Abstract
Purpose
Numerous publications during the COVID-19 pandemic recommended the use of hypofractionated radiation therapy. This project assessed aggregate changes in the quality of the evidence supporting these schedules to establish a comprehensive evidence base for future reference and highlight aspects for future study.
Methods and Materials
Based on a systematic review of published recommendations related to dose fractionation during the COVID-19 pandemic, 20 expert panelists assigned to 14 disease groups named and graded the highest quality of evidence schedule(s) used routinely for each condition and also graded all COVID-era recommended schedules. The American Society for Radiation Oncology quality of evidence criteria were used to rank the schedules. Process-related statistics and changes in distributions of quality ratings of the highest-rated versus recommended COVID-19 era schedules were described by disease groups and for specific clinical scenarios.
Results
From January to May 2020 there were 54 relevant publications, including 233 recommended COVID-19–adapted dose fractionations. For site-specific curative and site-specific palliative schedules, there was a significant shift from established higher-quality evidence to lower-quality evidence and expert opinions for the recommended schedules (P = .022 and P < .001, respectively). For curative-intent schedules, the distribution of quality scores was essentially reversed (highest levels of evidence "pre-COVID" vs "in-COVID": high quality, 51.4% vs 4.8%; expert opinion, 5.6% vs 49.3%), although there was variation in the magnitude of shifts between disease sites and among specific indications.
Conclusions
A large number of publications recommended hypofractionated radiation therapy schedules across numerous major disease sites during the COVID-19 pandemic, which were supported by a lower quality of evidence than the highest-quality routinely used dose fractionation schedules. This work provides an evidence-based assessment of these potentially practice-changing recommendations and informs individualized decision-making and counseling of patients. These data could also be used to support radiation therapy practices in the event of second waves or surges of the pandemic in new regions of the world.
Introduction
The coronavirus (SARS-CoV-2) outbreak was first reported in December 2019 and named COVID-19 by the World Health Organization in February 2020.1 By June 2020, there were an estimated 400,000 deaths from the disease worldwide, with approximately one-third of these in the United States and United Kingdom.2 As the COVID-19 pandemic expanded and matured, the pace of scientific investigation and publication related to the coronavirus and its effects also exponentially increased. In the spring of 2020 at the peak of the pandemic, the number of SARS-CoV-2–related research publications had an estimated doubling time of less than 14 days.3 This acceleration was distinct from patterns of publication during the outbreak of severe acute respiratory syndrome (SARS) in Asia in 2003, when only a small fraction of related articles were published during the time of the actual epidemic.4 This historic shift likely reflects (1) the rapid worldwide spread of the COVID-19 pandemic with a devastating global effect stimulating urgent actions in every nation, (2) a contemporary research, publishing, and social media infrastructure allowing for extremely rapid production and dissemination of information, and (3) the unprecedented open access of academic journals, societies, institutes, and companies, as demonstrated by the nearly 100 such entities that pledged to make coronavirus-related research freely available for the duration of the pandemic.5
Due to restrictions on services within radiation therapy departments and the need to minimize the exposure of patients at risk of severe infection from SARS-CoV-2 in heavily affected regions, numerous expert groups, professional societies, and other institutions early on in the COVID-19 pandemic proposed serious consideration of delay or alterations of regular radiation schedules.6 , 7 In the face of a need for immediate action, these recommendations were limited by a number of sizeable uncertainties, including (1) difficulty predicting the future prevalence of SARS-CoV-2 and the impact of the pandemic at a local or regional level, including over a prolonged course of fractionated radiation therapy, (2) variation in the nature of resource constraints and the need for prioritization between disease sites and hospital departments, and (3) limited data to personalize treatment decisions based on an individual patient’s risk from exposure to SARS-CoV-2 infection, because preliminary information only became available after the peak of the pandemic.8
Practice recommendations included the deferral of treatments for those at presumed lowest risk of cancer progression (eg, slowly growing cancers, or select cases of adjuvant radiation therapy) or where potentially reasonable alternatives (eg, extended duration of neoadjuvant hormone therapy) could be used for a limited period as a temporizing measure. Many suggested the use of hypofractionated radiation therapy schedules (those that are shorter overall but give a larger dose per fraction) to reduce patient exposures and optimize use of limited resources. These recommendations were frequently based on a theory of “shorter is better.” Although for some radiation therapy indications published trials have demonstrated that hypofractionation is a standard of care,9 data to support hypofractionation are not available for all sites and/or indications. Therefore, shorter courses were sometimes recommended to truncate treatment courses for indications for which data were lacking, perhaps with allowances that compromises were needed in the face of unavoidable circumstances.
Whether the rapid pace of information exchange, facilitated by social media outlets, professional society dissemination, and markedly accelerated peer review and publication processes,10 has resulted in a lower quality of research or practice recommendations is unknown.11 , 12 From the scientific perspective, expressions of editorial concern about duplicate reporting and high-profile retractions have raised doubts about the reproducibility and durability of aspects of the COVID-era research production.13, 14, 15, 16 However, the selection of a radiation therapy dose fractionation schedule is fundamental to high-quality cancer care, and the particular body of COVID-era recommendations for hypofractionation may stand as one of the enduring creations of the pandemic having lasting effect.
This project assessed the quality of treatment recommendations produced on the topic of hypofractionation during the COVID-19 era, directed at multiple objectives: first, to establish and disseminate an evidence base around this phenomenon for future reference; second, to comment on aggregate changes in the quality of evidence of these recommendations, either positive or negative, between and within disease groupings; and third, to highlight aspects of changes in quality requiring further, considered study post-COVID.
Methods and Materials
Literature search
With the intention of identifying all recommendations related to fractionation published during the early COVID-19 pandemic, a literature search was conducted in PubMed/MEDLINE for the terms “COVID” and “radiotherapy” to retrieve all relevant English-language articles appearing through June 1, 2020 (Literature Search and Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA] flow diagram, Appendix E1). An initial screening of titles and abstracts identified candidate publications and excluded duplicates or those that were clearly irrelevant (C.E., H.S.).
Brachytherapy and proton therapy recommendations were excluded due to their extremely limited appearance in this scientific literature and the lack of alteration from nonpandemic guidelines. The remaining publications were fully reviewed to identify any containing a recommendation of a specific schedule of radiation fractionation for a cancer or benign tumor condition (C.E., H.S., T.T., S.Y.), including treatments with palliative intent. This project did not address recommendations related to delay or omission of radiation therapy or adjustment of aspects of multidisciplinary care. Articles containing recommendations related to logistics of radiation therapy operations or COVID crisis management were excluded.
