Abstract
Senior individuals are more susceptible to the irreversible outcomes of endothelial barrier dysfunction, the hallmark of Acute Respiratory Distress Syndrome (ARDS). The Severe Acute Respiratory Syndrome Coronovirus 2 (SARS-CoV-2) - inflicted ARDS delivers the devastating outcomes of the COVID-19 worldwide. Endothelial hyperpermeability has been associated with both the progression and establishment of the COVID-19 - related respiratory failure. In the present study we investigated the in vitro effects of Metformin in the permeability of bovine pulmonary artery endothelial cells. Our preliminary results suggest that moderate doses (0.1, 0.5, 1.0 mM) of this anti-diabetic agent enhance the vascular barrier integrity, since it produces an increase in the transendothelial resistance of endothelial monolayers. Thus, we speculate that Metformin may deliver a new therapeutic possibility in ARDS, alone or in combination with other barrier enhancers.
Keywords: Acute lung injury, Acute respiratory distress syndrome, Growth hormone releasing hormone, P53, Heat shock protein 90
Lung endothelial barrier dysfunction is involved in the lethal consequences of Acute Respiratory Distress Syndrome (ARDS), as demonstrated in COVID-19 (Barabutis, 2019, Barabutis, 2020h). Ageing individuals are more susceptible to the consequences of the current pandemic. The increased permeability across the pulmonary endothelium leads to edema in the lung interstitium, and accumulation of proteins, fluid, red blood cells and neutrophils into the alveolar space (Matthay et al., 2019). Current therapeutics have not delivered the desired outcomes, and the anti-inflammatory effects of corticosteroids are linked with off target and side effects, as well as with induction of senescence (Matthay et al., 2019; Li and Ma, 2020; Carvalho et al., 2019). Recent evidence suggest that the EGR1, a candidate target of glucocorticoids, acts as a sensor of MAPK hyperactivity contributing to the triggering of senescence (Carvalho et al., 2019).
Research efforts have been intensified to uncover the molecular interrelations dictating the responses of the lung microvasculature in severe inflammation. Delineation of the corresponding network may propel exciting developments against the current pandemic, and research observations revealed the crucial role of P53 in the regulation of lung endothelial permeability (Barabutis, 2020a, Barabutis, 2020b).
P53 is a cell cycle regulator, able to trigger apoptosis or senescence (Barabutis, 2020c). The anti-inflammatory activities of this tumor suppressor has been associated with suppression of NFκB (Moiseeva et al., 2013). In the lungs, P53 protects against the LPS-induced endothelial barrier disruption (Barabutis et al., 2018a, Barabutis et al., 2015), partially via the reduction of the reactive oxygen species (ROS) (Akhter et al., 2020a). Furthermore, it suppresses the Ras homologous family member A (RhoA)/Myosin light chain 2 (MLC2) pathway, which in turn triggers the formation of the filamentous actin (increased permeability) (Barabutis, 2020a, Barabutis, 2020d). It appears that both cofilin and APE1/Ref1 participate in those events. Cofilin compromises the integrity of cytoskeleton, and APE1/Ref1 participates in the generation of ROS. Indeed, P53 is a target of both lipopolysaccharides (LPS) (Barabutis et al., 2019), and lipoteichoic acid (Kubra et al., 2020a) in the lungs. Treatment of endothelial cells with those toxins reduced P53 (Barabutis, 2020e). Furthermore, the LPS-induced ALI in P53 null mice were more severe than the lung damage inflicted in the wild type littermates (Uddin et al., 2020a), and in vitro P53 silencing by siRNA means has resulted to the collapse of the lung endothelium (Barabutis et al., 2015).
