Abstract
Antiviral drugs are a class of compounds developed specifically for the treatment of viral infections. In the development and subsequent application of antiviral drugs, like for any other class of drugs, quantitative analysis in biological matrix is important, e.g., to establish bioavailability, to study pharmacokinetics, and later on possibly for therapeutic drug monitoring. Liquid chromatography–mass spectrometry (LC–MS) with tandem mass spectrometry (MS–MS) operated in selected-reaction monitoring (SRM) mode is the method of choice in quantitative bioanalysis.
As information of the fragmentation of antiviral drugs in MS–MS is very much scattered in the scientific literature, it was decided to collect this information and to review it, not only to understand which product ions are actually used in SRM, but also to assist in other studies, e.g., in the identification of drug metabolites or (forced) degradation products. In this first study, attention is paid to antiviral agents used against HIV infection. The review provides fragmentation schemes of ca. 40 antiviral agents as well as several phosphorylated anabolites. The identity of the product ions used in SRM, i.e., elemental composition and exact-m/z, is tabulated, and more detailed fragmentation schemes are provided.
Keywords: Antiviral drugs, Tandem mass spectrometry, Fragmentation, HIV, Nucleoside reverse transcriptase inhibitors, Non-nucleoside reverse transcriptase inhibitors, Protein inhibitors, Non-peptide protein inhibitors, Integrase inhibitors, CCR5 inhibitors
Graphical abstract
Highlights
-
•
Detailed study on the fragmentation of ∼40 HIV-related antiviral drugs in MS–MS.
-
•
Tabulated product ions (with elemental composition) used in SRM.
-
•
Fragmentation scheme, confirmed using accurate-m/z data.
1. Introduction
Antiviral drugs are a class of compounds developed specifically for the treatment of viral infections. The initial development of antivirals started in the 1960s and especially targeted herpes viruses. The drug development was based on traditional medicinal chemistry (trial-and-error) approaches. With an increase of the understanding on how viruses work at genetic and molecular level, e.g., by unraveling the genetic sequences of viruses, major advances were made in the development of new antiviral drugs. From the early 1980s onwards, this development was boosted by the urge to deal with the acquired immunodeficiency syndrome (AIDS), which was caused by the human immunodeficiency virus (HIV). More recently, substantial attention has also been drawn by the Ebola, Zika, and the various Corona viruses. Viruses can be classified according to the Baltimore virus classification system [1], that groups viruses into families, depending on their type of genome (DNA, RNA, single-stranded (ss), double-stranded (ds), etc.) and their method of replication.
Most of the antiviral drugs currently available are designed to help in the treatment of infections with HIV, herpes viruses, the hepatitis B and C viruses, and influenza A and B viruses. A useful approach to classify antiviral drugs is by their stage of interference in the life cycle of a virus. There are several approaches to this. Drugs may act at or before the cell entry, that is interfere with the ability of a virus to infiltrate a target cell. Alternatively, drugs may act at the stage of viral replication, or at the release phase. Most antiviral agents interfere during viral synthesis, e.g., the reverse transcriptase inhibitors (RTIs), which alter and block viral replication, and the protease inhibitors (PIs), which obstruct viral maturation.
In the development of antivirals, as in the development of any other type of drug, and in the application of antivirals, selective, specific and sensitive analytical methods are of utmost importance. For the bioanalysis of antiviral agents, e.g., in bioavailability studies, drug metabolism studies as well as in therapeutic drug monitoring, liquid chromatography coupled to tandem mass spectrometry (LC–MS–MS) plays an important role. In most cases, LC–MS–MS is operated in selected-reaction monitoring (SRM) mode, using tandem quadrupole instruments. Then, the protonated molecule [M+H]+ (or in some cases the deprotonated molecule [M−H]–) of the target drug is selected as precursor ion in MS1, subjected to collision-induced dissociation (CID) in a collision cell, and one or more product ions are selected in MS2 and subsequently detected. Preferably, unique and structure-informative product ions are selected for each individual drug investigated. In principle, no structure information on the identity of the product ions is required to develop a reliable bioanalytical method and to validate it. In other instances, e.g., in drug metabolism studies, knowledge on the identity of the product ions is required. Based on our interest in the fragmentation of (de)protonated molecules in MS–MS [[2], [3], [4], [5]], it was decided to review the fragmentation of antivirals in MS–MS. In this study, the available knowledge on the fragmentation of HIV-related antivirals in MS–MS is reviewed, and whenever possible extended by interpretation of available MS–MS spectra. In a further studies, the fragmentation of other antivirals agents is discussed.