In addition, a manual search for articles in press that had not yet been indexed in PubMed/MEDLINE included the following radiation oncology-specific journals: International Journal of Radiation Oncology, Biology, Physics; Radiotherapy and Oncology; Practical Radiation Oncology; Advances in Radiation Oncology; and Clinical Oncology. Articles found to be in press were added if they contained fractionation recommendations and had not been captured by the initial search (C.E., S.Y.).
Finally, the websites of national or international organizations were searched for practice statements or other resources that included any unique COVID-specific fractionation recommendations. These organizations included the Royal College of Radiologists UK, National Institute for Health and Care Excellence, Royal Australian and New Zealand College of Radiologists, American Society for Radiation Oncology, European Society for Radiotherapy and Oncology, National Comprehensive Cancer Network, American Society of Clinical Oncology, International Lymphoma Radiation Oncology Group, and European Society for Paediatric Oncology. If a fractionation schedule was found in a document that had not previously been captured in the literature search, the publication was added to the search and the schedule was added to the list of regimens (C.E., S.Y.).
From this study set, all of the fractionation recommendations recommended in any of these publications were recorded, noting the number of publications in which each schedule had been mentioned and the references pertinent to each fractionation.
Rating procedures
An international team was assembled that included 20 disease site experts, of whom 1 to 3 were assigned to each disease group. Experts assigned to each group were asked to provide routinely used fractionation schedule(s) considered to be at the highest level of evidence for each specific condition in question and to provide references justifying their designation (Table E1).
The selected experts graded the quality of the evidence using the American Society of Radiation Oncology (ASTRO) classification.17 The ASTRO scale defines 4 levels of quality of evidence: high, moderate, low, and expert opinion. To be designated high quality, the fractionation schedule had to be supported by 2 or more well-conducted and highly generalizable randomized clinical trials or meta-analyses of such trials. Specifically for this project, a rating of high quality required intentional comparison of the attributes of that schedule’s fractionation as a randomization versus another fractionation schedule (eg, if the schedule was only incidentally used in 1 of the arms of the trial, the study was classified as observational, hence automatically reducing the quality level of the evidence supporting that schedule).
Experts named and graded the highest-quality schedule or schedules known to be routinely used for the specific clinical condition and graded all of the alternative or proposed fractionation schedules recommended for that clinical condition in publications identified from the COVID-era literature search. Experts first rated each of the fractionation schedules in isolation and then convened by assigned disease sites to determine a consensus quality score for the evidence supporting each fractionation schedule (Table E1). Each disease group also had the option of nominating schedules deemed most worthy of further development or dissemination.
Statistical analyses
Descriptive statistics were used for process metrics related to the number of participants, numbers of publications and recommendations evaluated, and the quality of evidence scores. Contingency tables with χ2 tests were used to evaluate the distribution of the quality of evidence of the highest-rated schedules compared with that of the COVID-era schedules. Analysis of variance methods were used to determine differences between disease groups.
Scatter regression plots were used to visualize the overall changes in quality from the highest-quality schedules for specific clinical scenarios to the quality of evidence of the alternative schedules proposed in the pandemic-era literature. The ASTRO quality scores were converted to an integer scale with units of 1, and regression lines were based on plotted shifts for each disease group. A diagonal slope of 1 represented no change in the quality of evidence from “pre-COVID” to “in-COVID.” Pooled t tests were used to measure differences of the regression slopes from 1.
The shifts in the quality of evidence from “pre-COVID” to the highest-ranked “in-COVID” site-specific recommendations were compared. The disease sites with less substantial shifts were compared with those with greater changes in quality using the adjusted χ2 test. Differences between disease sites were further compared using a weighted shift based on the “pre-COVID” evidence quality and the levels of evidentiary shift to the “in-COVID” ranking, with significance determined by the adjusted χ2 test. The weights were assigned according to a progressive hierarchy of the shifts—high to opinion, high to low, high to moderate, moderate to opinion, moderate to low, and low to opinion—receiving a numerical value from 6 to 1, respectively. Top-weighted shifts were compared with low-weighted shifts around the median. Significance for all tests was assessed at a P value < .05.
This study was granted exempt status (#20-30633) by the Institutional Review Board of the University of California, San Francisco.
Results
Literature search
The literature search was conducted without a start date and last run on June 1, 2020. The search retrieved a total of 238 articles. From February to May 2020, 2, 16, 89, and 110 articles appeared in the literature in each respective month, with another 21 preindexed for June. Of these, 36 were reviewed and found to be relevant to radiation therapy dose fractionation, and an additional 18 publications were found by manual searches, including 1 article in press and 17 practice recommendations or related resources issued by the National Comprehensive Cancer Network, the Royal College of Radiologists, and the Royal Australian and New Zealand College of Radiologists. In total, 54 selected publications were included as the evidence base for this systematic review. All radiation therapy dose fractionation schedules recommended in these publications are provided in Table E1.
Systematic review of “pre-COVID” and “in-COVID” evidence quality
Twenty panelists divided among 14 disease groups named the schedules with the highest level of evidence for each clinical indication and rated the quality of evidence supporting 233 recommended COVID-19–adapted dose fractionation schedules. The 14 disease sites and the respective number of COVID-19–adapted fractionation schedules (curative + palliative) were breast (28 + 3), central nervous system (CNS) (13 + 5), cutaneous (inclusive of melanoma [3 + 4] and nonmelanoma [11 + 3]), lung (22 + 8), upper gastrointestinal (UGI) (14 + 8), lower gastrointestinal (LGI) (6 + 3), genitourinary (GU) (15 + 3), gynecology (1 + 6), head and neck (11 + 7), hematologic (0 + 8), lymphoma (10 + 5), pediatrics (19 + 0), general palliative (0 + 11), and sarcoma (6 + 0). Aggregated quality ratings were generally grouped as disease site–specific curative, disease site–specific palliative, general palliative (eg, for bone metastases), and curative-intent cutaneous radiation therapy schedules (because skin cancer treatments often involve superficial, orthovoltage, or electron techniques, and there is frequent routine use of hypofractionation). The individual panelists’ scores and the disease group’s consensus scores for each of the dose fractionation schedules are cataloged in Table E1.
