandemic is an opportunity to describe and assess the impact of an emerging infectious disease on the exposome of individual urban dwellers and on the urban exposome of the place these individuals live, work and study and vice -versa (how the exposome might influence infectious diseases spread and control). Being exposed to any pathogen is only one step in a sequence of events that could lead to infection and ill health. However, the risk of exposure to the pathogen, the risks of ultimately acquiring the infection and the severity of the infection are a function of multiple parameters that could be grouped together, based on the three exposome domains, into the general external domain, specific external domain, and internal domain (Fig. 1).
The parameters of the human exposome also affect the characteristics of within-urban environments, maintaining an overarching role in shaping urban health and influence COVID-19 risk differentiation and, in general, the probable spread for any infectious disease. A study in New York city already showed that hospitalization and death rates associated with COVID-19 were disproportionally higher in certain boroughs compared to other areas of the city (Wadhera et al., 2020). In the same city, the number of residential units in each building, the mean assessed price of each unit and the neighborhood median household income were inversely related with the probability of infection with SARS-CoV-2, while the neighborhood unemployment rate, the neighborhood household membership and crowding rate all positively increased the probability of infection among pregnant women (Emeruwa et al., 2020). In another study using data from 50 cities around the globe, results showed that transmission in urban areas was restricted in specific latitude, temperature and humidity windows, suggesting that COVID-19 transmission might be partially driven by environmental determinants (Sajadi et al., 2020). Given the accumulating evidence from the COVID-19 pandemic, it becomes evident once more that it is important to simultaneously integrate the effects of multiple risk factors on public health measures and NPI strategies applied within the urban setting.
Following the urban exposome framework, inherent variability in the exposome components at the individual, neighborhood, or district levels ought to be comprehensively characterized at various time points (prior to, during and after a pandemic), if we are to improve our understanding of disease transmission dynamics and to better identify high-risk groups for infection (Table 1 ). The variability in individual and population risk of infection (and its severity) highlights the importance of considering methodological frameworks and approaches that go beyond the reductionist, one-size-fits-all approaches. The application of exposome-based approaches requires the integration of data from various sources and through different data flows. For example, already established ongoing routine surveillance or environmental tracking/monitoring systems could provide secondary data that can be combined with primary data from ad hoc studies. It should be emphasized here that proper harmonization of primary and secondary data sources should be adequately practiced when used in answering specific research questions or used for hypothesis generation. Comprehensive frameworks such as that of the exposome offer alternatives for better managing public health crises of the future. At the individual level, the new knowledge on COVID-19 reinforces the impact of co-morbidities in disproportionally increasing risk of infection (Grasselli et al., 2020, Richardson et al., 2020). At the population level, both susceptibility and broader co-existence of environmental exposures e.g. air pollution or different socioeconomic background could exacerbate disease risks and access to healthcare for certain vulnerable groups and confound, improve or affect the effectiveness of control options due to urban systems complexity (Azar et al., 2020, Khoury et al., 1989, Wu et al., 2020). For example, individuals in unique windows of susceptibility to health hazards, such as pregnancy, childhood, those with pre-existing illness, or elderly, usually present with higher disease risk due to non-genetic exogenous agents (chemicals, drugs, stress, etc.), which may further aggravate immune function or induce immunosuppression. This may render such individuals even more susceptible to additional downstream end points of disease, such as, lower routine vaccine antibodies counts following vaccination, or hormonal deficits, or occurrence of opportunistic infections (Grandjean et al., 2012, Woodruff et al., 2008). The exposome approach could find use in monitoring the phenotypic variance of the urban population characteristics before, during, and after epidemics. This would consider the numerous individual exposome components (e.g. unhealthy eating, smoking habits, alcohol consumption, occupation, socioeconomic status, disinfectant use, anxiety, other personal environmental exposures, etc.) and their potential clustering into focused exposure groups (e.g., climate, natural and built environment, air pollutants, diet, infectious agents, lifestyle, behaviors) (Haddad et al., 2019).
Table 1.