Heat shock protein 90 (Hsp90) is an important and widely abundant molecular chaperone, which assists towards the maturation of various client proteins, which may serve as transcriptions factors (Barabutis, 2020f). The inhibitors of that chaperone possess anti-cancer activities, thus exert anti-inflammatory effects. To explore the possibility that those compounds suppress lung inflammation, we employed in vivo models of ALI. We revealed that the beneficial effects of those agents in the lungs are not mediated exclusively via P53 induction and/or suppression of major inflammatory pathways (Barabutis et al., 2016; Barabutis and Siejka, 2020). Indeed, it now appears that induce the unfolded protein response element (Uddin et al., 2020b; Kubra et al., 2020b), which in turn affects P53 (Akhter et al., 2019). UPR activation induced 53, and its suppression reduced this endothelium defender (Akhter et al., 2019). UPR activation has been previously associated with beneficial outcome in cardiovascular disease and lung repair (Barabutis, 2020b; Kubra et al., 2020c). UPR inducers protects against the Kifunensine (UPR suppressor) -triggered barrier dysfunction (Kubra et al., 2020d; Akhter et al., 2020b). Moreover, UPR has been involved in mediating the effects of GHRH antagonists in the lung barrier function (Akhter et al., 2020c).
Growth Hormone Releasing Hormone (GHRH) regulates the release of growth hormone from the anterior pituitary gland, and has been involved in the progression of malignancies (Barabutis et al., 2018b). GHRH antagonists were developed to oppose cancers, but their applications studies are not limited to oncology. This class of compounds is holding the potential to suppress inflammatory pathways (Siejka et al., 2010; Barabutis et al., 2010), the production of ROS (Barabutis and Schally, 2008), and induce P53 (Barabutis and Schally, 2008; Barabutis and Schally, 2010). Their activities have also been associated with beneficial outcomes towards the lung microvasculature (Barabutis, 2020f; Uddin et al., 2019a), suggesting that they may represent exciting therapeutic possibilities against inflammatory lung diseases, including ARDS (Barabutis, 2020g). Remarkably, it was recently shown that GHRH antagonists protect against the hydrogen peroxide induced breakdown of the blood brain barrier, an effect associated with P53 induction (Barabutis et al., 2020).
Metformin is an anti-hyperglycemic first-choice drug for patients with type 2 diabetes. It reduces insulin resistance and it is also applied in the treatment of polycystic ovary syndrome presenting with metabolic abnormalities. The anti-cancer properties of Metformin have been associated with the activation of AMPK pathway (Wu and Zou, 2020). In primary human fibroblasts Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation, suggesting that it interacts with the senescence-related pathways (Moiseeva et al., 2013). In human lung endothelial cells Metformin suppressed senescence via an AMPK-dependent manner, and those effects were not associated with increased apoptosis (Cheng et al., 2017). Moreover, that compound was reported to increase health span and decreases mortality (Kulkarni et al., 2020), while other reports indicate that those effects may occur in a tissue- and model- specific manner (Glossmann and Lutz, 2019).
We have previously shown that GHRH antagonists stimulate AMPK activation in lung cancer cells (Siejka et al., 2011), and exert the capacity to inhibit endothelial VEGF secretion from endothelial cells (Siejka et al., 2003). AMPK seems a potential target for alleviating LPS-induced ALI (He et al., 2019). VEGF is increased in ARDS patients, while dexamethasone have shown to exert the opposite effects in an in vivo rat model of ARDS (Qin and Qiu, 2019).
P53 mediates the inhibitory effects of Metformin in cancers (Li et al., 2015), and this antidiabetic drug (Metformin) stabilizes P53 in malignancies (Lee et al., 2019). Hence, we investigated the effects of Metformin in the lung endothelial barrier function. To proceed with this task, we used bovine pulmonary artery endothelial cells (BPAEC) (#PB30205), from Genlantis (San Diego, CA), maintained per previously described procedures (Uddin et al., 2020b). The barrier function of the cells was measured with the electric cell-substrate impedance sensing (ECIS) model ΖΘ from Applied Biophysics (Troy, NY) (Akhter et al., 2020a; Uddin et al., 2019a). After the BPAECs formed confluent monolayers onto the ECIS arrays, they were exposed to vehicle (PBS) or Metformin (# TCM2009) (0.1, 0.5, 1 mM) from VWR (Radnor, PA). To assess statistics we employed two-way analysis of variance with Bonferroni correction, so to determine differences among groups. GraphPad Prism 5.01 from GraphPad (CA, USA) was used for data analysis.
