Abstract
Coronavirus disease 2019 (COVID-19) may present with pulmonary and extrapulmonary manifestations. We present a 41-year-old patient who presented with 1 week of increasing dyspnea and fever and initial chest radiography demonstrated bilateral diffuse infiltrates. Due to the patient's progressively worsening symptoms, he was intubated, paralyzed, sedated. He began proning, 100% fractional inspired oxygenation ventilation, and veno-venous extracorporeal membrane oxygenation. Computed tomography of the thorax revealed completely opacified lungs bilaterally with the exception of a small, aerated apicoposterior segment of the left lung. Computed tomography of the head demonstrated several areas of hemorrhage, areas of hypodensity consistent with posterior cerebral artery and middle cerebral artery territory infarcts, and findings consistent with transtentorial herniation. Given the radiologic findings and nonprogressive clinical status, the family placed the patient on comfort care and the patient died within minutes of extubation. As with our patient at the time of admission, presenting symptoms and clinical laboratory data provide reliable prognostic factors for patients with COVID-19.
Keywords: SARS-CoV-2, COVID-19, Acute respiratory distress syndrome, Cerebrovascular hemorrhage, Cerebral ischemia, Intramuscular hemorrhage
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the single-stranded RNA viral agent that leads to coronavirus disease 2019 (COVID-19). SARS-CoV-2 is highly contagious that is primarily spread through respiratory droplets, fomites, and as a nosocomial infection [1]. Due to the transmission through respiratory droplets, severe pulmonary manifestations, such as viral pneumonia, dyspnea, and hypoxia, and acute respiratory distress syndrome (ARDS) may be present. Patients with COVID-19 may suffer from extrapulmonary disease which has been shown to include neurologic, renal, gastrointestinal, cardiovascular, and hematologic manifestations [2]. With regard to neurologic disease, patients may develop cerebrovascular disease that may be both ischemic and hemorrhagic in nature [3], [4], [5], [6], [7], [8]. In this case report, we present a patient who developed severe ARDS and several areas of acute cerebral infarct and hemorrhage secondary to SARS-CoV-2 infection.
Case presentation
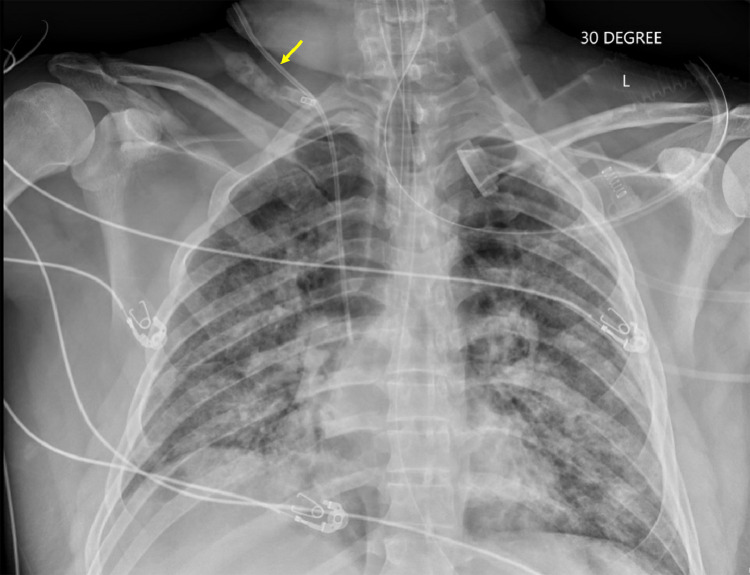
A 41-year-old Hispanic male with a history of obesity (body mass index 36.1) and binge-drinking (>30 alcoholic beverages during the weekend) presented in March 2020 to an urgent care complaining of 1 week of fever, dyspnea, and a nonproductive cough. Several of the patient's coworkers were experiencing similar symptoms, however only the patient's symptoms progressively worsened. The patient was transferred to an outside hospital for worsening symptoms. On arrival to an outside hospital, the patient was tachypneic, hypoxic with an oxygen saturation of 88% on room air, and a temperature of 38.3°C. C-reactive protein (CRP) and lactate dehydrogenase (LDH) were elevated at 273.6 mg/L (reference range: <8/0 mg/L) and 589 U/L (reference range: 122-225 U/L), respectively. Chest radiography showed bilateral patchy airspace consolidation consistent with pneumonia (Fig. 1). Due to the patient's pneumonia and possible ARDS and SARS-CoV-2 infection, the patient was started on hydroxychloroquine, zinc sulfate, azithromycin, vancomycin, and meropenem. The patient's condition began to acutely worsen. He was transferred to our hospital for possible proning and higher level of care.