Examples of public health response measures and non – pharmacological interventions used to contain and mitigate COVID-19, using the exposome concept, its domains and related tools. Their field deployment could be part of health prevention and promotion efforts, as well. Their application could take place either at baseline, during, and/or after a pandemic crisis, or periodically.
| Enhanced contact tracing | Quarantine and isolation | Use of personal protective equipment and personal hygiene | Closure of facilities (e.g. schools, universities, green/blue space) | Physical distancing and confinement (lockdowns) | ||
|---|---|---|---|---|---|---|
| Exposome domainsinvolved (i.e. internal, specific and general external) | Urban exposome | Internal: e.g. intra- and inter-city disease clusters | Internal and specific external: e.g. facilities for quarantine and isolation and services for those in quarantine or isolation | Internal and external: e.g. intra-urban variable availability of equipment, procurement and budget availability at national or global level | Internal: e.g. impact on learning opportunities, maintenance of closed facilities, city income due to decreased use of facilities | Internal, general and specific external domains: e.g. intra-urban impacts due to infrastructure/facilities/services capacity, mobility and availability of goods |
| Human exposome | Specific external: e.g. individuals affected depending on their habits/lifestyle/contacts | Internal: e.g. lifestyle and habits modified, as well as routine exposures | Internal and specific external: e.g. use of equipment might lead to differential exposure to infectious diseases or chemicals, as well as change in behaviors/habits | Internal and specific external: e.g. limiting access to facilities leads to increased time indoors and decreased time outdoors | Internal, and general externalor specific external: e.g. adhering to physical distancing rules might add on mental health burden, if services become unavailable, personal and group plans to be adjusted | |
| Intervention outcomes | Allow timely intervention in case of infection | Reduce risk of transmission | Reduce individual risk of infection and prevent transmission | Reduce risk of transmission and protect vulnerable groups (i.e. children) and those coming to contact with them | Reduce risk of transmission/infection | |
| Resolution (“unit”) of analysis | Individuals | Individuals/groups | Individuals and groups based on occupation (e.g. essential workers) | Individual, small area (e.g. neighborhood), group (e.g. specific age groups) | Individual, small area, city | |
| Study Designs | Surveys, network analysis | Surveys, trials, qualitative studies | Trials, cohorts/cross-sectional studies, surveys, qualitative studies | Trials, surveys, qualitative studies | ||
| Primary sample/data collection | Questionnaires, geocoded data travel/contacts history | Questionnaires, interviews | Questionnaire, policy analysis | |||
| Secondary data collection | Routine contact tracing and surveillance | Surveillance, geo-tracking data from devices/software | Procurement/orders/imports/manufacturing of equipment, records of entities, distribution of consumables (e.g. hospitals, schools) | Surveillance, other routinely collected information about use of facilities (e.g. school/university buildings) | Routine surveillance | |
| Tools (assigned to public health measures/intervention) | E-data collection, interviews or mixed methods data collection, sensors, biomonitoring, molecular biomarkers of exposure and effect, advanced biostatistical models | |||||
| Crowdsourcing, community/citizen science and social media | ||||||
| Open governmental data and infrastructure databases and/or policy documents | ||||||
2.2. Application of exposome tools and methods during epidemics
A suite of exposomic tools like biomonitoring, remote sensing, personal sensors, digital health, targeted/untargeted –omics platforms, high-dimensional-based statistical learning techniques, among others may prove useful in disentangling variation attributed to infectious disease severity and/or spread, while accounting for the urban environment and its characteristics in a comprehensive fashion. These tools have been quite frequently used in environmental health studies (Vermeulen et al., 2020), but little use of them has been practiced in the field of infectious disease epidemiology in relation to interactions with other urban structural, environmental and exposome variables. Exposomic tools can also be integrated in infectious diseases surveillance and monitoring, as well as in public health response to epidemics (Table 1). A few examples from the above-mentioned exposomic tools have already been used in the COVID-19 crisis, but rather in a fragmented mode, such as, digital contact tracing devices/wearables for tracking people, wastewater epidemiology for virus monitoring, select biomarkers of effect (cytokines, immunoglobulins, etc.), integration of various data sources to estimate indicators of vulnerability (Bringing Greater Precision to the COVID-19 Response, 2020, Coperchini et al., 2020, Hart and Halden, 2020, Servick et al., 2020).