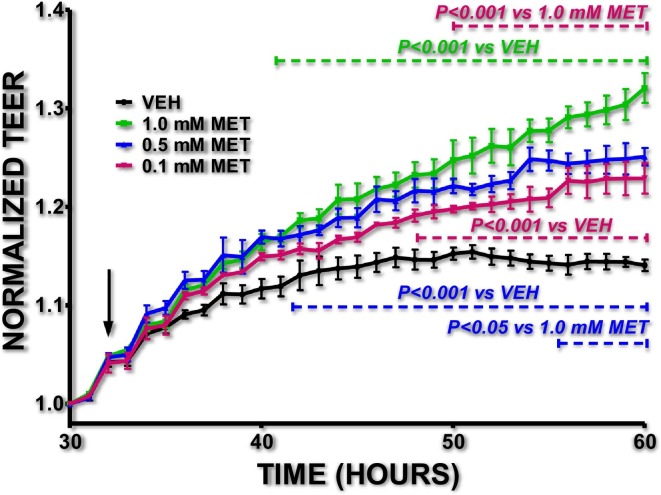
The results depicted in Fig. 1 suggest that moderate doses of Metformin enhances the lung endothelial barrier function, since the Metformin-treated cells presented higher transendothelial resistance (TEER) values compared to the vehicle-treated cells. It was previously reported by others that the barrier-enhancing effects of Metformin in the vascular barrier are associated with the AMPK-α1 (Jian et al., 2013). Recently it was suggested that Metformin may help against COVID-19 (Sharma et al., 2020), and it was revealed that it decreased deaths due to COVID-19 in diabetic patients (Luo et al., 2020). Others have suggested that metformin opposed COVID-19 by reducing TNF-alpha in females over males (Bramante et al., 2020), but the possibility of severe side effects exists (Palermo et al., 2020; Chamorro-Pareja et al., 2020; Bornstein et al., 2020). Dehydration and lactic acidosis may occur due to Metformin treatment, and renal function is carefully monitored because of the risk to develop chronic kidney disease or acute kidney injury (Bornstein et al., 2020).
Fig. 1.
Metformin enhances lung endothelial barrier function.
BPAEC were exposed to vehicle (VEH) (PBS) or Metformin (MET) (0.1, 0.5, 1 mM) at the time point indicated by the black arrow. A gradual decrease in BPAEC permeability (increased TEER) was observed in the Metformin-treated cells. N = 3 per group; means ± SEM.
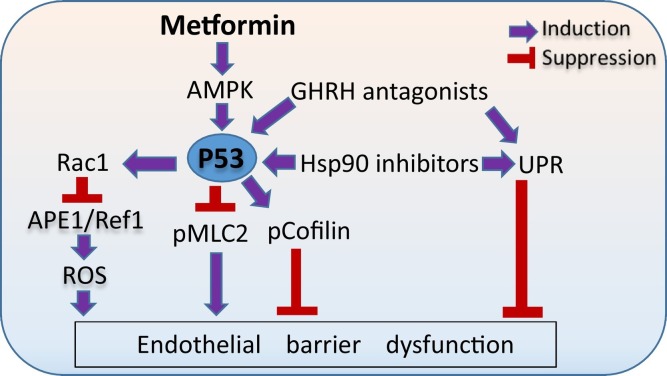
Endothelial barrier enhancement has been previously reported in lung monolayers treated with the P53 inducers Hsp90 inhibitors (Uddin et al., 2019b) and GHRH antagonists (Uddin et al., 2019a). Since Phase 3 of COVID-19 is marked by the disruption of the pulmonary vascular endothelium (Romagnoli et al., 2020), we feel that the application of Metformin in combination with moderate doses of other anti-inflammatory and barrier-enhancing agents (e.g. Hsp90 inhibitors and GHRH antagonists) might be an efficient approach to sabotage the ferocious COVID-19 - related cytokine storm in the SARS-CoV-2 triggered ARDS and reduce the corresponding complications, especially in the elderly. In case of higher doses of those compounds, non-desirable toxic effects may occur. It was shown that low doses of Hsp90 inhibitors (1–10 μM) enhance endothelial barrier function without affecting cellular viability. On the other hand, higher doses (50–100 μM) of those compounds lead to toxic effects and cellular death (Kubra et al., 2020a; Uddin et al., 2020b; Kubra et al., 2020d). In a similar fashion, low doses of GHRH antagonists have been associated with anti-oxidant (Barabutis and Schally, 2008) and barrier-enhancing activities (Uddin et al., 2019a), while higher doses induce apoptosis (Barabutis and Schally, 2010; Rick et al., 2012). P53 may serve as a powerful modulator in those events. Fig. 2 depicts the beneficial effects of metformin, GHRH antagonists and Hsp90 inhibitors in endothelial barrier function.