The human immunodeficiency viruses (HIV-1 and HIV-2) are retroviruses, that is: they contain two copies of positive-sense single-stranded RNA, that is replicated in infected cells. The HIV may infect vital cells in the human immune system, such as helper T cells (specifically CD4+ T cells), macrophages, and dendritic cells. Over time, they may cause the acquired immunodeficiency syndrome (AIDS). In most cases, HIV is a sexually transmitted infection.
Numerous papers have been published on the bioanalysis of HIV-related antivirals, as demonstrated in a two review papers [6,7]. Often, therapeutic drug monitoring (TDM) is performed to patients administered with HIV-related antivirals [8,9]. An important issue in TDM is the frequent use of combination therapies in HIV treatment, wherein several antiviral agents are administered in combination [10]. To this end, the development and application of numerous multianalyte methods have been reported as well, involving the analysis of as many as 16 HIV-related antivirals [[11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]]. In most cases, one (or more recently two) SRM transition have been used. Alternatively, high-resolution MS can be used in quantification, based on narrow-window extracted-ion chromatograms from full-spectrum MS data [24]. No attempts have been made to achieve a comprehensive review of the available literature on this. Our focus was on the fragmentation of the antivirals in MS–MS, for which data were acquired from MS–MS spectra reported in literature and available in mass spectral library collections. Whenever possible, accurate-m/z data from high-resolution mass spectrometry instruments was used to confirm our interpretation.
Antiviral drugs targeting HIV infection can be classified in basically six classes: (1) nucleoside reverse transcriptase inhibitors (NRTIs) and some of their anabolite analogues, (2) non-nucleoside reverse transcriptase inhibitors (nNRTIs), (3) protein inhibitors (PI), (4) non-peptide protein inhibitors (npPI), (5) integrase inhibitors (INIs), and (6) C–C motif chemokine receptor CCR5 inhibitors. The various compounds discussed below are grouped along this classification. For each compound class, a Table is presented, summarizing the SRM transitions that are used in bioanalysis. Although the tandem quadrupole instruments used in bioanalysis are operated in unit-mass resolution, exact-m/z values are given in these Tables, together with the elemental composition, derived from the fragmentation scheme, discussed for each individual compound.
2. Methods
The m/z data to interpret the fragmentation in MS–MS, to derive elemental composition and accurate-m/z for the product ions used in SRM and to develop the fragmentation schemes, have been collected by searching the literature using the PubMed search website with search terms like “compound name MS” or “compound name metabolites”. The papers found in this way were screened for relevant MS–MS data, i.e., MS–MS spectra, (tabulated) m/z-values for SRM transitions, and, when available, structure proposals for the product ions. Special attention was paid to finding accurate-m/z data, mostly from literature dealing with identification of metabolites or (forced) degradation products. For some compounds, accurate-m/z data were available in an available MS–MS spectral library for toxicological unknown screening [25].
In most cases, the interpretation of the spectrum is initially performed using nominal m/z values of the product ions. The elemental composition of the proposed structures are then verified against and/or confirmed by the accurate-m/z data. If no structure proposal is reached for a particular product ion, the process is reversed: a plausible structure proposal is derived from elemental compositions calculated from the accurate-m/z data. Accurate-m/z data of product ions can be used to calculate their possible elemental composition. Such calculations were performed using a ±3 mDa window, which generally (but not always) is appropriate to the available data. The fact that the elemental composition of a product ion is restricted by the elemental composition of the precursor ion, i.e., the antiviral drug investigated, greatly reduces the number of possible hits. In this way, the proposed structures of the product ions have been evaluated, at least in terms of elemental composition. Structure proposals for the product ions are based on their elemental composition and on logical neutral losses from the structure of the compound investigated [5]. Whenever needed, names of product ions or neutral losses were generated with the “Generate Name for Structure” option available in the ACD/ChemSketch software (version 2018.1.1; www.acdlabs.com). In some cases, the elemental composition derived from available accurate-m/z data is not in agreement with the elemental composition of the literature-proposed structure of a product ion; this is indicated in the text.
In the Tables, for most SRM transitions, a limited number of references is given (in order to limit the size of the table). In most cases, an early paper is selected, whenever possible one providing an MS–MS spectrum of the compound, and more recent papers dealing with multi-analyte methods. This gives insight in which SRM transitions are used most frequently for each compound. As SRM transitions in the Tables have been acquired on different instruments from various manufacturers, it was decided not to add information on the collision energy applied, as a particular set collision energy value may yield different levels of fragmentation between instruments from different manufacturers.