Fig. 1.
Thirty-degree anteroposterior radiograph of the chest on the day of admission demonstrating bilateral patchy airspace opacities, consistent with multifocal pneumonia. A right internal jugular central venous catheter is labeled with a yellow arrow. (Color version of figure is available online.)
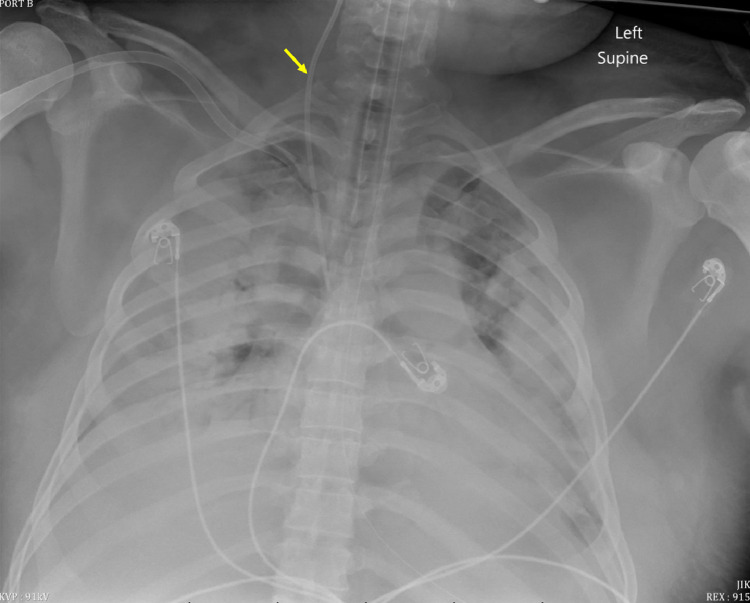
At the time of admission, the patient's nasopharyngeal swabbing for SARS-CoV-2 from the outside hospital was still pending. The patient was intubated, paralyzed with cisatracurium besilate, and sedated with propofol and fentanyl due to progressive respiratory failure. An arterial blood gas revealed a blood pH of 7.37 and a partial pressure of oxygen of 113 on fraction of inspired oxygen (FiO2) of 60%. Laboratory values for procalcitonin was 0.66 ng/mL (reference range: <0.1 ng/mL), CRP of 273.6 mg/L, LDH of 589 U/L, ferritin of 1217 ng/mL (reference range: 30-400 ng/mL), and D-dimer of 1.01 mcg/mL (reference range: <0.5 mcg/mL). Chest radiography revealed worsening diffuse, bilateral patchy opacities (Fig. 2). Norepinephrine was initiated due to a mean arterial pressure of 64 on admission.
Fig. 2.
Supine anteroposterior radiograph of the chest 2 days after initial presentation demonstrating interval worsening of the diffuse bilateral patchy airspace opacities, now with apical aeration of the lungs bilaterally and large bilateral pleural effusions. A right internal jugular central venous catheter is labeled with a yellow arrow. (Color version of figure is available online.)
One day later, the patient's SARS-CoV-2 test came back positive. The patient's ratio of partial pressure of oxygen to FiO2 worsened to 88 despite proning and high airway pressure release ventilation settings. Trial supination results in profound hypoxemia within seconds. The patient began spiking fevers over 39°C and experienced episodic hypoglycemia. The decision to begin venous-venous extracorporeal membrane oxygenation (V-V-ECMO) was made due to poor lung compliance and oxygenation. The patient was not a candidate for veno-arterial-venous ECMO. No atrial or ventricular wall defects were identified on transthoracic echocardiography. Despite 100% FiO2 ventilation and V-V-ECMO, the patient continued to have episodes of significant deoxygenation resulting in an average oxygen saturation level of ∼75% with several hypoxic events as low as 41%. The patient began to experience significant diuresis of almost 7 L of fluid. Chest radiography and computed tomography (CT) of the head, thorax, and abdomen were ordered to investigate the cause of massive diuresis and furthered clinical deterioration.