Fig. 2.
Metformin, GHRH antagonists and Hsp90 inhibitors in endothelial barrier function.
Metformin affects P53 via AMPK activation, which in turn induces the activation of MLC2 and deactivate cofilin, supporting endothelial integrity. Induction of P53 by either Metformin, Hsp90 inhibitors and GHRH antagonists downregulates the ROS inducer APE1/Ref1 via Rac1 induction, and reduces endothelial permeability. Moreover, GHRH antagonists and Hsp90 inhibitors activate UPR, which in turn enhances endothelial barrier function.
Authors' contribution
MAU and KTK executed experiments and prepared the figures, MSA prepared the figures, AS critically edited the paper, NB drafted, edited the paper, provided funds and conceived the project. All authors approve the content of the paper.
Funding
Our research is supported by the R&D, Research Competitiveness Subprogram (RCS) of the Louisiana Board of Regents through the Board of Regents Support Fund (LEQSF(2019-22)-RD-A-26) to NB.
Declaration of competing interest
None.
References
- Akhter M.S., Uddin M.A., Barabutis N. Unfolded protein response regulates P53 expression in the pulmonary endothelium. J. Biochem. Mol. Toxicol. 2019;33(10) doi: 10.1002/jbt.22380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter M.S., Uddin M.A., Barabutis N. P53 regulates the redox status of lung endothelial cells. Inflammation. 2020;43(2):686–691. doi: 10.1007/s10753-019-01150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter M.S. Kifunensine compromises lung endothelial barrier function. Microvasc. Res. 2020;132:104051. doi: 10.1016/j.mvr.2020.104051. [DOI] [PubMed] [Google Scholar]
- Akhter M.S. Involvement of the unfolded protein response in the protective effects of growth hormone releasing hormone antagonists in the lungs. J. Cell Commun. Signal. 2020;13:1–5. doi: 10.1007/s12079-020-00593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabutis N. Unfolded protein response in acute respiratory distress syndrome. Lung. 2019;197(6):827–828. doi: 10.1007/s00408-019-00279-4. [DOI] [PubMed] [Google Scholar]
- Barabutis N. Regulation of lung endothelial permeability by NEK kinases. IUBMB Life. 2020;72(4):801–804. doi: 10.1002/iub.2251. [DOI] [PubMed] [Google Scholar]
- Barabutis N. Unfolded protein response in lung health and disease. Front. Med. 2020;7:344. doi: 10.3389/fmed.2020.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabutis N. P53 in acute respiratory distress syndrome. Cell. Mol. Life Sci. 2020;77(22):4725–4727. doi: 10.1007/s00018-020-03629-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabutis N. P53 in RhoA regulation. Cytoskeleton (Hoboken) 2020;77(5-6):197–201. doi: 10.1002/cm.21604. [DOI] [PubMed] [Google Scholar]
- Barabutis N. P53 in lung vascular barrier dysfunction. Vasc. Biol. 2020;2(1):E1–E2. doi: 10.1530/VB-20-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabutis N. Heat shock protein 90 inhibition in the inflamed lungs. Cell Stress Chaperones. 2020;25(2):195–197. doi: 10.1007/s12192-020-01069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabutis N. Growth hormone releasing hormone in the unfolded protein response context. Endocrine. 2020;67(2):291–293. doi: 10.1007/s12020-020-02205-8. [DOI] [PubMed] [Google Scholar]
- Barabutis Nektarios. Unfolded Protein Response in the COVID-19 Context. Aging and Health Research. 2020 doi: 10.1016/j.ahr.2020.100001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabutis N., Schally A.V. Antioxidant activity of growth hormone-releasing hormone antagonists in LNCaP human prostate cancer line. Proc. Natl. Acad. Sci. U. S. A. 2008;105(51):20470–20475. doi: 10.1073/pnas.0811209106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabutis N., Schally A.V. Growth hormone-releasing hormone: extrapituitary effects in physiology and pathology. Cell Cycle. 2010;9(20):4110–4116. doi: 10.4161/cc.9.20.13787. [DOI] [PubMed] [Google Scholar]
