The coronavirus disease-2019 (COVID-19) pandemic has created unprecedented challenges in clinical research activities; its effects on ongoing clinical trials has not been yet quantified (1,2). In this report, we provide a description of the effect of the COVID-19 pandemic on ongoing clinical trials.
Historical metadata of all trials reported on ClinicalTrials.gov from January 1, 2017, to May 31, 2020, were queried by using Python (Python Software Foundation, Beaverton, Oregon). The months from January 1 to May 31, 2020, were defined as the COVID-19 pandemic period. Non–COVID-19–related trials were identified by excluding “COVID,” “coronavirus,” and “SARS-CoV-2” in trial titles. Stopping a trial was defined as a switch in trial status from “recruiting” to “active and not recruiting,” “completed,” “suspended,” “terminated,” or “withdrawn.” COVID-19 infection counts by country were obtained from the Johns Hopkins Coronavirus Resource Center. The number of infections was adjusted by population size and reported as the number of cases per million inhabitants. Correlations between the number of COVID-19 cases per million inhabitants and the number of trials were calculated by using Pearson correlation coefficients. Categorical variables are reported as counts and percentages and compared with the chi-square test. Poisson models were used to compare trends over time.
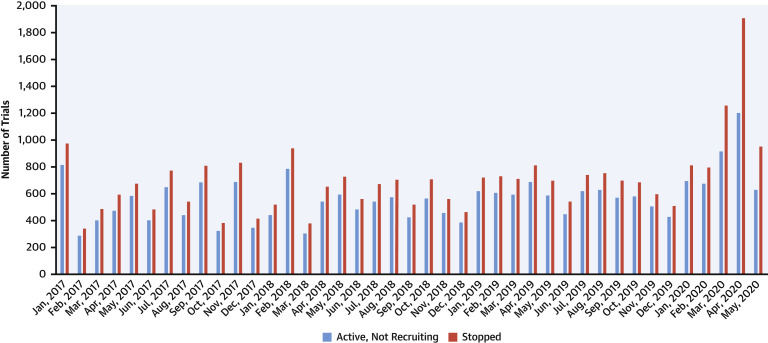
A total of 321,218 non–COVID-19–related trials were queried, of which 28,672 (8.9%) were reported as stopped. Of these, 22,934 trials were stopped from January 1, 2017, to December 31, 2019, at an average rate of 638 trials/month, and 5,758 were stopped from January 1, 2020, to May 31, 2020 (period of the COVID-19 global spread) at an average rate of 1,147 trials/month. During the period of the COVID-19 pandemic, the number of stopped trials increased significantly over time (p for trend <0.001) (Figure 1 ). The majority of the trials that were stopped during the COVID-19 pandemic had nongovernmental funding (95.4%). During the pandemic, the cumulative proportion of trials stopped by country ranged from 1.0% to 17.1%, weakly correlating with the national population–adjusted numbers of COVID-19 cases through the end of May 2020 (r = 0.21).
Figure 1.
Temporal Trends for Active But Not Recruiting and Stopped Trials on Clinicaltrials.gov From January 2017 to May 2020
We found that during the initial months of the COVID-19 pandemic, the rate of clinical trials that were stopped increased significantly compared with the pre-pandemic era. Of concern, the number of trials stopped per month increased significantly with time during the pandemic, suggesting that the consequences of the crisis may be worse than suggested by our data.
The COVID-19 pandemic has led to unprecedented challenges for clinical research activity. Mitigation efforts and social distancing as well as resource redeployment have disrupted essential aspects of clinical trial activity, including accrual and randomization, intervention delivery, and outcome collection (1).
A survey of 61 cardiac surgery units on 4 continents reported substantial modifications of research activity in almost one-quarter of them (3). One adult Cancer Center Network in the United Sates reported a decrease from 20% to 46% in enrollment during the pandemic (4), and a survey of the American Society of Clinical Oncology showed that during the crisis, nearly 60% of the units suspended research activity (5).
A key limitation of the present report is the possibility of a delay in the update of trial status on ClinicalTrials.gov; thus, these findings likely represent an underestimate of the true impact of the pandemic. In conclusion, the COVID-19 pandemic has had profound effects on active non-COVID-19 clinical trials. Long-term data will determine the full extent of the disruption the pandemic imposes on clinical research.
Footnotes
Please note: Dr. Bhatt serves on the Advisory Board of Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, PhaseBio, PLx Pharma, and Regado Biosciences; serves on the Board of Directors for Boston VA Research Institute, Society of Cardiovascular Patient Care, and TobeSoft; is chair of the American Heart Association Quality Oversight Committee; serves on Data Monitoring Committees for Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi-Sankyo), and Population Health Research Institute; receives honoraria from the American College of Cardiology (senior associate editor, Clinical Trials and News, ACC.org; vice-chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor-in-Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor-in-Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (guest editor, associate editor), K2P (cochair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (continuing medical education [CME] steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and U.S. national coleader, funded by Bayer), Slack Publications (chief medical editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (secretary/treasurer), WebMD (CME steering committees); has relationships with Clinical Cardiology (deputy editor), the NCDR-ACTION Registry Steering Committee (chair), and the VA CART Research and Publications Committee (chair); has received research funding from Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi-Aventis, Synaptic, and The Medicines Company; receives royalties from Elsevier (editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); serves as site coinvestigator for Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), and Svelte; is a trustee for the American College of Cardiology; and performs unfunded research for FlowCo, Merck, Novo Nordisk, and Takeda. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Harvey D. White, DSc, served as Guest Associate Editor for this paper. P.K. Shah, MD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACCauthor instructions page.
References
- 1.Bagiella E., Bhatt D.L., Gaudino M. The consequences of the COVID-19 pandemic on non-COVID-19 clinical trials. J Am Coll Cardiol. 2020;76:342–345. doi: 10.1016/j.jacc.2020.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrd J.B., Bello N., Meyer M.N. Pandemic pandemonium: pausing clinical research during the COVID-19 outbreak. Circulation. 2020;141:2045–2047. doi: 10.1161/CIRCULATIONAHA.120.047347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaudino M., Chikwe J., Hameed I., Robinson N.B., Fremes S.E., Ruel M. Response of cardiac surgery units to COVID-19: an internationally-based quantitative survey. Circulation. 2020;142:300–302. doi: 10.1161/CIRCULATIONAHA.120.047865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unger J.M., Nghiem V.T., Hershman D.L., Vaidya R., LeBlanc M., Blanke C.D. Association of National Cancer Institute-sponsored clinical trial network group studies with guideline care and new drug indications. JAMA Netw Open. 2019;2 [Google Scholar]
- 5.Waterhouse D.M., Harvey R.D., Hurley P. Early impact of COVID-19 on the conduct of oncology clinical trials and long-term opportunities for transformation: findings from an American Society of Clinical Oncology Survey. JCO Oncol Pract. 2020;16:417–421. doi: 10.1200/OP.20.00275. [DOI] [PubMed] [Google Scholar]