The accurate-m/z values in the Tables (or more precisely exact-m/z values, as these are calculated values) are given with three significant digits. With the current mass accuracy of high-resolution MS instruments, i.e., typically 1–3 ppm, and ions with m/z below 1000, the error is in the third decimal place.
3. Nucleoside reverse transcriptase inhibitors (NRTIs)
There are two classes of reverse transcriptase inhibitors, i.e., compounds that are similar to nucleosides, called nucleoside reverse transcriptase inhibitors (NRTIs), or compounds that are not similar to nucleosides, called non-nucleoside reverse transcriptase inhibitors (nNRTIs).
Nucleoside reverse transcriptase inhibitors (NRTIs) are analogues of the naturally occurring deoxynucleotides needed to synthesize the viral DNA. They inhibit the reverse transcriptase process, thereby effectively preventing HIV from multiplying. Their mode of action is to compete for incorporation into the viral DNA chain synthesized by an infected cell. As the NRTI lacks a 3′-hydroxyl group on the deoxyribose moiety, the next incoming deoxynucleotide cannot form the next 5′–3′ phosphodiester bond needed to extend the DNA chain. As a result, viral DNA replication is terminated.
Several multianalyte methods for the bioanalysis of NRTIs have been reported as well [26,27]. The bioanalysis of NRTIs and their phosphorylated anabolites has been reviewed [28]. Most NRTIs can be analyzed in both positive-ion and negative-ion mode. In bioanalysis of the NRTIs, the positive-ion mode is generally preferred, where as their phosphorylated anabolites are often analyzed in negative-ion mode. The SRM transitions used in bioanalysis are summarized in Table 1 for the positive-ion mode and Table 2 for the negative-ion mode.
Table 1.
SRM transitions used in the bioanalysis of nucleoside reverse transcriptase inhibitor (NRTIs) antiviral drugs in positive-ion mode. MP, DP, and TP are mono-, di-, and tri-phosphorylated anabolites.
| Compound | m/z of [M+H]+ | Formula | m/z of SRM product-ion | Formula | Literature |
|---|---|---|---|---|---|
| abacivir (ABC, Ziagen) | 287.161 | [C14H19N6O]+ | 191.104 | [C8H11N6]+ | [11,26,27,29] |
| 151.073 | [C5H7N6]+ | [23] | |||
| carbovir (CBV) | 248.114 | [C11H14N5O2]+ | 152.057 | [C5H6N5O]+ | [30,31] |
| CBV-TP | 488.013 | [C11H17N5O11P3]+ | 152.057 | [C5H6N5O]+ | [32,33] |
| didanosine (ddI) | 237.098 | [C10H13N4O3]+ | 137.046 | [C5H5N4O]+ | [11,27] |
| valdidanosine, prodrug of ddI | 336.167 | [C15H22N5O4]+ | 200.128 | [C10H18NO3]+ | [34] |
| dideoxyadenosine (ddA) | 236.114 | [C10H14N5O2]+ | 136.072 | [C5H6N5]+ | [30] |
| emtricitabine (FTC) | 248.050 | [C8H10FN3O3S]+ | 130.041 | [C4H5FN3O]+ | [23,27] |
| FTC-TP | 487.949 | [C8H14FN3O12P3S]+ | 130.041 | [C4H5FN3O]+ | [35,32] |
| lamivudine (3 TC) | 230.059 | [C8H12N3O3S]+ | 112.050 | [C4H6N3O]+ | [11,20,23,26,27,36,30,31,37] |
| 3 TC-MP | 310.026 | [C8H13N3O6PS]+ | 112.050 | [C4H6N3O]+ | [32] |
| 3 TC-TP | 469.958 | [C8H14N3O12P3S]+ | 112.050 | [C4H6N3O]+ | [35,32,33] |
| stavudine (d4T) | 225.087 | [C10H13N2O4]+ | 127.050 | [C5H7N2O2]+ | [27,36] |
| tenofovir (TFV) | 288.086 | [C9H15N5O4P]+ | 176.093 | [C8H10N5]+ | [18,20,23,30,32] |
| 270.075 | [C9H13N5O3P]+ | [38] | |||
| TFV-DP | 448.018 | [C9H17N5O10P3]+ | 176.093 | [C8H10N5]+ | [35,33] |
| 270.075 | [C9H13N5O3P]+ | [35,32,39] | |||
| tenofovir disoproxil (TDF) | 520.180 | [C19H31N5O10P]+ | 176.093 | [C8H10N5]+ | [30] |
| tenofovir alafenamide (TAF) | 477.201 | [C21H30N6O6P]+ | 176.093 | [C8H10N5]+ | [40] |
| 346.106 | [C15H17N5O3P]+ | [39] | |||
| zalcitabine (ddC) | 212.103 | [C9H14N3O3]+ | 112.051 | [C4H6N3O]+ | [11,26,27] |
| zidovudine (AZT, ZDV) | 268.104 | [C10H14N5O4]+ | 127.050 | [C5H7N2O2]+ | [27,31] |
| AZT-MP | 348.070 | [C10H15N5O7P]+ | 80.974 | [H2O3P]+ | [32] |
Table 2.