- Barabutis N., Siejka A. The highly interrelated GHRH, p53, and Hsp90 universe. Cell Biol. Int. 2020;44(8):1558–1563. doi: 10.1002/cbin.11356. [DOI] [PubMed] [Google Scholar]
- Barabutis N. Activation of mitogen-activated protein kinases by a splice variant of GHRH receptor. J. Mol. Endocrinol. 2010;44(2):127–134. doi: 10.1677/JME-09-0121. [DOI] [PubMed] [Google Scholar]
- Barabutis N. p53 protects against LPS-induced lung endothelial barrier dysfunction. Am. J. Phys. Lung Cell. Mol. Phys. 2015;308(8):L776–L787. doi: 10.1152/ajplung.00334.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabutis N., Verin A., Catravas J.D. Regulation of pulmonary endothelial barrier function by kinases. Am. J. Phys. Lung Cell. Mol. Phys. 2016;311(5):L832–L845. doi: 10.1152/ajplung.00233.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabutis N. Wild-type p53 enhances endothelial barrier function by mediating RAC1 signalling and RhoA inhibition. J. Cell. Mol. Med. 2018;22(3):1792–1804. doi: 10.1111/jcmm.13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabutis N., Schally A.V., Siejka A. P53, GHRH, inflammation and cancer. EBioMedicine. 2018;37:557–562. doi: 10.1016/j.ebiom.2018.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabutis N., Uddin M.A., Catravas J.D. Hsp90 inhibitors suppress P53 phosphorylation in LPS - induced endothelial inflammation. Cytokine. 2019;113:427–432. doi: 10.1016/j.cyto.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabutis N. GHRH antagonists protect against hydrogen peroxide-induced breakdown of brain microvascular endothelium integrity. Horm. Metab. Res. 2020;52(5):336–339. doi: 10.1055/a-1149-9347. [DOI] [PubMed] [Google Scholar]
- Bornstein S.R. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8(6):546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramante C. medRxiv. 2020. Observational study of metformin and risk of mortality in patients hospitalized with Covid-19. [Google Scholar]
- Carvalho C. Glucocorticoids delay RAF-induced senescence promoted by EGR1. J. Cell Sci. 2019;132(16) doi: 10.1242/jcs.230748. [DOI] [PubMed] [Google Scholar]
- Chamorro-Pareja N. Letter to the editor: unexpected high mortality in COVID-19 and diabetic ketoacidosis. Metabolism. 2020;110:154301. doi: 10.1016/j.metabol.2020.154301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X.Y. AMP-activated protein kinase reduces inflammatory responses and cellular senescence in pulmonary emphysema. Oncotarget. 2017;8(14):22513–22523. doi: 10.18632/oncotarget.15116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glossmann H.H., Lutz O.M.D. Metformin and aging: a review. Gerontology. 2019;65(6):581–590. doi: 10.1159/000502257. [DOI] [PubMed] [Google Scholar]
- He Y. AMPK as a potential pharmacological target for alleviating LPS-induced acute lung injury partly via NLRC4 inflammasome pathway inhibition. Exp. Gerontol. 2019;125:110661. doi: 10.1016/j.exger.2019.110661. [DOI] [PubMed] [Google Scholar]
- Jian M.Y. Metformin-stimulated AMPK-alpha1 promotes microvascular repair in acute lung injury. Am. J. Phys. Lung Cell. Mol. Phys. 2013;305(11):L844–L855. doi: 10.1152/ajplung.00173.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubra K.T. P53 is subjected to lipoteichoic acid-induced phosphorylation in the lungs. TH Open. 2020;4(3):e173–e174. doi: 10.1055/s-0040-1714695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubra K.T. Hsp90 inhibitors induce the unfolded protein response in bovine and mice lung cells. Cell. Signal. 2020;67:109500. doi: 10.1016/j.cellsig.2019.109500. [DOI] [PubMed] [Google Scholar]
- Kubra K.T. Unfolded protein response in cardiovascular disease. Cell. Signal. 2020;73:109699. doi: 10.1016/j.cellsig.2020.109699. [DOI] [PubMed] [Google Scholar]