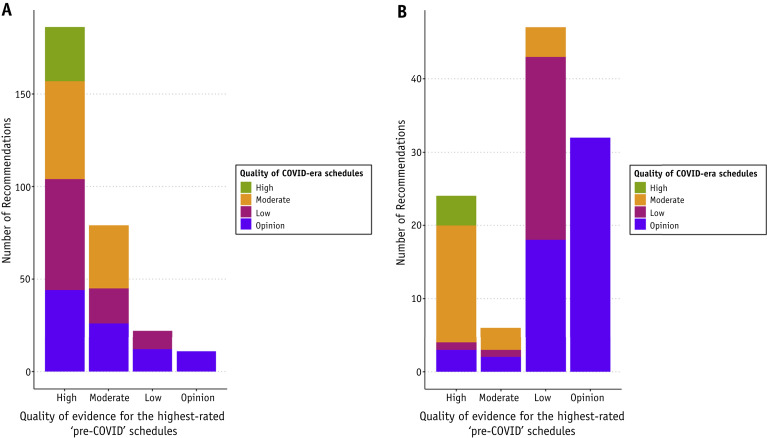
For site-specific curative and site-specific palliative schedules, there was a significant shift in the publishing record from established higher-quality evidence schedules to lower-quality evidence and opinions for schedules recommended in the COVID era (Figs. 1 A, B; P = .022 and P < .001, respectively). For curative-intent schedules, the overall distribution of quality scores was essentially reversed for the highest levels of evidence "pre-COVID" versus "in COVID": high quality, 51.4% versus 4.8% and expert opinion, 5.6% versus 49.3% (Table 1 ). Cutaneous curative-intent radiation therapy was an outlier because schedules commonly used before the COVID era were all already at a low-quality evidence level, with pandemic recommendations consistently ranked even further downward to the level of opinion (Fig. E1). Although this shift was significant due to the consistent pattern (P = .008), it was attributable to the pre-existing low quality of evidence rather than indicative of a major shift in quality. Conversely, for the general palliative schedules, there was high-quality evidence supporting the use of hypofractionated radiation therapy as standard, with 5 of 11 schedules rated as high quality, including the use of a single fraction for spinal cord compression and bone metastases. Because these schedules were already hypofractionated before the COVID era, there was no impetus for alteration, and the quality level was unchanged (Fig. E2; P = .09).
Fig. 1.
(A) Curative-intent recommendations: number of COVID-era recommendations grouped by quality of evidence, plotted against the quality of evidence of the corresponding routinely used highest-quality schedule (P = .022). Site-specific palliative, general palliative, and cutaneous recommendations are excluded. (B) Site-specific palliative recommendations: number of COVID-era recommendations grouped by quality of evidence, plotted against the quality of evidence of the corresponding routinely used highest-quality schedule (P < .001).
Table 1.
Percentages of consensus scores ranking the quality of evidence of the highest-rated routinely used fractionation schedules compared with the recommended COVID-era schedules for curative and palliative treatments
| ASTRO quality of evidence | Curative |
Curative, cutaneous |
Palliative, disease-site specific |
Palliative, general |
||||
|---|---|---|---|---|---|---|---|---|
| Highest quality | COVID era (N = 146) | Highest quality | COVID era (N = 14) | Highest quality | COVID era (N = 65) | Highest quality | COVID era (N = 9) | |
| High | 51.4% | 4.8% | 0% | 0% | 16.1% | 1.5% | 55.6% | 33.3% |
| Moderate | 33.3% | 17.1% | 0% | 0% | 5.4% | 13.9% | 11.1% | 33.3% |
| Low | 9.7% | 28.8% | 83.3% | 83.3% | 39.3% | 21.5% | 33.3% | 22.2% |
| Opinion | 5.6% | 49.3% | 16.7% | 16.7% | 39.3% | 63.1% | 0% | 11.1% |
Abbreviations: ASTRO = American Society of Radiation Oncology; N = number of recommended dose fractionation schedules.
For most disease groups, high-quality evidence supported the routine use of conventionally fractionated radiation therapy. There was moderate-to-low quality evidence supporting conventionally fractionated regimens for some gastrointestinal indications, such as borderline or inoperable pancreatic cancers (25-30 fractions of 2 Gy, moderate-quality evidence) or preoperative esophageal cancer (28 fractions of 1.8 Gy, moderate-quality evidence) and anal cancer (28-30 fractions of 1.8 Gy, low-quality evidence) if treated without concurrent chemotherapy. On the other hand, there was high-quality evidence supporting hypofractionated schedules for specific indications such as intact prostate (20 fractions of 3 Gy),18 adjuvant breast (15-16 fractions of 2.67-2.66 Gy),19 , 20 preoperative rectal (5 fractions of 5 Gy),21, 22, 23, 24 and T1 larynx (28 fractions of 2.25 Gy) cancers,25 and glioblastoma in older or less fit patients (15 fractions of 2.67 Gy).26 , 27 Lastly, for some disease groups, there was lower-level evidence supporting hypofractionated treatments, including stereotactic body radiation therapy in a single fraction for peripheral T1-2 non-small cell lung cancer (NSCLC) (moderate-quality evidence),28 20 fractions of 2.75 Gy for organ preservation in bladder cancer (moderate-quality evidence),29 , 30 and various fractionations (eg, 10 fractions of 4 Gy, 15 fractions of 3.33 Gy, and 20 fractions of 2.75 Gy) for cutaneous cancers (low-quality evidence).