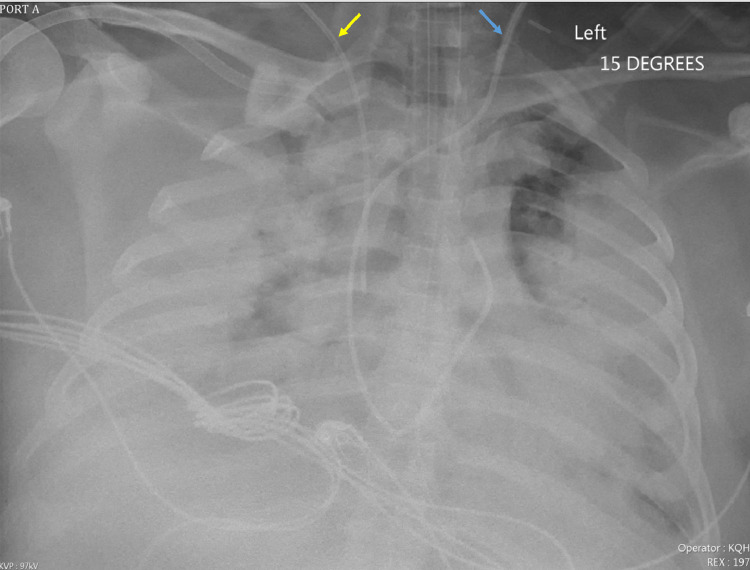
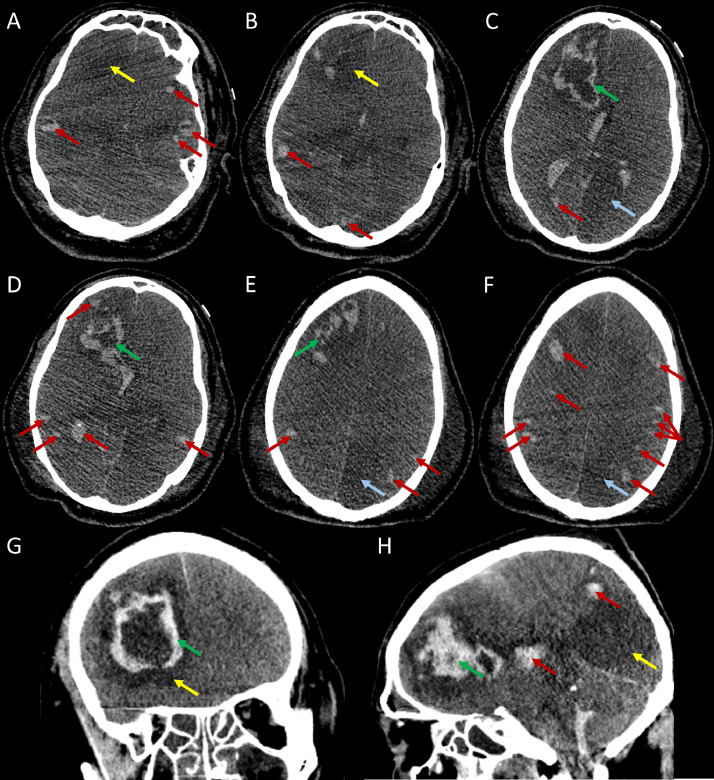
Chest radiography demonstrated confluent parenchymal densities that occupied the entirety of the left and right lungs, with relative sparing of the left lung apex (Fig. 3). CT of the head (Fig. 4) revealed a hypodensity in the posterior cerebral artery territory, likely representing an acute infarct. Another hypodensity was located in the left thalamus, which may have represented additional posterior cerebral artery territory infarction or leukoencephalopathy secondary to COVID-19. An additional area of hypodensity within the right temporal lobe may have represented a right middle cerebral artery territory infarction. There were numerous hemorrhagic lesions at the gray-white matter junction that appear embolic in nature. A large hypodense region within the left frontal lobe with peripheral hemorrhage and edema was present. The hemorrhage extended to the ventricular system, though the ventricular system did not appear dilated. Mass effect with an approximate 5 mm midline shift toward the left was noted. There was apparent effacement of the basal cisterns with dilation of the contralateral temporal horn which was consistent with transtentorial herniation.