Up to date methodological assessments of population susceptibility to both chemical or biological agents have been largely focused on characterizing the genetic variation, for example in genome-wide association studies and using tools such as off-the-shelf chip technologies, or single-molecule real-time sequencing technologies. The variance of external exposome components (e.g. air or water quality, green space availability) is also usually assessed independently while routinely collected data (e.g. from registries or surveillance systems) or data from environmental monitoring (e.g. for air and water quality) add to the data sources used in population health studies. The above methods and data sources shall be concomitantly employed in an urban health monitoring study, provided there is proper stratification to include small area features of the city, allowing for scaling to larger administrative city units. Different study designs e.g. (multi) cross-sectional surveys or (nested in) cohorts or experimental trials, with qualitative and quantitative methodologies could be considered, integrating data on multi-level city stressors (environmental, behavioral/lifestyle, climatic or other, distal or proximal parameters) with data from secondary data sources (e.g. routine surveillance, crowdsourcing, citizen science, open governmental data, infrastructure databases) on infectious disease risk or determinants of disease incidence and control (Table 1). Exposome-based urban studies could be articulated to exploit infectious diseases surveillance data, while benefiting from the comprehensive spatiotemporal assessment of pathogen/microbial, environmental (e.g. chemical), and lifestyle/behavioral exposures, using novel exposomic tools at the small area (neighborhood) level. Therefore, the application of the exposome approach based on the integration of tools and methods, can address both the probability of exposures and their impact based on fragility and vulnerability of populations in urban settings (Operational tool on rapid risk assessment methodology - ECDC 2019,” 2019). Then, operationally, the exposome could aid in risk assessment, preparedness and response to events that have a public health impact.
The prospective benefits of applying the exposome concept to contain or mitigate infectious diseases in urban settings are expected to be high. Most of the tools of the human exposome, as depicted in Table 1, have been extensively used in research, e.g. in chronic diseases or exposure assessment/exposome studies. Tools for proper classification of continuous measurement of exposures (e.g. using wearables) in indoor or outdoor environments, location activity trackers, modeling of environmental exposures and population dynamics, and the use of early biomarkers of response to infection and non-invasive biomarkers of exposure (skin or urine or exhaled breath condensates) are among the tools used in exposome research that can be considered mature (with extensive application in different contexts). As such, these tools can be used in the study of infectious diseases under the exposome umbrella not only in urban settings, but also in rural settings and, in general, in occasions that call for a comprehensive approach.
Some of the benefits of the aforementioned applications of the exposome or the use of “exposome tools” to address public health challenges related to infectious diseases can be summarized in the following points:
-
(i)
An exposomic approach that integrates multiple data sources in a concise and timely manner will allow for the better understanding of the variability in exposures related to each city’s demographic, infrastructure, climate, built and natural environment characteristics, as these relate to disease spread patterns.
-
(ii)
Monitoring both infectious disease dynamics and the burden of chronic diseases in smaller areas of urban settings will provide information of greater granularity and allow for hypotheses generation towards the identification of urban networks or clusters of determinants and indicators of higher disease risk and/or higher vulnerability.
-
(iii)
Research on subsets of exposome variables with larger impact on public health will be prioritized and then aid the development of site-specific public health measures and their evaluation when they are deployed either in terms of a response to an epidemic or in tackling underlying chronic disease burden (either communicable or non-communicable diseases).
-
(iv)
Research findings of the application of wider exposomic approaches that integrate the study of infectious diseases dynamics could feed into current guidance measures or model trajectories and risk assessment efforts, further refining or informing preparedness and response to epidemics.
This list of benefits may not be exhaustive, but it provides the basis for a broader scientific dialogue and exchange of information and tools between disciplines. The ample availability of tools and methods and the benefit of integrated exposome approaches come along with a great responsibility for researchers and stakeholders as they are in charge of identifying opportunities, performing operational and basic research, communicating limitations and uncertainty and translating results to public health practices. It might not be feasible to deploy all available technological and methodological innovations due to data availability or the lack of technical capacity in different settings. When tools and methods are not available to build capacity or to develop the necessary tools and foster innovation for new methods, prioritization and hierarchy of needs should be warranted depending on the local/regional needs. Using a case by case scenario, the cost and logistics of organizing and coordinating such efforts need to be balanced against anticipated benefits. Additionally, all new technologies that seem promising in terms of data collection efficiency, e.g. (geo)tracking technologies and personal identification systems or digital contact tracing could bring forward sensitive issues with respect to privacy, data protection and ethical risks that must not be neglected. A cost-benefit analysis was out of scope for this article, however, it is obvious that when the wider use of such exposomic tools and technologies is practiced, then their cost is expected to decrease. Lastly, communication and interpretation of data and results with various stakeholders needs to be done in a timely and trustful manner that fosters further collaborations and efficient implementation of outputs in practice.