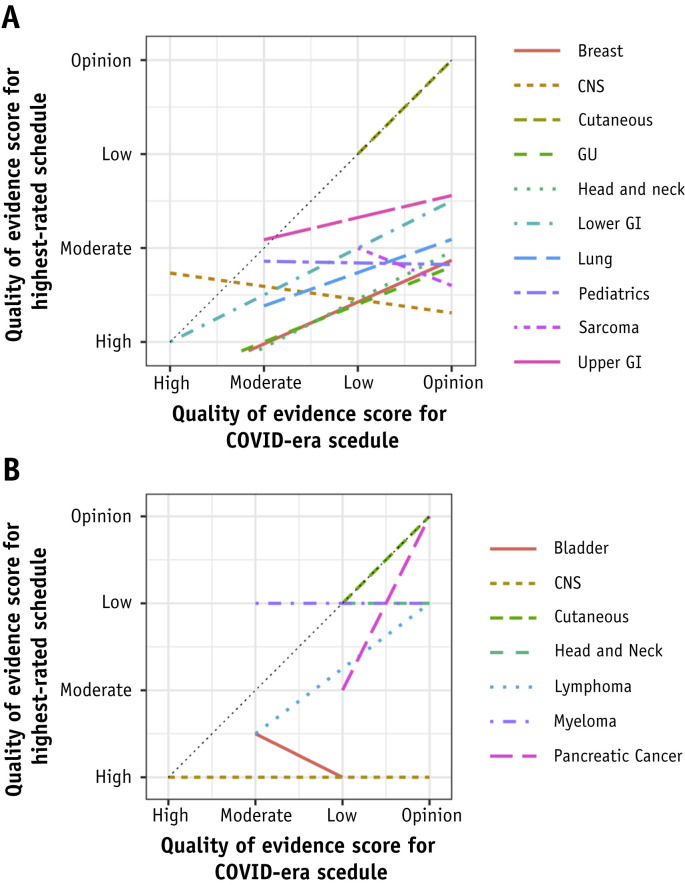
The overall shifts in the quality of evidence between the highest-rated fractionation schedules and the COVID-era recommended schedules were organized in scatter plots by disease groups (Fig. E3). These shifts were further mapped by disease groups in forest plots demonstrating the range of experts’ quality rankings for each of the COVID-era schedules (Fig. E4). The overall differences in shifts across disease groups were found to be not significant across the curative-intent disease groupings (P = .422) but were significant for the disease-specific palliative groupings (P = .005). However, the difference between curative-intent disease groupings with shifts of ≤1 (lower and upper GI, renal) from those with shifts >1 was significant (P = .001).
In scatter regression plots, the diagonal with a slope of 1 represented no change in the evidence level from “pre-COVID” to “in-COVID” and was visually compared against the regression slope for each disease group (Figs. E5 and E6). All plot points for curative-intent schedules (except cutaneous) fell below the diagonal, confirming a universal shift to a lower quality of evidence (P value < .01) (Fig. 2 A). Sarcoma, CNS, and pediatrics showed the most negative slopes, but relatively reduced slopes were seen for upper GI, lung, lower GI, GU, breast, and head and neck. Results for site-specific palliative schedules were mixed, but all were located on or inferior to the diagonal with lung and head and neck sloping negatively (Fig. 2B).
Fig. 2.
(A) Curative-intent (including cutaneous) consensus scores: multiple regression lines, each representing a disease group, of the shifts in the quality of evidence from routinely used highest-quality curative-intent schedules to COVID-era schedules. Dotted black line (slope of 1) represents no change in the quality of evidence. Paired t test comparing the regression lines’ slopes to the diagonal slope of 1 was significant (P < .01). Lines are truncated to avoid extrapolation outside of known data points. (B) Site-specific palliative consensus scores: multiple regression lines, each representing a disease group, of the shifts in the quality of evidence from routinely used highest-quality, site-specific palliative schedules to COVID-era schedules. Dotted black line (slope of 1) represents no change in the quality of evidence. Paired t test comparing the regression lines’ slopes to the diagonal slope of 1 showed mixed results (eg, cutaneous had a slope of 1 and CNS had a slope of 0). Lines are truncated to avoid extrapolation outside of known data points. Abbreviations: CNS = central nervous system; GI = gastrointestinal; GU = genitourinary.
For some disease groups, the recommendation to use shorter than routinely applied fractionated schedules was underpinned by high- to moderate-quality evidence, including those for adjuvant whole breast (eg, 5 fractions of 5.2 Gy),31 intact prostate (eg, 7 fractions of 6.1 Gy or stereotactic body radiation therapy at 5 fractions of 7.25-8.0 Gy),32 , 33 salvage prostate (20 fractions of 2.625 Gy),34 and NSCLC (20 fractions of 2.75 Gy).35 For others, the shift was from high- to low-quality evidence (eg, head and neck cancer, 20 fractions of 2.75 Gy36 , 37 or 30 fractions of 2.17 Gy38; or limited-stage small cell lung cancer, 15-16 fractions of 2.67-2.81 Gy39 , 40) or from high-quality evidence to expert opinion (eg, glioblastoma, grade III glioma, or low-grade glioma, 15 fractions of 2.67 Gy).
Because there was a nonlinear relationship between the integral change in the quality of evidence and the potential significance of the evidentiary shift (eg, high to moderate vs low to opinion or high to low vs moderate to opinion), we also assessed disease sites by weighted shifts based on the highest-quality schedule’s score and the number of levels of shift separating it from the COVID-era recommendation (Figs. E7 and E8). For curative radiation therapy, there was heterogeneity in the highest levels of evidence within disease group sites (eg, preoperative rectal vs anal cancer) or indications (eg, adjuvant whole breast vs adjuvant breast boost or head and neck definitive vs head and neck postoperative), and between the groups there was variation in the magnitude and weight of the shifts to the COVID-era quality of evidence. Less impact was seen for disease groups already having a lower quality of evidence for their highest-ranked schedules (eg, upper and lower GI) or those that experienced minimal change (eg, pediatrics), and more for those with a pre-existing higher quality of evidence that resulted in larger decreases toward the COVID-era quality of evidence (eg, lymphoma, GU, CNS, HN, and breast). The distribution of weighted shifts falling below the median value of 3 (gynecologic, hematologic, lower GI, pediatrics, renal, and upper GI) versus those above was significant (P < .00001). For disease site–specific palliative schedules, the change associated with the COVID-era schedules was less, because of the lower quality of pre-existing evidence. Exceptions to this generalization were GU (bladder) and CNS, in which there were notable COVID-era reductions in quality for site-specific palliative recommendations due to the pre-existing high-quality evidence for palliative schedules.