Fig. 3.
Fifteen-degree anteroposterior radiograph of the chest 4 days after initial presentation showing confluent parenchymal densities occupying almost the entirety of the right lung. The left lung is almost entirely opacified with a small amount of aerated lung remaining within the apicoposterior segment of the left upper lobe. A right internal jugular central venous catheter is labeled with a yellow arrow. The large bore left-sided catheter, likely representing an ECMO catheter, is labeled with a blue arrow. (Color version of figure is available online.)
Fig. 4.
Nonenhanced CT of the head 6 days after initial presentation demonstrates a geographic hypodensity involving the left posterior cerebral artery territory (blue arrows in axial images of C, E, and F). Another hypodensity of the right frontal lobe is shown with a yellow arrow in A, B, and G. This area also reveals a peripheral hemorrhagic component that extends into the ventricular system (green arrows in axial images C, D, and E and coronal and sagittal images of G and H). Numerous, scattered hemorrhagic lesions are visualized near the gray-white matter junctions which are consistent with an embolic etiology (red arrows). (Color version of figure is available online.)
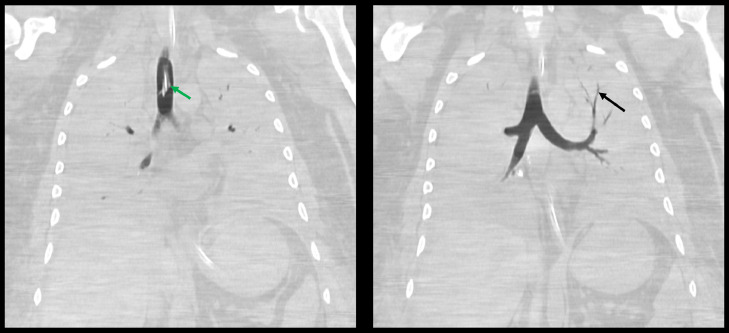
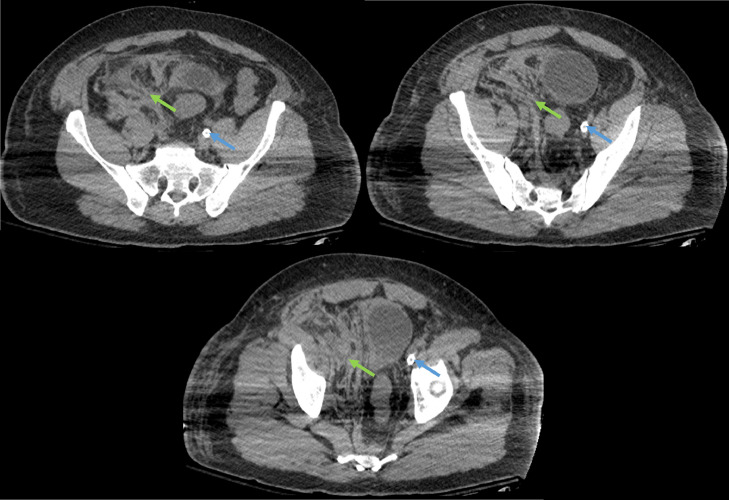
Occlusion of the posterior segmental branch of the right upper lobe bronchus, segmental branches of the right lower lobe bronchus, and left lower lobe bronchus and its segmental branches were noted on CT of the thorax (Fig. 5). The right lung was completely opaque. Right-sided air bronchograms were present. The majority of the left lung was opacified with the exception of aerated lung in the apicoposterior segment of the left upper lobe. Small-to-moderate bilateral pleural effusions were present. CT of the abdomen and pelvis revealed hyperdense fluid in the right retroperitoneum and right extraperitoneal pelvis and presacral space with asymmetric enlargement of the right psoas muscle (Fig. 6). This finding was worrisome for intramuscular hemorrhage.
