Early in March 2020, the SARS-CoV-2 (COVID-19) pandemic struck Belgium [1]. Our large radiation oncology department took immediate precautionary measures to prevent COVID-19-induced saturation of health resources that may lead to delays and possibly the inability to provide radiation therapy to our patients. As part of the emergency adjustments, we implemented an ultrahypofractionation scheme of 26 Gy in five consecutive fractions, based on the FAST-Forward trial, for postoperative radiation therapy of breast cancer patients [2,3]. Prior to the COVID-19 pandemic, our protocol was 40 Gy in 15 daily fractions for 3 consecutive weeks. As 30% of our patients are breast cancer patients, we anticipated that adopting ultrahypofractionation in this population would have the largest impact on reducing infection risks for patients and staff.
Here we describe the measures taken to introduce and apply this protocol, treatment delivery experiences and early toxicity of treated patients.
Methods
Ultrahypofractionation was offered to all eligible patients and was therefore not regarded as a prospective trial. Informed consent was achieved for radiation therapy. Data were recorded in a prospective database. The reporting of outcomes was approved by the institutional ethics committee (CTOR20067GZA).
Prior to the introduction of the protocol, the following measures were taken by the radiation therapy team:
-
•
The protocol was discussed and accepted within the multidisciplinary breast cancer team.
-
•
Radiation therapy planning and delivery procedures were reviewed by the radiation therapy team [3]. Our routine computed tomography-based isocentric intensity-modulated radiation therapy sliding window treatment technique (i.e. irregular surface correction) was used, based on clinical target volumes according to the European Society for Radiotherapy and Oncology (ESTRO) guidelines [4]. If indicated (n = 4), axillary lymph node levels 1 and 2 up until the caudal border of axillary vessels were included. Respiratory control was considered in all left-sided breast cancer patients. A single boost dose of 6 Gy was delivered using an intensity-modulated radiation therapy technique for deeply seated tumours and a single electron field for superficial tumours [cut-off: Euclidian skin-to-caudal planning target volume distance <3 cm, i.e. maximum 9 MeV]. The dose prescription was according to ICRU 50 [5]. Plans were evaluated according to the FAST-Forward trial planning objectives. Daily kV/kV-based position and transit electronic portal image device (EPID) dosimetric verification was carried out.
-
•
After reviewing and discussing the protocol with the FAST-Forward trial team (the full paper was still pending publication), we identified the need for a tumour bed boost as the FAST-Forward trial population was relatively low risk. While the FAST-Forward trial allowed for a sequential fractionated boost, we preferred to avoid further treatment prolongation during COVID-19 times. Using the linear-quadratic model with an α/β value of 3, we estimated 6 Gy in one fraction to the tumour bed being equivalent to 10 Gy in five fractions.
-
•
The protocol adopted by our department was planned for all node-negative patients referred for postoperative whole breast radiation therapy or patients ≥70 years with low-risk node-positive disease. A sequential boost was delivered to all patients <70 years.
-
•
As elective patient contacts were discouraged during the pandemic, we carried out tele-medicine follow-up [6].
-
•
Despite available evidence, concerns existed that ultrahypofractionation would lead to more severe or longer-lasting early skin reactions compared with 40 Gy in 15 fractions, especially including a hypofractionated boost. Therefore, we decided to carry out a tele-medicine follow-up weekly after completing radiation therapy. Data on side-effects were recorded using the Common Terminology Criteria for Adverse Events (CTCAE) v.5 and the European Organization for Research and Treatment of Cancer (EORTC)-Breast (QLQ-BR23) questionnaires (see Table 1 ). A descriptive statistical analysis of toxicity was carried out; the significance of differences was assessed using the Pearson chi-squared test and P-values < 0.05 were taken to indicate statistical significance.
Table 1.