3. Conclusion and recommendations
The dynamically evolving and perplexed urban environment of the 21st century will be the key setting dealing with the COVID-19 pandemic repercussions, as well as likely/possible future epidemics. The unprecedented crisis of the COVID-19 pandemic brought up changes in the way we live our lives, the way we work/study, and the way we socialize, thus, impacting on the human exposome and the resilience of cities to manage epidemics of high magnitude. New epidemic waves of COVID-19 are anticipated along with the emergence of other new infectious diseases that threaten global health and security, particularly in urban settings.
Public health measures and response interventions, such as enhanced contact tracing, personal protective equipment usage, quarantine, and lockdowns will most likely continue to be deployed but perhaps at different scales, depending on the epidemiology of COVID-19, available resources, and technical capacity. Given the experiences acquired so far and the capacity built while responding to COVID-19, large scale, population-wide measures may not be practical or necessary, while more site-specific approaches will be considered based on the so far generated knowledge. The COVID-19 pandemic emphasized the need to better understand the risk variation and the cost-efficacy of measures and interventions against the disease spread that might present with area-stratified or socioeconomic status-stratified population risk of infection within urban settings. In order for the interventions to become more effective and efficient in protecting susceptible/high risk groups (e.g., elderly), all exposome components that shape the urban environment and define public health response within a city need to be integrated to offer science-based guidance for risk assessment and site-specific decision making and to provide tailored responses that can quickly become operational. Therefore, the application of a comprehensive framework, such as that of the exposome, could lead to better tailoring and evaluating the effectiveness of public health measures. This may be achieved via the integrated description and evaluation of a suite of exposome components in various areas of a city where health disparities and other risk factors (e.g. co-exposure to chemicals, pollution etc.) often prevail.
Anticipated changes in public health systems and in the organization of disease and health surveillance systems at the urban community level will require the design and (re)testing of existing or novel public health measures. Such efforts will inevitably be proven useful to accompany already established prevention and control measures for infectious diseases. To aid response in urban settings and understand the dynamics that shape health in cities, the environmental features of small areas (e.g. at neighborhood level) need to be fully characterized using the urban exposome framework. The interactions of risk factors of the urban community with the local determinants of infectious disease spread and containment should be better delineated to establish improved response programs for high-risk and vulnerable subpopulation groups. The exposome framework is the featured concept that could simultaneously integrate both environmental and (epi)genetic variants and other health determinants in the same urban study. Such approaches could entail the spatiotemporal integration of hierarchically important clusters of infectious disease risk factors and their linked networks of urban parameters, feeding into preparedness and response measures. The exposome utility and its tools may also find use in the formulation of site-specific public health measures in urban communities of varying risk and exposure profiles.
A great opportunity emerges for the public health research community to exploit the exposome tools in upgrading site-specific public health measures in urban communities. More research efforts in testing and developing specific NPI using the exposome concept are warranted, to efficiently prepare and respond to future epidemics without disproportionally affecting health disparities and equity efforts in urban settings.
Declaration of Competing Interest
None.
Acknowledgments
Anjoeka Pronk, Karen S. Galea, Rob Stierum, Miranda Loh, and Konstantinos C. Makris would like to thank the EXPOSOGAS project that received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 810995.