Fig. 5.
Coronal nonenhanced CT images of the thorax 6 days after initial presentation demonstrates complete opacification of the lungs, with relative sparing of the apicoposterior segment of the left upper lobe. Air-filled left upper lobe bronchi are indicated by the black arrow. The endotracheal tube is indicated by the green arrow. (Color version of figure is available online.)
Fig. 6.
Axial nonenhanced CT images of the abdomen and pelvis 6 days after initial presentation demonstrating hyperdense fluid in the right retroperitoneum extending to the right hemipelvis (orange arrow), displacing the pelvic organs laterally to the left. The right psoas muscle is enlarged as compared to the contralateral side, and the distal iliopsoas muscle contains a large hematoma (green arrows). The large bore left-sided catheter, likely representing an ECMO catheter, is labeled with a blue arrow. (Color version of figure is available online.)
Due to the severe neurologic abnormalities demonstrating extensive ischemic and hemorrhagic disease in the brain, as well as evidence of transtentorial herniation, the medical team felt the patient's prognosis was grim. The patient's family was informed of the imaging findings and felt it was necessary to pursue comfort measures. The patient was extubated, and the patient was pronounced dead several minutes later.
Discussion
SARS-CoV-2, a single-stranded RNA virus that leads to COVID-19, was labeled a global pandemic by the World Health Organization on March 11, 2020. It was found to have significantly greater transmission rate but lower case fatality ratio than the 2003 SARS-CoV pandemic [1,9,10]. The SARS-CoV-2 infects human cells through its densely glycosylated spike proteins which binds to the angiotensin-converting enzyme 2 receptor with a much higher affinity than SARS-CoV [11]. These receptors are found predominantly on human alveolar epithelial cells, though they may also be found in other organ systems such as endothelial cells, esophageal and intestinal epithelium, and cardiac myocytes [12]. These findings may explain the extrapulmonary manifestations of SARS-CoV-2 infection.
The majority of patients with SARS-CoV-2 infection are either asymptomatic or present with mild upper respiratory symptoms (sneezing, coughing, dyspnea, rhinorrhea, fatigue, and fever) approximately 2-4 days following infection [13]. However, patients may progress to developing symptoms of severe dyspnea or hypoxia, and ARDS, which has a high mortality rate due to the necessitation for mechanical ventilatory support, multiorgan involvement, shock, septicemia, and multiple organ dysfunction syndrome [1]. Pathology specimens reveal damage to the type II pneumocytes of the alveolar epithelium leads to fibromyxoid exudates, fibrinous cords, and mucus plugging within the bronchioles. These changes impair oxygen exchange at the level of the alveolar membranes. Coinciding with these changes, there are findings consistent with ARDS, which include widespread interstitial inflammatory infiltrates, severe epithelial damage, and diffuse hyperplasia of type II pneumocytes [14,15]. Other findings, such as acute fibrinous and organizing pneumonia and pulmonary intravascular coagulopathy, may also be present [1]. Furthermore, infection of the alveolar membrane leads to a local and a systemic inflammatory response from excessive cytokine synthesis and distribution. This mechanism of the cytokine is thought to be due to activation of inflammatory cells as a result of the accumulation of uncleaved angiotensin II. The excessive cytokines not only contribute to widespread diffuse alveolar damage, but also play an important role in the development of a systemic inflammatory response syndrome. Proinflammatory cytokines lead to widespread activation of procoagulant factors which lead to microthrombi, ARDS, multiple organ dysfunction syndrome, ischemia and/or infarction, and the high rate of mortality in patients with severe COVID-19 [1,16].