SRM transitions used in the bioanalysis of nucleoside reverse transcriptase inhibitor (NRTI) antiviral drugs in negative-ion mode. MP, DP, and TP are mono-, di-, and tri-phosphorylated anabolites.
| Compound | m/z of [M−H]– | Formula | m/z of SRM product-ion | Formula | Literature |
|---|---|---|---|---|---|
| didanosine (ddI) | 235.084 | [C10H11N4O3]– | 135.031 | [C5H3N4O]– | [26,30] |
| dideoxyadenosine-TP (ddA-TP) | 473.999 | [C10H15N5O11P3]– | 158.925 | [HO6P2]– | [27,41] |
| 143.006 | [C7H5ClF]+ | [42] | |||
| emtricitabine-TP (FTC-TP) | 485.934 | [C8H12FN3O12P3S]– | 158.925 | [HO6P2]– | [35] |
| lamivudine-MP (3 TC-MP) | 308.011 | [C8H11N3O6PS]– | 78.959 | [O3P]– | [27] |
| lamivudine-TP (3 TC-TP) | 467.944 | [C8H13N3O12P3S]– | 158.925 | [HO6P2]– | [27,35,41] |
| stavudine (d4T) | 223.072 | [C10H11N2O4]– | 125.036 | [C5H5N2O2]– | [26] |
| 41.999 | [CNO]– | [11] | |||
| d4T-MP | 303.039 | [C10H12N2O7P]– | 176.996 | [C5H6O5P]– | [27] |
| d4T-DP | 383.005 | [C10H13N2O10P2]– | 256.962 | [C5H7O8P2]– | [27] |
| d4T-TP | 462.971 | [C10H14N2O13P3]– | 158.925 | [HO6P2]– | [27,41] |
| tenofovir (TFV) | 286.071 | [C9H13N5O4P]– | 134.047 | [C5H4N5]– | [27] |
| TFV-MP | 366.037 | [C9H14N5O7P2]– | 78.959 | [O3P]– | [27] |
| TFV-DP | 446.004 | [C9H15N5O10P3]– | 158.925 | [HO6P2]– | [27,35] |
| zidovudine (AZT, ZDV) | 266.089 | [C10H12N5O4]– | 223.072 | [C10H11N2O4]– | [11,26,30] |
| 41.999 | [CNO]– | [37] | |||
| 42.010 | [N3]– | ||||
| AZT-MP | 346.056 | [C10H13N5O7P]– | 176.996 | [C5H6O5P]– | [27] |
| AZT-DP | 426.022 | [C10H14N5O10P2]– | 176.996 | [C5H6O5P]– | [27] |
| AZT-TP | 505.988 | [C10H15N5O13P3]– | 379.946 | [C5H9N3O11P3]– | [32] |
| 158.925 | [HO6P2]– | [27] |
3.1. Positive-ion mode fragmentation of NRTIs
In positive-ion mode, the loss of the nucleoside N-substituent, with charge retention on the nucleobase is generally the most important fragmentation route [26,[36], [43], [44], [45]]. For didanosine (ddI; [M+H]+ with m/z 237), for example, this results in protonated 9H-purin-6-ol ([C5H5N4O]+) with m/z 137. These compound-specific product ions are used in SRM, as indicated for the various NRTIs in Table 1 and Fig. 1 . This fragmentation behavior is similar to what is observed for nucleosides [46]. Multianalyte methods for the bioanalysis of NRTIs and their phosphorylated anabolites have been reported as well [26,27,[30], [31], [32], [35]].
Fig. 1.
Structures and major product ions of the nucleoside reverse transcriptase inhibitor (NRTI) antiviral drugs in positive-ion mode.