Handling editor: Thanh Nguyen
References
- Andrianou X.D., Makris K.C. The framework of urban exposome: Application of the exposome concept in urban health studies. Sci. Total Environ. 2018;636:963–967. doi: 10.1016/j.scitotenv.2018.04.329. [DOI] [PubMed] [Google Scholar]
- Azar K.M.J., Shen Z., Romanelli R.J., Lockhart S.H., Smits K., Robinson S., Brown S., Pressman A.R. Disparities In Outcomes Among COVID-19 Patients In A Large Health Care System In California. Health Aff. 2020;39:1253–1262. doi: 10.1377/hlthaff.2020.00598. https://doi.org/10/ggx4mf. [DOI] [PubMed] [Google Scholar]
- Bouffanais, R., Lim, S.S., 2020. Cities — try to predict superspreading hotspots for COVID-19 [WWW Document]. URL https://www.nature.com/articles/d41586-020-02072-3 (accessed 7.17.20). [DOI] [PubMed]
- Bringing Greater Precision to the COVID-19 Response [WWW Document], 2020. Africa COVID-19 Community Vulnerability Index. URL https://precisionforcovid.org/africa (accessed 7.12.20).
- CDC COVID-19 Response Team, 2020. Geographic Differences in COVID-19 Cases, Deaths, and Incidence — United States, February 12–April 7, 2020. MMWR Morb Mortal Wkly Rep 69. https://doi.org/10.15585/mmwr.mm6915e4. [DOI] [PMC free article] [PubMed]
- Chu D.K., Akl E.A., Duda S., Solo K., Yaacoub S., Schünemann H.J., Chu D.K., Akl E.A., El-harakeh A., Bognanni A., Lotfi T., Loeb M., Hajizadeh A., Bak A., Izcovich A., Cuello-Garcia C.A., Chen C., Harris D.J., Borowiack E., Chamseddine F., Schünemann F., Morgano G.P., Schünemann G.E.U.M., Chen G., Zhao H., Neumann I., Chan J., Khabsa J., Hneiny L., Harrison L., Smith M., Rizk N., Rossi P.G., AbiHanna P., El-khoury R., Stalteri R., Baldeh T., Piggott T., Zhang Y., Saad Z., Khamis A., Reinap M., Duda S., Solo K., Yaacoub S., Schünemann H.J. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. The Lancet. 2020 doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cissé O.H., Ma L., Jiang C., Snyder M., Kovacs J.A. Humans Are Selectively Exposed to Pneumocystis jirovecii. mBio. 2020;11 doi: 10.1128/mBio.03138-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeruwa U.N., Ona S., Shaman J.L., Turitz A., Wright J.D., Gyamfi-Bannerman C., Melamed A. Associations between Built Environment, Neighborhood Socioeconomic Status, and SARS-CoV-2 Infection among Pregnant Women in New York City. JAMA. 2020;324:390–392. doi: 10.1001/jama.2020.11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P., Andersen E.W., Budtz-Jørgensen E., Nielsen F., Mølbak K., Weihe P., Heilmann C. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA. 2012;307:391–397. doi: 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli, G., Zangrillo, A., Zanella, A., Antonelli, M., Cabrini, L., Castelli, A., Cereda, D., Coluccello, A., Foti, G., Fumagalli, R., Iotti, G., Latronico, N., Lorini, L., Merler, S., Natalini, G., Piatti, A., Ranieri, M.V., Scandroglio, A.M., Storti, E., Cecconi, M., Pesenti, A., COVID-19 Lombardy ICU Network, Nailescu, A., Corona, A., Zangrillo, A., Protti, A., Albertin, A., Forastieri Molinari, A., Lombardo, A., Pezzi, A., Benini, A., Scandroglio, A.M., Malara, A., Castelli, A., Coluccello, A., Micucci, A., Pesenti, A., Sala, A., Alborghetti, A., Antonini, B., Capra, C., Troiano, C., Roscitano, C., Radrizzani, D., Chiumello, D., Coppini, D., Guzzon, D., Costantini, E., Malpetti, E., Zoia, E., Catena, E., Agosteo, E., Barbara, E., Beretta, E., Boselli, E., Storti, E., Harizay, F., Della Mura, F., Lorini, F.L., Donato Sigurtà, F., Marino, F., Mojoli, F., Rasulo, F., Grasselli, G., Casella, G., De Filippi, G., Castelli, G., Aldegheri, G., Gallioli, G., Lotti, G., Albano, G., Landoni, G., Marino, G., Vitale, G., Battista Perego, G., Evasi, G., Citerio, G., Foti, G., Natalini, G., Merli, G., Sforzini, I., Bianciardi, L., Carnevale, L., Grazioli, L., Cabrini, L., Guatteri, L., Salvi, L., Dei Poli, M., Galletti, M., Gemma, M., Ranucci, M., Riccio, M., Borelli, M., Zambon, M., Subert, M., Cecconi, M., Mazzoni, M.G., Raimondi, M., Panigada, M., Belliato, M., Bronzini, N., Latronico, N., Petrucci, N., Belgiorno, N., Tagliabue, P., Cortellazzi, P., Gnesin, P., Grosso, P., Gritti, P., Perazzo, P., Severgnini, P., Ruggeri, P., Sebastiano, P., Covello, R.D., Fernandez-Olmos, R., Fumagalli, R., Keim, R., Rona, R., Valsecchi, R., Cattaneo, S., Colombo, S., Cirri, S., Bonazzi, S., Greco, S., Muttini, S., Langer, T., Alaimo, V., Viola, U., 2020. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. https://doi.org/10.1001/jama.2020.5394.