Since SARS-CoV-2 is transmitted primarily through droplets, a significant portion of symptomatic patients will experience pulmonary radiologic findings. Patients with suspected COVID-19 will typically receive chest radiography in the initial workup. The majority of SARS-CoV-2-positive patients have bilateral alterations (~73%), and more specifically have findings of reticular alterations (~63%) or ground-glass opacities (~69%) [17]. However, since chest radiographic findings may be absent (~25%) during the first 0-5 days after testing positive, CT is considered the most reliable imaging modality for patients with suspected cases of COVID-19 [17]. Patients referred for CT should undergo noncontrast material-enhanced chest CT, unless patients require CT pulmonary angiography for a suspected pulmonary embolism [18]. Although, chest radiography may be used for follow-up imaging. Similar to chest radiography, patients within the first 4-5 days of infection may have normal CT findings. A small, but important, subset of patients (1.2%-4.0%) are observed to have a normal CT of the thorax despite being symptomatic in the later stage of their infection [19], [20], [21]. Chest CT findings with high incidence (>70%) in SARS-CoV-2-positive patients include ground glass opacities, vascular enlargement, bilateral abnormalities, lower lobe involvement, and has a posterior abnormality predilection [18]. Other findings that are less prominent (<70%) may range from consolidation, opacities, septal, bronchial wall, or pleural thickening, air bronchograms, cavitating lung lesions, or pleural or pericardial effusions [18]. Patients may rapidly progress to ARDS, especially in high-risk patients. ARDS, which is characterized by acute noncardiogenic pulmonary edema, hypoxemia, and necessitates mechanical ventilation, occurs in 33% of hospitalized COVID-19 patients [22]. Of these patients, approximately 16% die [22].
Extrapulmonary features of COVID-19 may complicate or even be the cause of death for patients infected with SARS-CoV-2. Patients may develop complications involving renal dysfunction, gastrointestinal complications, liver dysfunction, cardiac and mediastinal abnormalities, neurologic complications, and hematologic abnormalities [1]. Neurological manifestations may be benign or severe. Benign neurologic presentations involve headache, hyposmia, and hypogeusia. However, severe symptoms such as dizziness, ataxia, confusion, acute ischemic stroke, cerebral venous sinus thrombosis, or cerebral hemorrhage may occur with the primary infection [23]. Patients may secondarily develop postinfectious demyelinating neurological complications such as acute myelitis and Guillain-Barre syndrome [24,25]. Neuroradiologic imaging may reveal diffuse white matter abnormalities and acute cerebrovascular disease including intracranial hemorrhage, large-vessel occlusion, acute or subacute ischemic stroke, and dural venous sinus thrombosis [3], [4], [5], [6], [7], [8],26,27]. The presence of acute stroke is a strong prognostic marker for a poor outcome in patients hospitalized with COVID-19 [28].
We presented a patient who arrived to our institution and required rapid advancement of his care based on his acutely worsening condition. Despite aggressive and invasive measures to oxygenate the patient with mechanical ventilation with 100% FiO2 and V-V-ECMO, the patient's oxygen saturation levels and partial pressure of oxygen remained inadequate.
Prognostication of COVID-19 mortality is currently being heavily investigated such that patient risk and outcomes may be further stratified. Some prognostic factors associated with mortality in patients infected with SARS-CoV-2 include elevated CRP, LDH, and aminotransferases, acute onset of symptoms, fever and dyspnea, tachypnea, low oxygen saturation on admission, and lower platelet count [29,30]. Although these data were published several months following the death of our patient, it appears that our patient had several significant prognostic factors associated with increased mortality, such as elevated CRP, LDH, tachypnea, cerebrovascular disease, low oxygen saturation on admission, and acutely progressive disease.
Conclusion
SARS-CoV-2 leads to the development of COVID-19, which predominantly affects the respiratory system. However, increasing evidence demonstrates multisystem disease either through direct viral infection of systemic organs, via a systemic inflammatory response, or a combination of both mechanisms. In the presented patient, aggressive techniques to improve the patient's status failed. This patient's treatment course highlights the difficulty in treating patients with COVID-19, especially those who are at higher risk or those with prognostic factors associated with mortality.
Patient consent statement
No consent obtained for this case report as this is a retrospective study with no patient identifiers.
Formal consents are not required for the use of entirely anonymized images from which the individual cannot be identified—for example, x-rays, ultrasound images, pathology slides, or laparoscopic images, provided that these do not contain any identifying marks and are not accompanied by text that might identify the individual concerned.
Footnotes
Declarations of interest: None.