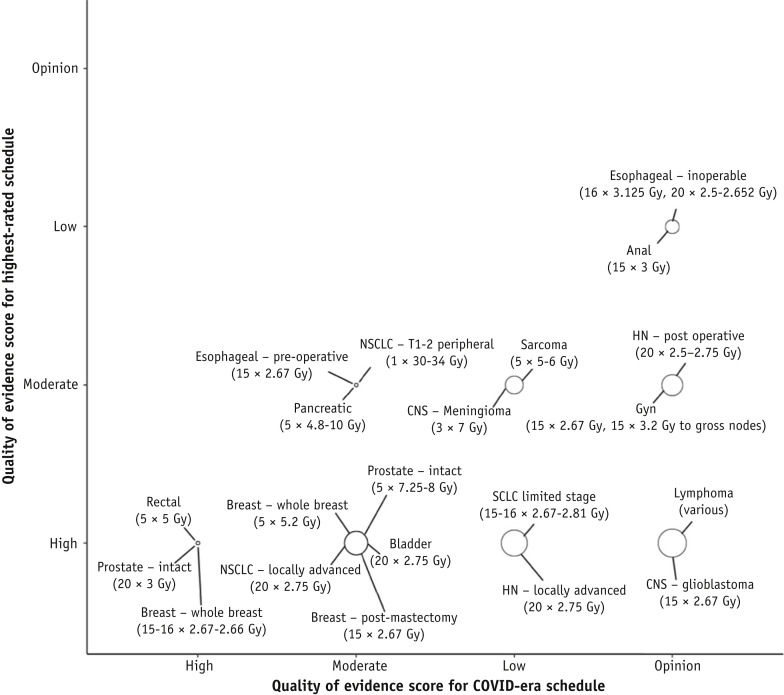
Due to the heterogeneity of the surveyed curative-intent scenarios, we also assessed disease-specific shifts in evidence quality by comparing the highest-quality “pre-COVID” schedules to the highest-rated and most frequently recommended (1-10 recommendations; Fig. E9) COVID-era hypofractionated schedules for a specific cancer condition (Fig. 3 ). This analysis was not meant to designate preferred or endorsed schedules (the intent was not to conflate frequency of recommendation with quality of evidence) but to test for differences in evidence shifts that might separate the most commonly recommended schedules. There were no differences among weighted shifts overall for these chosen specific indications, but the distribution of the indications having weighted shifts above and below 3 was significant (P < .0001). It was apparent that for some indications, such as limited-stage small cell lung cancer, locoregionally advanced head and neck cancer, glioblastoma, and lymphoma, there were substantial declines in the quality of the evidence in the COVID era. On the other hand, for adjuvant whole breast or intact prostate cancer treatments, there was pre-existing high-quality evidence supporting 3- to 4-week schedules (which are routinely used in some parts of the world) and moderate-quality evidence for 1-week schedules, such that the shifts from the highest-quality schedules to COVID-era schedules were minimal.
Fig. 3.
Curative-intent consensus scores: shifts in the quality of evidence from the highest-quality curative-intent schedules to the highest-rated and most frequently recommended COVID-era schedules within each disease site. The size of the bubble is proportional to the weight of the shift, with the weight determined from a 6-point scale incorporating the highest-ranked schedule’s quality of evidence and the number of levels of shift separating it from the COVID-era recommendation. Variance among the weights was not significant (P = .074), but the difference above and below the median of 3 was significant (P < .0001). Abbreviations: CNS = central nervous system; Gyn = gynecologic; HN = head and neck; NSCLC = non-small cell lung cancer; SCLC = small cell lung cancer.
Among some groups, panelists demarcated certain schedules as worthy of further development or dissemination if not already part of the standard of care (Table E1). Numerous fractionation schedules were deemed highly acceptable in the treatment of breast cancer (eg, 15 fractions of 2.67 Gy for various scenarios or 5 fractions of 5.2 Gy postoperatively). Other schedules pertained to specific groups, such as older or less fit patients (eg, 15 fractions of 2.67 Gy for glioblastoma and 8 fractions of 5 Gy for definitive skin cancer therapy). Some groups noted a need for testing of hypofractionation in combination with chemotherapy (eg, 20 fractions of 2.75 Gy for definitive-intent head and neck and NSCLC treatments). Although some disease groups declined to nominate specific schedules, participants expressed a universal sentiment that evidence-based assessment should be the basis of any such process.
Discussion
To safeguard treatment capacity from staff shortages and mitigate the risk of patient infection by SARS-CoV-2 from daily hospital attendances, a large number of publications early on in the COVID-19 pandemic recommended consideration of hypofractionated radiation therapy under the premise that “shorter is better.”7 , 41 The unpredictable nature of the pandemic and the prolonged course of most radiation therapy treatments meant that these schedules were often proposed in advance of an actual critical resource constraint or surge in SARS-CoV-2 community prevalence. Early in the pandemic, the potential impacts on cancer care could not be predicted given the lack of evidence to guide any formalized risk assessment. This systematic review was aimed at a large-scale evaluation of this phenomenon, assessing across all disease groups the shifts in the quality of evidence for these “in-COVID” recommended schedules compared with those defined by experts to be in routine use in the “pre-COVID” era that were supported by the highest quality of evidence. A schedule at the highest quality of evidence was considered to be the most objective reference point that would be independent of the variation and subjectivity that might be incurred in defining a “standard of care,” which did not clearly exist for some specific disease indications.
Over the course of just a few months during the early and peak pandemic, 54 published articles from the radiation therapy community specifically recommended the use of hypofractionation during COVID-19. The large number of articles and speed of publication were notable and resulted from the perception of an urgent need to support radiation oncologists, many of whom may have been unfamiliar with the use of such schedules or the evidence supporting them, particularly in an international context. The resulting body of literature as summarized in this paper laid out a large menu of dose fractionation schedules from the worldwide radiation therapy community, but the range of the underlying evidence base for these recommendations varied widely from randomized trials to opinion. Because these publications constitute a major scholarly response of the radiation therapy community to the COVID-19 pandemic, it is important to critically evaluate this literature in light of the consistently stronger evidence that has long supported historically accepted conventional regimens.
For some disease sites such as head and neck cancer and high-grade glioma, there were large shifts from high- to low-quality evidence or expert opinion, which was acknowledged in published consensus statements, leading to advice in most cases to maintain standard practices except where impossible due to severely reduced resources.42 , 43 For pediatric cancers, in which the mitigation of late effects is a priority, there was only low-quality evidence or opinion supporting the use of hypofractionated radiation therapy. This was reflected in a practice recommendation to continue standard treatments, reserving hypofractionated radiation therapy for selected poor-prognosis patients for whom radiation therapy could not be safely deferred.44
For other situations, including adjuvant whole breast and intact prostate treatments, there was pre-existing high-quality evidence to support the recommended use of moderate hypofractionation over 3 and 4 weeks, respectively, and moderate evidence to support further shortening over 1 week. In the setting of resource constraints, such recommendations have the potential for greatest impact because these are high-volume cancers. For example, adjuvant whole or partial breast radiation therapy may account for approximately 30% of all delivered fractions within a radiation therapy department,45 , 46 and the use of 15 to 1620 , 47 or 5 fractions31 , 48 , 49 instead of 25 fractions would reduce the overall demand for delivered fractions by an estimated 10% to 25%.