- Kubra K.T. Luminespib counteracts the Kifunensine-induced lung endothelial barrier dysfunction. Curr. Res. Toxicol. 2020;1:111–115. doi: 10.1016/j.crtox.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni A.S., Gubbi S., Barzilai N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metab. 2020;32(1):15–30. doi: 10.1016/j.cmet.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.B. Metformin and tenovin-6 synergistically induces apoptosis through LKB1-independent SIRT1 down-regulation in non-small cell lung cancer cells. J. Cell. Mol. Med. 2019;23(4):2872–2889. doi: 10.1111/jcmm.14194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit. Care. 2020;24(1):198. doi: 10.1186/s13054-020-02911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P. p53 is required for metformin-induced growth inhibition, senescence and apoptosis in breast cancer cells. Biochem. Biophys. Res. Commun. 2015;464(4):1267–1274. doi: 10.1016/j.bbrc.2015.07.117. [DOI] [PubMed] [Google Scholar]
- Luo P. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am. J. Trop. Med. Hyg. 2020;103(1):69–72. doi: 10.4269/ajtmh.20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay M.A. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers. 2019;5(1):18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseeva O. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-kappaB activation. Aging Cell. 2013;12(3):489–498. doi: 10.1111/acel.12075. [DOI] [PubMed] [Google Scholar]
- Palermo N.E., Sadhu A.R., McDonnell M.E. Diabetic ketoacidosis in COVID-19: unique concerns and considerations. J. Clin. Endocrinol. Metab. 2020;105(8) doi: 10.1210/clinem/dgaa360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M., Qiu Z. Changes in TNF-alpha, IL-6, IL-10 and VEGF in rats with ARDS and the effects of dexamethasone. Exp. Ther. Med. 2019;17(1):383–387. doi: 10.3892/etm.2018.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick F.G. GHRH antagonist when combined with cytotoxic agents induces S-phase arrest and additive growth inhibition of human colon cancer. Cell Cycle. 2012;11(22):4203–4210. doi: 10.4161/cc.22498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli S. SARS-CoV-2 and COVID-19: from the bench to the bedside. Physiol. Rev. 2020;100(4):1455–1466. doi: 10.1152/physrev.00020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Ray A., Sadasivam B. Metformin in COVID-19: a possible role beyond diabetes. Diabetes Res. Clin. Pract. 2020;164:108183. doi: 10.1016/j.diabres.2020.108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siejka A. GH-RH antagonist (MZ-4-71) inhibits VEGF secretion and proliferation of murine endothelial cells. Life Sci. 2003;72(22):2473–2479. doi: 10.1016/s0024-3205(03)00164-4. [DOI] [PubMed] [Google Scholar]
- Siejka A., Schally A.V., Barabutis N. Activation of Janus kinase/signal transducer and activator of transcription 3 pathway by growth hormone-releasing hormone. Cell. Mol. Life Sci. 2010;67(6):959–964. doi: 10.1007/s00018-009-0224-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siejka A., Barabutis N., Schally A.V. GHRH antagonist MZ-5-156 increases the expression of AMPK in A549 lung cancer cells. Cell Cycle. 2011;10(21):3714–3718. doi: 10.4161/cc.10.21.17904. [DOI] [PubMed] [Google Scholar]
- Uddin M.A. GHRH antagonists support lung endothelial barrier function. Tissue Barriers. 2019;7(4):1669989. doi: 10.1080/21688370.2019.1669989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M.A. P53 supports endothelial barrier function via APE1/Ref1 suppression. Immunobiology. 2019;224(4):532–538. doi: 10.1016/j.imbio.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M.A. P53 deficiency potentiates LPS-induced acue lung injury. Curr. Res. Physiol. 2020;3:30–33. doi: 10.1016/j.crphys.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M. Effects of heat shock protein 90 inhibition in the lungs. Med. Drug Discov. 2020;6:100046. doi: 10.1016/j.medidd.2020.100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Zou M.H. AMPK, mitochondrial function, and cardiovascular disease. Int. J. Mol. Sci. 2020;21(14) doi: 10.3390/ijms21144987. [DOI] [PMC free article] [PubMed] [Google Scholar]