References
- 1.Shanmugam C, Mohammed AR, Ravuri S, Luthra V, Rajagopal N, Karre S. COVID-2019—a comprehensive pathology insight. Pathol Res Pract. 2020;216(10) doi: 10.1016/j.prp.2020.153222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behzad S, Aghaghazvini L, Radmard AR, Gholamrezanezhad A. Extrapulmonary manifestations of COVID-19: radiologic and clinical overview. Clin Imaging. 2020;66:35–41. doi: 10.1016/j.clinimag.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altschul DJ, Unda SR, de La Garza Ramos R, Zampolin R, Benton J, Holland R. Hemorrhagic presentations of COVID-19: risk factors for mortality. Clin Neurol Neurosurg. 2020;198 doi: 10.1016/j.clineuro.2020.106112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morassi M, Bagatto D, Cobelli M, D'Agostini S, Gigli GL, Bna C. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. 2020;267(8):2185–2192. doi: 10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy ST, Garg T, Shah C, Nascimento FA, Imran R, Kan P. Cerebrovascular disease in patients with COVID-19: a review of the literature and case series. Case Rep Neurol. 2020;12(2):199–209. doi: 10.1159/000508958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheruiyot I, Sehmi P, Ominde B, Bundi P, Mislani M, Ngure B. Intracranial hemorrhage in coronavirus disease 2019 (COVID-19) patients. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2020:1–9. doi: 10.1007/s10072-020-04870-z. . E-publication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruan S. Likelihood of survival of coronavirus disease 2019. Lancet Infect Dis. 2020;20(6):630–631. doi: 10.1016/S1473-3099(20)30257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahase E. Coronavirus Covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ. 2020;368:m641. doi: 10.1136/bmj.m641. [DOI] [PubMed] [Google Scholar]
- 11.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng T, Cao H, Zhang H, Kang Z, Xu D, Gong H, et al. The insert sequence in SARS-CoV-2 enhances spike protein cleavage by TMPRSS. bioRxiv. 2020:2020.02.08.926006. doi:10.1101/2020.02.08.926006
- 13.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 14.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33(6):1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vancheri SG, Savietto G, Ballati F, Maggi A, Canino C, Bortolotto C. Radiographic findings in 240 patients with COVID-19 pneumonia: time-dependence after the onset of symptoms. Eur Radiol. 2020;30(11):6161–6169. doi: 10.1007/s00330-020-06967-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwee TC, Kwee RM. Chest CT in COVID-19: what the radiologist needs to know. Radiography. 2020;40(7):1848–1865. doi: 10.1148/rg.2020200159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Dong C, Hu Y, Li C, Ren Q, Zhang X. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020;296(2):E55–E64. doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding X, Xu J, Zhou J, Long Q. Chest CT findings of COVID-19 pneumonia by duration of symptoms. Eur J Radiol. 2020;127 doi: 10.1016/j.ejrad.2020.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295(3) doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tzotzos SJ, Fischer B, Fischer H, Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care. 2020;24(1):516. doi: 10.1186/s13054-020-03240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lahiri D, Ardila A. COVID-19 pandemic: a neurological perspective. Cureus. 2020;12(4):e7889. doi: 10.7759/cureus.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chow CCN, Magnussen J, Ip J, Su Y. Acute transverse myelitis in COVID-19 infection. BMJ Case Rep. 2020;13(8) doi: 10.1136/bcr-2020-236720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radmanesh A, Raz E, Zan E, Derman A, Kaminetzky M. Brain imaging use and findings in COVID-19: a single academic center experience in the epicenter of disease in the United States. AJNR Am J Neuroradiol. 2020;41(7):1179–1183. doi: 10.3174/ajnr.A6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain R, Young M, Dogra S, Kennedy H, Nguyen V, Jones S. COVID-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci. 2020;414 doi: 10.1016/j.jns.2020.116923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mostaza JM, García-Iglesias F, González-Alegre T, Blanco F, Varas M, Hernandez-Blanco C. Clinical course and prognostic factors of COVID-19 infection in an elderly hospitalized population. Arch Gerontol Geriatr. 2020;91 doi: 10.1016/j.archger.2020.104204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, He W, Yu X, Hu D, Bao M, Liu H. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80(6):639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]