- Haddad N., Andrianou X.D., Makris K.C. A Scoping Review on the Characteristics of Human Exposome Studies. Curr. Pollut. Rep. 2019 doi: 10.1007/s40726-019-00130-7. [DOI] [Google Scholar]
- Hart O.E., Halden R.U. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: Feasibility, economy, opportunities and challenges. Sci. Total Environ. 2020;730 doi: 10.1016/j.scitotenv.2020.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S., Han S.H., Rhee J.-Y. Cluster of Coronavirus Disease Associated with Fitness Dance Classes, South Korea. Emerg Infect. Dis. 2020;26 doi: 10.3201/eid2608.200633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C., Wang X., Li X., Inlora J., Wang T., Liu Q., Snyder M. Dynamic Human Environmental Exposome Revealed by Longitudinal Personal Monitoring. Cell. 2018;175:277–291.e31. doi: 10.1016/j.cell.2018.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury M.J., Flanders W.D., Greenland S., Adams M.J. On the measurement of susceptibility in epidemiologic studies. Am. J. Epidemiol. 1989;129:183–190. doi: 10.1093/oxfordjournals.aje.a115107. [DOI] [PubMed] [Google Scholar]
- Kim A.W., Nyengerai T., Mendenhall E. Evaluating the Mental Health Impacts of the COVID-19 Pandemic in Urban South Africa: Perceived Risk of COVID-19 Infection and Childhood Trauma Predict Adult Depressive Symptoms. medRxiv. 2020 doi: 10.1017/S0033291720003414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land W.G. Role of Damage-Associated Molecular Patterns in Light of Modern Environmental Research: A Tautological Approach. Int. J. Environ. Res. 2020:1–22. doi: 10.1007/s41742-020-00276-z. https://doi.org/10/ghbg9g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Sangion A., Li L. Evaluating consumer exposure to disinfecting chemicals against coronavirus disease 2019 (COVID-19) and associated health risks. Environ. Int. 2020;145 doi: 10.1016/j.envint.2020.106108. https://doi.org/10/ghbwh8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton S.X., Raval U., Harary J.M., Pasinetti G.M. The role of the exposome in promoting resilience or susceptibility after SARS-CoV-2 infection. J. Expo Sci. Environ. Epidemiol. 2020;30:776–777. doi: 10.1038/s41370-020-0232-4. https://doi.org/10/ghbg9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Operational tool on rapid risk assessment methodology - ECDC 2019 [WWW Document], 2019. European Centre for Disease Prevention and Control. URL https://www.ecdc.europa.eu/en/publications-data/operational-tool-rapid-risk-assessment-methodology-ecdc-2019 (accessed 7.18.20).
- Riccardo, F., Ajelli, M., Andrianou, X., Bella, A., Manso, M.D., Fabiani, M., Bellino, S., Boros, S., Urdiales, A.M., Marziano, V., Rota, M.C., Filia, A., D’Ancona, F. (Paolo), Siddu, A., Punzo, O., Trentini, F., Guzzetta, G., Poletti, P., Stefanelli, P., Castrucci, M.R., Ciervo, A., Benedetto, C.D., Tallon, M., Piccioli, A., Brusaferro, S., Rezza, G., Merler, S., Pezzotti, P., Group, C.-19 working, 2020. Epidemiological characteristics of COVID-19 cases in Italy and estimates of the reproductive numbers one month into the epidemic. medRxiv 2020.04.08.20056861. https://doi.org/10.1101/2020.04.08.20056861.