Outline of the tele-medicine questionnaire, based on the Common Terminology Criteria for Adverse Events (CTCAE) v.5 (upper part) and European Organization for Research and Treatment of Cancer (EORTC) QLQ-BR23 (lower part) questionnaires
| 0 | 1 | 2 | 3 | |
|---|---|---|---|---|
| Radiation dermatitis | None | Faint erythema Dry desquamation |
Moderate to brisk erythema, mostly in skin folds Moderate oedema |
Confluent moist desquamation, other than skin folds Bleeding induced by minor trauma |
| Breast oedema | Asymptomatic | Symptomatic (pain or psychosocial impact) | Severe symptoms Intervention needed Enlarge radiation therapy fields |
|
| Pain of skin | Mild | Moderate pain Limiting instrumental ADL |
Severe pain Limiting self-care ADL |
|
| Fatigue |
Relieved by rest |
Fatigue not relieved by rest Limiting instrumental ADL |
Fatigue not relieved by rest Limiting self-care ADL |
|
| 0 |
1 |
2 |
3 |
|
| Did you have any pain in your arm or shoulder? | Not at all | A little bit | Quite a bit | Very much |
| Did you have a swollen arm or hand? | ||||
| Was it difficult to raise your arm or to move it sideways? | ||||
| Have you had any breast pain? | ||||
| Have you had any increased breast sensitivity? | ||||
| Have you had skin problems on the breast (e.g. itchy, dry, flaky)? | ||||
ADL, activities of daily living.
Results
Between 24 March and 2 June 2020,102 breast cancer patients were treated with ultrahypofractionation. Treatment preparation and delivery went as planned. Of the 544 delivered fractions, only 20 fractions (3.7%) did not pass the transit dosimetry tolerance level of 98%, mostly related to respiration and/or swelling of the breast.
Clinical and Treatment Outcomes
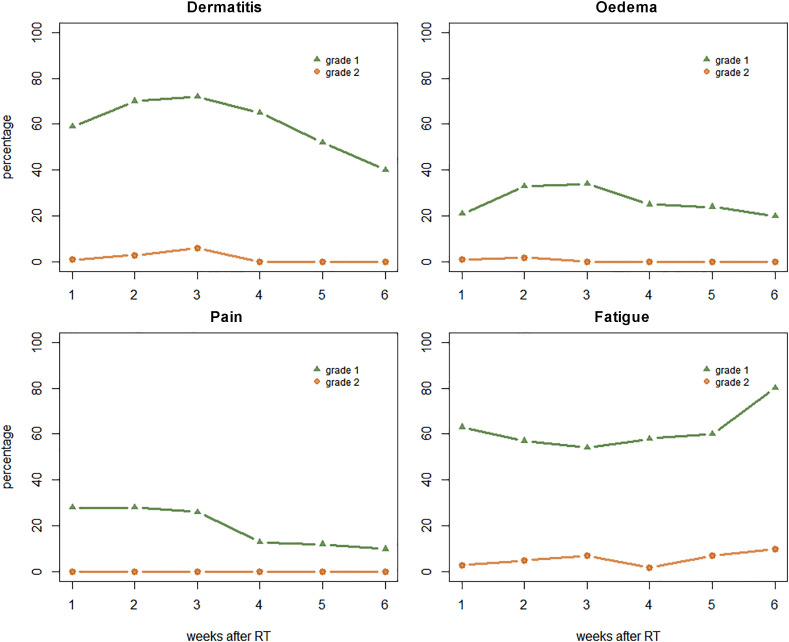
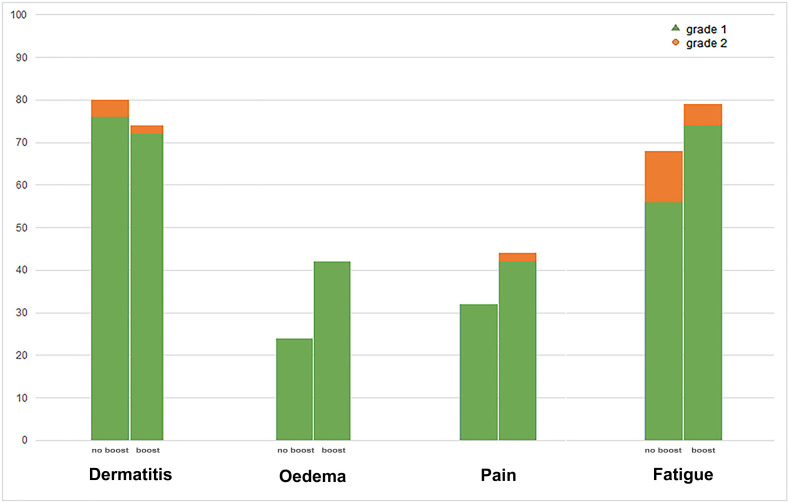
All 102 patients successfully completed radiation therapy. Sixty-eight patients had already had at least one tele-medicine follow-up and were included for toxicity analysis. The median follow-up was 32 days (range 8–54). Patient-, tumour- and treatment-related characteristics are listed in Table 2 . The prevalence of the most-reported grade 1 early breast toxicity after treatment was 74% for dermatitis, 68% for fatigue