Within disease sites, there was some variation in the quality of evidence across specific clinical scenarios, partly influenced by restricted access to operating rooms or rationing of surgical resources during the pandemic, resulting in the need for alternative treatments as a temporizing measure before surgery (eg, preoperative whole breast radiation therapy, where there is no option for preoperative systemic therapy, low-quality evidence).50, 51, 52 In some cases there was variation within the same disease-specific indication, where there were recommended hypofractionated schedules supported by higher- or lower-quality evidence. Examples of such situations and the schedules with lower-quality evidence or expert opinion included adjuvant partial breast (4.0 Gy × 10 fractions, expert opinion; 7.0-8.8 Gy × 5 alternating daily fractions for radiation therapy alone, expert opinion; 3.0 Gy × 18 fractions, expert opinion),42 , 53 , 54 NSCLC (2.5 Gy × 20 fractions for sequential chemoradiation therapy, expert opinion),55 and intact prostate cancer (2.7 Gy × 26 fractions, low-quality evidence).56 , 57
This systematic evaluation of hypofractionated schedules by standardized quality of evidence ratings informs comparisons between treatments and provides evidence-based assessment of potentially practice-changing recommendations. This project’s compilation of all recommended fractionation schedules from the early and peak pandemic will serve as a useful reference. At a time of constrained resources and on a policy-making level, this evidence-based assessment enables prioritization decisions between and within disease sites; for the practitioner, it supports individual treatment discussions and informed consent processes with patients. In a situation of adequate resources but risk mitigation, decision-making processes for an individual patient would likely require a more deliberative conversation than might be possible in a situation of resource inadequacy. It should also be noted that our project found that in some cases, quality ratings may support the use of hypofractionated regimens over current commonly used regimens.
A potential lesson learned from the COVID-19 pandemic is the value of coordinating and integrating responses from the worldwide radiation therapy community to provide rationalization and harmonization of recommendations. In this respect, there are limitations to this particular work: the literature search had to be stopped before the pandemic had truly ceased and was limited only to English-language publications, and the schedules were rated by a relatively small number of disease specialists using only 1 system from ASTRO. In addition, the COVID-era literature itself may be incomplete and not represent a true catalog of all the best hypofractionated regimens, and the level of peer review may have been less rigorous than in usual times, especially for urgently issued practice statements and editorials. In this project, analyses were necessarily in aggregate without detailed focus on the nuances of specific disease conditions.
A lesser emphasis of this work was to consider which of the suggested dose fractionation schedules might be worthy of further study or dissemination. It is important to recognize that although the COVID-era schedules were generally concerned with shortening treatment time while maintaining similar levels of local tumor control (isoeffectiveness), future studies might also be concerned with dose intensification or isotoxicity. The COVID-era literature is not exhaustive of all schedules under investigation; for example, ongoing trials are evaluating the use of hypofractionated schedules to address resource constraints48 and patient convenience.49 Some disease group panelists opted not to select particular schedules for further study, but all commented on the importance of systematic evaluation of the outcomes of patients treated during the COVID-19 pandemic to inform clinical practice and the design of future research.
It is unclear to what extent the international oncology community has actually implemented practice-changing recommendations based on lower levels of evidence. It is also unknown whether individual oncologic or toxicity outcomes were compromised for purposes of risk mitigation or to manage constrained resources. The real-world application of hypofractionated schedules during the pandemic and any impact on patient outcomes will be a subject of future work. Examples of such initiatives include the National Cancer Research Institute’s COVID RT registry in the United Kingdom,58 the COVID-19 and Cancer Consortium (CCC19) registry, the National Cancer Institute's COVID-19 in Cancer Patients Study (N-CCaPS), and the American Society of Clinical Oncology’s Survey on COVID-19 in Oncology Registry, supported by ASTRO in the United States.59 We should harness the tangible observed benefits of collaboration and rapid research outputs to streamline and accelerate innovation. There may be novel opportunities to learn from patients treated with nonstandard dose fractionations during the COVID-19 pandemic, either to set aside certain fractionation practices or to inform future rational clinical trial designs. These data could also be used to support radiation therapy practices in the event of second waves or surges of the pandemic in new regions of the world.
Footnotes
Note—An online CME test for this article can be taken at https://academy.astro.org.
David J. Thomson, Sue S. Yom, and Hina Saeed made equal contributions to this study.
A.C., D.J.T., and P.H. are supported by NIHR Manchester Biomedical Centre. C.E.C. is supported by NIHR Cambridge Biomedical Centre. D.J.T. is supported by a grant from the Taylor Family Foundation and Cancer Research UK. S.M. is partly supported by the NIHR Oxford Biomedical Center.
Disclosures: S.T.C. reports personal fees from Varian Medical Systems, outside the submitted work. A.C. reports grants from the National Institute of Health Research, Manchester Biomedical Research Centre, Cancer Research, UK, Medical Research Council, UK, Prostate Cancer, UK, and Bayer, UK; personal fees from Janssen Pharmaceutical; nonfinancial support from ASCO; and grants and nonfinancial support from Elekta AB, outside the submitted work. I.E.N. reports grants from the National Institutes of Health, Endectra LLC, and Resero AI LLC, outside the submitted work. C.E. reports other from Novocure, outside the submitted work. M.G. reports grants from Varian and AstraZeneca and personal fees from AstraZeneca, outside the submitted work. M.S.K. owns common stock in Dr Reddy’s Laboratories, Healthcare Services Group, Mazor Robotics, and U.S. Physical Therapy. S.K.J. reports grants, personal fees, and nonfinancial support from Merck & Co, outside the submitted work. S.S.Y. reports grants from Genentech, Bristol-Myers Squibb, Merck, and BioMimetix and personal fees from Springer and UpToDate, outside the submitted work.
All data generated and analyzed during this study are included in this published article (and its supplementary information files).
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2020.06.054.