- Richardson, S., Hirsch, J.S., Narasimhan, M., Crawford, J.M., McGinn, T., Davidson, K.W., and the Northwell COVID-19 Research Consortium, Barnaby, D.P., Becker, L.B., Chelico, J.D., Cohen, S.L., Cookingham, J., Coppa, K., Diefenbach, M.A., Dominello, A.J., Duer-Hefele, J., Falzon, L., Gitlin, J., Hajizadeh, N., Harvin, T.G., Hirschwerk, D.A., Kim, E.J., Kozel, Z.M., Marrast, L.M., Mogavero, J.N., Osorio, G.A., Qiu, M., Zanos, T.P., 2020. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. https://doi.org/10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed]
- Sajadi M.M., Habibzadeh P., Vintzileos A., Shokouhi S., Miralles-Wilhelm F., Amoroso A. Temperature, Humidity, and Latitude Analysis to Estimate Potential Spread and Seasonality of Coronavirus Disease 2019 (COVID-19) JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salje H., Kiem C.T., Lefrancq N., Courtejoie N., Bosetti P., Paireau J., Andronico A., Hozé N., Richet J., Dubost C.-L., Strat Y.L., Lessler J., Levy-Bruhl D., Fontanet A., Opatowski L., Boelle P.-Y., Cauchemez S. Estimating the burden of SARS-CoV-2 in France. Science. 2020 doi: 10.1126/science.abc3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servick K., Cho A., Couzin-Frankel J., Guglielmi G. Coronavirus disruptions reverberate through research. Science. 2020;367:1289–1290. doi: 10.1126/science.367.6484.1289. [DOI] [PubMed] [Google Scholar]
- Urban Development Overview [WWW Document], n.d. URL http://www.worldbank.org/en/topic/urbandevelopment/overview#3 (accessed 7.9.18).
- Vermeulen R., Schymanski E.L., Barabási A.-L., Miller G.W. The exposome and health: where chemistry meets biology. Science. 2020;367:392–396. doi: 10.1126/science.aay3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhera R.K., Wadhera P., Gaba P., Figueroa J.F., Joynt Maddox K.E., Yeh R.W., Shen C. Variation in COVID-19 hospitalizations and deaths across New York City boroughs. JAMA. 2020;323:2192–2195. doi: 10.1001/jama.2020.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2020. Strengthening Preparedness for COVID-19 in Cities and Urban Settings [WWW Document]. URL https://www.who.int/publications-detail/strengthening-preparedness-for-covid-19-in-cities-and-urban-settings (accessed 6.2.20).
- Wild C.P. The exposome: from concept to utility. Int. J. Epidemiol. 2012;41:24–32. doi: 10.1093/ije/dyr236. [DOI] [PubMed] [Google Scholar]
- Wild C.P. Complementing the Genome with an “Exposome”: The Outstanding Challenge of Environmental Exposure Measurement in Molecular Epidemiology. Cancer Epidemiol. Biomark. Prev. 2005;14:1847–1850. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- Woodruff T.J., Zeise L., Axelrad D.A., Guyton K.Z., Janssen S., Miller M., Miller G.G., Schwartz J.M., Alexeeff G., Anderson H., Birnbaum L., Bois F., Cogliano V.J., Crofton K., Euling S.Y., Foster P.M.D., Germolec D.R., Gray E., Hattis D.B., Kyle A.D., Luebke R.W., Luster M.I., Portier C., Rice D.C., Solomon G., Vandenberg J., Zoeller R.T. Meeting report: moving upstream-evaluating adverse upstream end points for improved risk assessment and decision-making. Environ. Health Perspect. 2008;116:1568–1575. doi: 10.1289/ehp.11516. https://doi.org/10/d2qz28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X., Nethery, R.C., Sabath, B.M., Braun, D., Dominici, F., 2020. Exposure to air pollution and COVID-19 mortality in the United States: A nationwide cross-sectional study. medRxiv 2020.04.05.20054502. https://doi.org/10.1101/2020.04.05.20054502.
- Zhang X.Q. United Nations Human Settlements Programme; Nairobi: 2011. The economic role of cities. [Google Scholar]