and 38% for oedema (Table 3 ). Grade 2 toxicity was reported by 7% for fatigue and 3% for dermatitis. No grade ≥3 toxicity was seen. Most toxicity was seen 3 weeks after the completion of radiation therapy (Figure 1 ). The prevalence of CTCAE toxicity is demonstrated in Figure 2 , showing no significant differences between patients receiving a boost and patients not receiving a boost.
Table 2.
Patient-, tumour- and treatment-related characteristics
| Patients (n) | % | Patients (n) | % | ||
|---|---|---|---|---|---|
| Age (years) | Hormone receptor status | ||||
| ≤40 | 0 | 0 | ER | ||
| 41–50 | 7 | 10 | Unknown | 1 | 1 |
| 51–60 | 8 | 12 | Positive (Allred >2) | 60 | 88 |
| >60 | 53 | 78 | Negative | 7 | 10 |
| WHO status | PR | ||||
| Unknown | 18 | 26 | Unknown | 1 | 1 |
| 0 | 34 | 50 | Positive (Allred >2) | 50 | 74 |
| 1 | 14 | 21 | Negative | 17 | 25 |
| ≥2 | 2 | 3 | |||
| Tumour size (mm) | |||||
| Unknown | 5 | 7 | Her2 | ||
| <5 | 9 | 13 | Unknown | 3 | 4 |
| 6–10 | 18 | 27 | 0 | 10 | 15 |
| 11–20 | 26 | 38 | 1+ | 31 | 46 |
| 21–50 | 10 | 15 | 2+ | 18 | 26 |
| >50 | 0 | 0 | 3+ | 6 | 9 |
| Nodal assessment | |||||
| Histological type | |||||
| IDC | 47 | 69 | Unknown | 1 | 1 |
| ILC | 11 | 16 | Sentinel node biopsy | 62 | 91 |
| IDC/ILC | 1 | 1 | Axillary node dissection | 1 | 1 |
| DCIS | 4 | 6 | None (DCIS) | 4 | 6 |
| Other | 5 | 7 | |||
| Number of positive nodes | |||||
| Histological grade | Unknown | 2 | 3 | ||
| Unknown | 1 | 1 | 0 | 59 | 87 |
| 1 | 22 | 32 | 1–3 | 7 | 10 |
| 2 | 31 | 46 | 4+ | 0 | 0 |
| 3 | 14 | 21 | |||
| Radiation treatment | |||||
| Presence of LVI | Tumour bed boost | 43 | 63 | ||
| Unknown | 2 | 3 | Regional lymph node irradiation | 4 | 6 |
| Yes | 0 | 0 | |||
| No | 66 | 97 | Adjuvant therapy | ||
| Endocrine therapy | 62 | 91 | |||
| Presence of PNI | Chemotherapy | 3 | 4 | ||
| Unknown | 2 | 3 | No adjuvant therapy | 3 | 4 |
| Yes | 1 | 1 | |||
| No | 65 | 96 | |||
| Tumour focality | |||||
| Unifocal | 64 | 94 | |||
| Multifocal | 4 | 6 | |||
DCIS, ductal carcinoma in situ; ER, oestrogen receptor; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; LVI, lymphovascular invasion; PNI, perineural Invasion; PR, progesterone receptor.