For most compounds, additional product ions have been reported. For abacavir (ABC; [M+H]+ with m/z 287), secondary fragmentation of the protonated nucleobase with m/z 191 leads to product ions with m/z 174 due to the loss of ammonia (NH3), with m/z 164 due to the loss of hydrogen cyanide (HCN), and with m/z 151 due to the loss of cyclopropene (C3H4) [44]. For lamivudine (3 TC; [M+H]+ with m/z 230), secondary fragmentation of the protonated nucleobase with m/z 112 leads to product ions with m/z 95 due to the loss of ammonia and with m/z 69 due to the loss of hydrogen isocyanate (HNCO) [43]. For stavudine (d4T; [M+H]+ with m/z 225), secondary fragmentation of the protonated nucleobase with m/z 127 leads to product ions with m/z 110 due to the loss of ammonia and m/z 84 due to the loss of hydrogen isocyanate. The product ions with m/z 69 ([C4H5O]+), m/z 81 ([C5H5O]+) are due to secondary fragmentation of the sugar moiety (with m/z 99 ([C5H7O2]+).
3.2. Negative-ion mode fragmentation of NRTIs
In some studies, some of the NRTIs have been analyzed in negative-ion mode, using [M−H]– as precursor ion. Again, the loss of the nucleoside N-substituent, with charge retention on the nucleobase is generally the most important fragmentation route. Relevant information is collected in Table 2. For zidovudine (AZT; [M−H]– with m/z 266), two product ions have been used in SRM, i.e., the ion with m/z 233 due to the loss of 43 Da, either hydrazoic acid (HN3) or hydrogen isocyanate (HNCO), and the ion with m/z 42. [15N]-labelling of the azide moiety in zidovudine suggests that this ion is an isobaric mixture of the azide anion ([N3]–) and the isocyanate anion ([NCO]–) [37].
The biologically-active forms of these NRTIs are the intracellular phosphorylated anabolites. Their analysis in, for instance, peripheral blood mononuclear cells (PBMC) poses challenges in LC–MS, because ion-pair or ion-exchange LC is required in most cases [41]. One approach is the selective isolation of the phosphate anabolites from the biological matrix by anion-exchange cartridges, followed dephosphorylation by an acid phosphatase, cleanup, and analysis of the resulting nucleosides (the parent drugs) by reversed-phase LC–MS [31]. The di- and triphosphorylated anabolites are analyzed in negative-ion mode using SRM with transitions involving fragmentation of [M−H]– to a common product ion with m/z 159 ([HO6P2]–) or m/z 177 ([C5H6O5P]–) [27,41]. However, analysis in positive-ion mode was also found to be possible, using the protonated nucleobases as product ions in SRM [33]. Typical product ions observed for some of these anabolites in either positive-ion or negative-ion mode are given in Table 1, Table 2, respectively.
3.3. Tenofovir
Tenofovir (TFV; [M+H]+ with m/z 288) is a monophosphorylated antiviral drug, which is often used in combination with emtricitabine. In positive-ion mode, it shows product ions with m/z 270 due to the loss of water, m/z 206 due to the loss of phosphoric acid (H3PO3), m/z 176 due to a subsequent loss of formaldehyde (H2C O), and of m/z 136 due to protonated 6-amino-9H-purine ([C5H6N5]+) (Fig. 1). The ion with m/z 176 is mostly used as product ion in SRM. Secondary fragmentation of the ion with m/z 176 involves the loss of ammonia (NH3) to m/z 159 and the loss of propene (C3H6) to m/z 136 [47]. Tenofovir is frequently administered in the form of a prodrug, e.g., tenofovir disoproxil fumarate (TDF; [M+H]+ with m/z 520) or tenofovir alafenamide fumarate (TAF; [M+H]+ with m/z 477), and in combination therapies. The major product ions of TDF and TAF are the ions with m/z 288, involving the loss of the disoproxil moiety, and with m/z 176, similar to tenofovir. Detailed studies on the fragmentation for these two prodrugs have been reported [48,49].
Some NRTIs are not only applied in HIV treatment, but also in treatment of the Hepatitis B Virus (HBV), e.g., entecavir, lamivudine, telbivudine and tenofovir. The fragmentation of entecavir and telbivudine is discussed in Part 2 [50].