Supplementary Data
References
- 1.World Health Organization Naming the coronavirus disease (COVID-19) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it Available at:
- 2.World Health Organization Coronavirus disease (COVID-19). Situation report-137. https://www.who.int/docs/default-source/sri-lanka-documents/20200605-covid-19-sitrep-137.pdf?sfvrsn=a13df572_2 Available at:
- 3.COVID-19 primer. https://covid19primer.com/dashboard Available at:
- 4.Xing W., Hejblum G., Leung G.M., et al. Anatomy of the epidemiological literature on the 2003 SARS outbreaks in Hong Kong and Toronto: A time-stratified review. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000272. e1000272-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasinski E. News opinion journals open access to coronavirus resources. https://www.the-scientist.com/news-opinion/journals-open-access-to-coronavirus-resources–67105 Available at:
- 6.Filippi AR, Russi E, Magrini SM, et al. Letter from Italy: First practical indications for radiation therapy departments during COVID-19 outbreak [e-pub ahead of print]. Int J Radiat Oncol Biol Phys. 10.1016/j.ijrobp.2020.03.007. Accessed July 7, 2020. [DOI] [PMC free article] [PubMed]
- 7.Achard V., Tsoutsou P., Zilli T. Letter from Switzerland. Int J Radiat Oncol Biol Phys. 2020;107:600–601. doi: 10.1016/j.ijrobp.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai M., Liu D., Liu M., et al. Patients with cancer appear more vulnerable to SARS-CoV-2: A multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dearnaley D., Syndikus I., Mossop H., et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047–1060. doi: 10.1016/S1470-2045(16)30102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.COVID-19 rapid communications. https://www.sciencedirect.com/journal/radiotherapy-and-oncology/special-issue/1086W2WWCC6 Available at:
- 11.Flynn J.F. Science has an ugly, complicated dark side. And the coronavirus is bringing it out. Mother Jones. https://www.motherjones.com/politics/2020/04/coronavirus-science-rush-to-publish-retractions/ Available at:
- 12.Dagens A., Sigfrid L., Cai E., et al. Scope, quality, and inclusivity of clinical guidelines produced early in the Covid-19 pandemic: Rapid review. BMJ. 2020;369:m1936. doi: 10.1136/bmj.m1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehra M.R., Desai S.S., Kuy S., et al. Retraction: Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020;382:2582. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Mehra M.R., Ruschitzka F., Patel A.N. Retraction—Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis. Lancet. 2020;395:1820. doi: 10.1016/S0140-6736(20)31324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauchner H., Golub R.M., Zylke J. Editorial concern—Possible reporting of the same patients with COVID-19 in different reports. JAMA. 2020;323:1256. doi: 10.1001/jama.2020.3980. [DOI] [PubMed] [Google Scholar]
- 16.Tingley K. Coronavirus is forcing medical research to speed up. New York Times. 2020 2020/04/16/;Sect.
- 17.Patton C., Bradfield L. ASTRO clinical practice guideline methodology guide. https://www.astro.org/ASTRO/media/ASTRO/Patient%20Care%20and%20Research/PDFs/ASTRO_GuidelineMethodology.pdf Available at:
- 18.Catton C.N., Lukka H., Gu C.S., et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35:1884–1890. doi: 10.1200/JCO.2016.71.7397. [DOI] [PubMed] [Google Scholar]
- 19.START Trialists’ Group. Bentzen S.M., Agrawal R.K., et al. The UK standardisation of breast radiotherapy (START) trial B of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet. 2008;371:1098–1107. doi: 10.1016/S0140-6736(08)60348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whelan T.J., Pignol J.P., Levine M.N., et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 21.Bujko K., Nowacki M.P., Nasierowska-Guttmejer A., et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215–1223. doi: 10.1002/bjs.5506. [DOI] [PubMed] [Google Scholar]
- 22.Ngan S.Y., Burmeister B., Fisher R.J., et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol. 2012;30:3827–3833. doi: 10.1200/JCO.2012.42.9597. [DOI] [PubMed] [Google Scholar]
- 23.Erlandsson J., Holm T., Pettersson D., et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): A multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017;18:336–346. doi: 10.1016/S1470-2045(17)30086-4. [DOI] [PubMed] [Google Scholar]
- 24.Bujko K., Wyrwicz L., Rutkowski A., et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: Results of a randomized phase III study. Ann Oncol. 2016;27:834–842. doi: 10.1093/annonc/mdw062. [DOI] [PubMed] [Google Scholar]
- 25.Yamazaki H., Nishiyama K., Tanaka E., et al. Radiotherapy for early glottic carcinoma (T1N0M0): Results of prospective randomized study of radiation fraction size and overall treatment time. Int J Radiat Oncol Biol Phys. 2006;64:77–82. doi: 10.1016/j.ijrobp.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Roa W., Brasher P.M., Bauman G., et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: A prospective randomized clinical trial. J Clin Oncol. 2004;22:1583–1588. doi: 10.1200/JCO.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 27.Malmström A., Grønberg B.H., Marosi C., et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 28.Singh A.K., Gomez-Suescun J.A., Stephans K.L., et al. One versus three fractions of stereotactic body radiation therapy for peripheral stage I to II non-small cell lung cancer: A randomized, multi-institution, phase 2 trial. Int J Radiat Oncol Biol Phys. 2019;105:752–759. doi: 10.1016/j.ijrobp.2019.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall E., Hussain S.A., Porta N., et al. BC2001 long-term outcomes: A phase III randomized trial of chemoradiotherapy versus radiotherapy (RT) alone and standard RT versus reduced high-dose volume RT in muscle-invasive bladder cancer. J Clin Oncol. 2017;35(6_suppl):280. [Google Scholar]
- 30.James N.D., Hussain S.A., Hall E., et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366:1477–1488. doi: 10.1056/NEJMoa1106106. [DOI] [PubMed] [Google Scholar]
- 31.Murray Brunt A., Haviland J.S., Wheatley D.A., et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020;395:1613–1626. doi: 10.1016/S0140-6736(20)30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Widmark A., Gunnlaugsson A., Beckman L., et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394:385–395. doi: 10.1016/S0140-6736(19)31131-6. [DOI] [PubMed] [Google Scholar]