Table 3.
Incidence of highest reported radiation toxicity and breast irradiation side-effects
| Grade 1 [n (%)] | Grade 2 [n (%)] | Grade 3 [n (%)] | |
|---|---|---|---|
| Dermatitis | 50 (74) | 2 (3) | 0 (0) |
| Oedema | 26 (38) | 1 (1) | 0 (0) |
| Pain | 24 (35) | 0 (0) | 0 (0) |
| Fatigue | 46 (68) | 5 (7) | 0 (0) |
| Not at all [n (%)] |
A little bit [n (%)] |
Quite a bit [n (%)] |
|
| Pain in arm/shoulder | 64 (94) | 4 (6) | 0 (0) |
| Swollen arm/hand | 65 (95) | 3 (4) | 0 (0) |
| Difficult arm movement | 64 (94) | 4 (6) | 0 (0) |
| Breast pain | 26 (38) | 36 (53) | 6 (9) |
| Increased breast sensitivity | 28 (41) | 36 (53) | 4 (6) |
| Skin problems (e.g. itchy, dry, flaky) | 19 (28) | 42 (62) | 5 (7) |
Fig 1.
Prevalence of grade 1 and grade 2 Common Terminology Criteria for Adverse Events (CTCAE) toxicity (v.5).
Fig 2.
Prevalence of highest reported Common Terminology Criteria for Adverse Events (CTCAE) toxicity for patients receiving a tumour bed boost versus no tumour bed boost (right and left column, respectively). No significant differences were found between groups.
Discussion
Here we report the accelerated implementation of ultrahypofractionation for postoperative breast radiation therapy at the time of a major worldwide health crisis. Early in the COVID-19 pandemic in Belgium, we found that rapid adjustments were needed to ensure continuity of care for patients without compromising oncological outcome and to reduce the potential infectious risks for patients and staff. As our centre treats about 1000 breast cancer patients a year, we decided to adopt ultrahypofractionation for postoperative radiation therapy for breast cancer to significantly reduce potential exposure of patients and staff, while providing treatment without delay. The low rates of early toxicity, as previously shown in the FAST-Forward trial, encouraged us to make this choice, while efficacy results were then still pending publication [3,6].
We aimed to select comparable patients with those eligible for the FAST-Forward trial; nonetheless, when resources at our department decreased further due to staffing problems, we also included selected node-positive patients, avoiding irradiation of the brachial plexus by limiting the nodal target volume up until the caudal part of the axillary vessels.
Importantly, the implementation of a radiobiological equivalent hypofractionated tumour bed boost was unique. Similar to other studies about hypofractionation of whole-breast radiation therapy, hypofractionation of the tumour bed boost was lacking behind in the FAST-Forward trial protocol. We did not expect increased early toxicity rates as the biological equivalent dose remained the same as with conventional fractionation. This was confirmed by the unchanged early toxicity rates of patients treated with a boost versus those treated without a boost in our cohort (Figure 2). Regarding late toxicity, this hypofractionated boost might hypothetically impact cosmetic outcome adversely as the EqD2 dose is 2 Gy higher when assuming an α/β value of 2 for late effects. Before routine implementation post-COVID-19, this should be further investigated.
We aimed to keep the workload for staff as low as reasonably achievable. Therefore, most of the treatment preparation of ultrahypofractionation was identical to our former radiation therapy breast protocol of 40 Gy in 15 fractions. No difficulties were noted during treatment preparation. Two adaptations made were the implementation of a daily kV/kV set-up verification instead of an eNAL procedure, and daily transit EPID dosimetry with integrated images, for quality assurance [7]. Of the delivered fractions, only 20 (3.7%) did not pass the tolerance level of 98%. This is lower than the observed 8% not passing the tolerance level in a historical cohort of node-negative patients treated at our department pre-COVID with the 40/15 scheme. Probable explanations are the reduced overall treatment time and daily kV/kV set-up verification.
After completing radiation therapy, low rates of clinically significant early toxicity were seen, similar to the FAST-Forward trial [6]. A peculiar finding is the lack of grade 3 toxicity, possibly partially explained by the use of tele-medicine, which might induce – despite thoroughly questioning the patient – an underestimation of toxicity. The use of tele-medicine is also the most important limitation of our report, but because our primary goal was to reduce patient exposure to COVID-19 in crowded hospitals, this was inevitable. Nevertheless, studies have shown that these tele-medicine follow-ups can be equally effective as routine hospital visits [8,9].
In conclusion, ultrahypofractionation was fluidly implemented in our departmental workflow. We also noted no negative influence on general staff and patient satisfaction. It allowed a reduction in potential exposure of patients and staff to COVID-19 and enabled treatment delivery without delay. Furthermore, improved treatment accuracy and low early toxicity rates were shown. These findings endorse our continued use of ultrahypofractionation for all node-negative patients after the COVID-19 pandemic, while for the ultrahypofractionated boost we will contribute to initiating prospective research.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author (MM). The data are not publicly available due to institutional ethics policy, as they contain information that could compromise the privacy of research participants.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgements
The authors thank the FAST-Forward team members for their availability to discuss our protocol during the preparatory phase.
References
- 1.https://www.worldometers.info/coronavirus/country/belgium/n.d
- 2.Coles C.E., Aristei C., Bliss J., Boersma L., Brunt A.M., Chatterjee S. International guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. Clin Oncol. 2020;32:279–281. doi: 10.1016/j.clon.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray Brunt A., Haviland J.S., Wheatley D.A., Sydenham M.A., Alhasso A., Bloomfield D.J. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020;6736:1–14. doi: 10.1016/S0140-6736(20)30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Offersen B.V., Boersma L.J., Kirkove C., Hol S., Aznar M.C., Biete Sola A. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol. 2015;114:3–10. doi: 10.1016/j.radonc.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 5.International Commission of Radiation Units and Measurements ICRU Report 50. Prescribing, recoding, and reporting photon beam therapy. Med Phys. 1994;21:833–834. doi: 10.1016/S0360-3016(03)01373-7. [DOI] [Google Scholar]
- 6.Brunt A.M., Wheatley D., Yarnold J., Somaiah N., Kelly S., Harnett A. Acute skin toxicity associated with a 1-week schedule of whole breast radiotherapy compared with a standard 3-week regimen delivered in the UK FAST-Forward trial. Radiother Oncol. 2016;120:114–118. doi: 10.1016/j.radonc.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Boer H.C.J., Heijmen B.J.M. eNAL: an extension of the NAL setup correction protocol for effective use of weekly follow-up measurements. Int J Radiat Oncol Biol Phys. 2007;67:1586–1595. doi: 10.1016/j.ijrobp.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 8.Kimman M.L., Dirksen C.D., Voogd A.C., Falger P., Gijsen B.C.M., Thuring M. Nurse-led telephone follow-up and an educational group programme after breast cancer treatment: results of a 2 x 2 randomised controlled trial. Eur J Cancer. 2011;47:1027–1036. doi: 10.1016/j.ejca.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Beaver K., Tysver-Robinson D., Campbell M., Twomey M., Williamson S., Hindley A. Comparing hospital and telephone follow-up after treatment for breast cancer: randomised equivalence trial. BMJ. 2009;338:a3147. doi: 10.1136/bmj.a3147. [DOI] [PMC free article] [PubMed] [Google Scholar]