- 33.Jackson W.C., Silva J., Hartman H.E., et al. Stereotactic body radiation therapy for localized prostate cancer: A systematic review and meta-analysis of over 6,000 patients treated on prospective studies. Int J Radiat Oncol Biol Phys. 2019;104:778–789. doi: 10.1016/j.ijrobp.2019.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chin S., Fatimilehin A., Walshaw R., et al. Ten-year outcomes of moderately hypofractionated salvage postprostatectomy radiation therapy and external validation of a contemporary multivariable nomogram for biochemical failure. Int J Radiat Oncol Biol Phys. 2020;107:288–296. doi: 10.1016/j.ijrobp.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Maguire J., Khan I., McMenemin R., et al. SOCCAR: A randomised phase II trial comparing sequential versus concurrent chemotherapy and radical hypofractionated radiotherapy in patients with inoperable stage III non-small cell lung cancer and good performance status. Eur J Cancer. 2014;50:2939–2949. doi: 10.1016/j.ejca.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Tobias J.S., Monson K., Gupta N., et al. Chemoradiotherapy for locally advanced head and neck cancer: 10-year follow-up of the UK head and neck (UKHAN1) trial. Lancet Oncol. 2010;11:66–74. doi: 10.1016/S1470-2045(09)70306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benghiat H., Sanghera P., Cashmore J., et al. Four week hypofractionated accelerated intensity modulated radiotherapy and synchronous carboplatin or cetuximab in biologically staged oropharyngeal carcinoma. Cancer Clin Oncol. 2014;3 [Google Scholar]
- 38.Nutting C.M., Morden J.P., Harrington K.J., et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turgeon G.A., Souhami L., Kopek N., et al. Thoracic irradiation in 3 weeks for limited-stage small cell lung cancer: Is twice a day fractionation really needed? Cancer Radiother. 2017;21:89–98. doi: 10.1016/j.canrad.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 40.Sculier J.P., Lafitte J.J., Efremidis A., et al. A phase III randomised study of concomitant induction radiochemotherapy testing two modalities of radiosensitisation by cisplatin (standard versus daily) for limited small-cell lung cancer. Ann Oncol. 2008;19:1691–1697. doi: 10.1093/annonc/mdn354. [DOI] [PubMed] [Google Scholar]
- 41.Simcock R., Thomas T.V., Estes C., et al. COVID-19: Global radiation oncology’s targeted response for pandemic preparedness. Clin Transl Radiat Oncol. 2020;22:55–68. doi: 10.1016/j.ctro.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomson D.J., Palma D., Guckenberger M., et al. Practice recommendations for risk-adapted head and neck cancer radiation therapy during the COVID-19 pandemic: An ASTRO-ESTRO consensus statement. Int J Radiat Oncol Biol Phys. 2020;107:618–627. doi: 10.1016/j.ijrobp.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernhardt D, Wick W, Weiss SE, et al. Neuro-oncology management during the COVID-19 pandemic with a focus on WHO grades III and IV gliomas [e-pub ahead of print]. Neuro Oncol. https://doi.org/10.1093/neuonc/noaa113. Accessed July 10, 2020. [DOI] [PMC free article] [PubMed]
- 44.Janssens G.O., Mandeville H.C., Timmermann B., et al. A rapid review of evidence and recommendations from the SIOPE radiation oncology working group to help mitigate for reduced paediatric radiotherapy capacity during the COVID-19 pandemic or other crises. Radiother Oncol. 2020;148:216–222. doi: 10.1016/j.radonc.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Rashdan A, Roumeliotis M, Quirk S, et al. Adapting radiation therapy treatments for patients with breast cancer during the COVID-19 pandemic: Hypo-fractionation and accelerated partial breast irradiation to address World Health Organization recommendations [e-pub ahead of print]. Adv Radiat Oncol. https://doi.org/10.1016/j.adro.2020.03.011. Accessed July 10, 2020. [DOI] [PMC free article] [PubMed]
- 46.Coles C.E., Aristei C., Bliss J., et al. International guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. Clin Oncol. 2020;32:279–281. doi: 10.1016/j.clon.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haviland J.S., Owen J.R., Dewar J.A., et al. The UK standardisation of breast radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 48.Whelan T.J., Julian J.A., Berrang T.S., et al. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): A randomised controlled trial. Lancet. 2019;394:2165–2172. doi: 10.1016/S0140-6736(19)32515-2. [DOI] [PubMed] [Google Scholar]
- 49.Vicini F.A., Cecchini R.S., White J.R., et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: A randomised, phase 3, equivalence trial. Lancet. 2019;394:2155–2164. doi: 10.1016/S0140-6736(19)32514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lightowlers S.V., Boersma L.J., Fourquet A., et al. Preoperative breast radiation therapy: Indications and perspectives. Eur J Cancer. 2017;82:184–192. doi: 10.1016/j.ejca.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 51.US National Library of Medicine NeoRad breast cancer study. https://clinicaltrials.gov/ct2/show/NCT04261244 Available at:
- 52.US National Library of Medicine Pre- versus postoperative accelerated partial breast irradiation in early stage breast cancer patients. https://clinicaltrials.gov/ct2/show/NCT02913729 Available at:
- 53.Braunstein LZ, Gillespie EF, Hong L, et al. Breast radiotherapy under COVID-19 pandemic resource constraints—Approaches to defer or shorten treatment from a comprehensive cancer center in the United States [e-pub ahead of print]. Adv Radiat Oncol. https://doi.org/10.1016/j.adro.2020.03.013. Accessed July 10, 2020. [DOI] [PMC free article] [PubMed]
- 54.Parashar B., Chen W.C., Herman J.M., et al. Disease site-specific guidelines for curative radiation treatment during ‘limited surgery’ and ‘hospital avoidance’: A radiation oncology perspective from the epicenter of COVID-19 pandemic. Cureus. 2020;12 doi: 10.7759/cureus.8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rathod S., Dubey A., Bashir B., et al. Bracing for impact with new 4R’s in the COVID-19 pandemic—A provincial thoracic radiation oncology consensus. Radiother Oncol. 2020;149:124–127. doi: 10.1016/j.radonc.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Resource Sparing Curative Radiotherapy for Locally Advanced Squamous Cell Cancer of the Head and Neck: The HYPNO Trial (HYPNO). Available at: https://clinicaltrials.gov/ct2/show/NCT02765503. Accessed July 29, 2020.
- 57.Shorter course, hypofractionated pre-surgery radiation therapy in treating patients with localized, resectable soft tissue sarcoma of the extremity of superficial trunk. Available at: https://clinicaltrials.gov/ct2/show/NCT03819985. Accessed July 29, 2020.
- 58.National Cancer Research Institute NCRI’s CTRad to lead COVID RT: A UK-wide initiative to study the impact of COVID-19 on radiotherapy services and patient outcomes. https://www.ncri.org.uk/news/covid19-radiotherapy-initiative/ Available at:
- 59.American Society of Clinical Oncology New COVID-19 cancer registry aims to understand impact on patients during pandemic, inform future care. https://www.asco.org/about-asco/press-center/news-releases/new-covid-19-cancer-registry-aims-understand-impact-patients Available at:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.