4. Non-nucleoside reverse transcriptase inhibitors (nNRTIs)
The nNRTIs have the same clinical effect as the NRTIs, that is: inhibiting the reverse transcriptase enzyme. However, their mode of action is different: they inhibit reverse transcriptase by binding to the enzyme involved, preventing DNA synthesis from the viral RNA. A number of compounds have been described. Characteristic SRM transitions used in the bioanalysis of nNRTIs are summarized in Table 3 a for the positive-ion mode and Table 3 b for the negative-ion mode.
Table 3a.
SRM transitions for non-nucleoside reverse transcriptase inhibitor (nNRTI) antiviral drugs in the positive-ion mode. A question mark (?) in the column “formula” indicates that the proposed elemental composition is uncertain, as it is not confirmed by accurate-m/z data.
| Compound | m/z of [M+H]+ | Formula | m/z of SRM product-ion | Formula | Literature |
|---|---|---|---|---|---|
| dapivirine | 330.171 | [C20H20N5]+ | 158.059 | [C8H6N4]+• ? | [51,52] |
| 119.086 | [C9H11]+ | [51,52] | |||
| delavirdine | 457.202 | [C22H29N6O3S]+ | 221.176 | [C12H21N4]+ | [11] |
| doravirine | 426.058 | [C17H12ClF3N5O3]+ | 112.051 | [C4H6N3O]+ | [53] |
| 315.014 | [C13H7ClF3N2O2]+ | [53] | |||
| efavirenz | 316.035 | [C14H10ClF3NO2]+ | 244.014 | [C11H6ClF3N]+ | [14,54,55] |
| 232.014 | [C10H6ClF3N]+ | [55] | |||
| 168.081 | [C12H10N]+ | [55] | |||
| etravirine | 435.056 | [C20H16BrN6O]+ | 144.056 | [C8H5N3]+ | [17,42] |
| 303.983 | [C11H7BrN5O]+ | [17,21,23,56] | |||
| 164.948 | [C3H481BrN2O]+ | [57] | |||
| 162.950 | [C3H4BrN2O]+ | [[14], [16], [18], [42], [58]] | |||
| nevirapine | 267.124 | [C15H15N4O]+ | 226.085 | [C12H10N4O]+• | [11,14,18,20,21,42,54,55] |
| 198.091 | [C11H10N4]+• | [23,55] | |||
| 197.082 | [C11H9N4]+ ? | [17,55] | |||
| 80.049 | [C5H6N]+ | [19] | |||
| rilpivirine (RPV) | 367.167 | [C22H19N6]+ | 350.140 | [C22H16N5]+ ? | [19] |
| 224.118 | [C14H14N3]+ ? | [19,23] | |||
| 195.092 | [C13H11N2]+ | [17,22] | |||
| 192 | ? | [17] | |||
| 128.062 | [C10H8]+• | [22,59] |
Table 3b.
SRM transitions for non-nucleoside reverse transcriptase inhibitor (nNRTI) antiviral drugs in the negative-ion mode.
4.1. Dapivirine
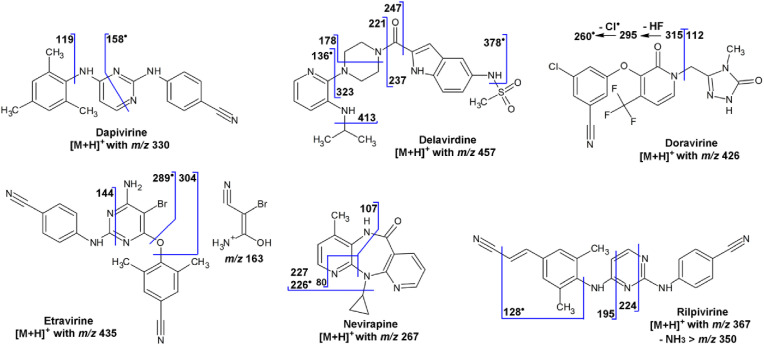
Dapivirine ([C20H20N5]+; [M+H]+ with m/z 330) shows product ions with m/z 158, which (most likely) is an odd-electron ion ([C8H6N4]+• with m/z 158.059), and with m/z 119, which is the 2,4,6-trimethylphen-1-ylium ion ([C9H11]+) (Fig. 2 ) [51,52].
Fig. 2.
Structures and fragmentation scheme of the non-nucleoside reverse transcriptase inhibitor (nNRTI) antiviral drugs in positive-ion mode.
4.2. Delavirdine
The fragmentation of delavirdine ([M+H]+ with m/z 457) has been discussed in the course of metabolite studies [[60], [61], [62]]. At the high-m/z end of the MS–MS spectrum, product ions are observed with m/z 378, which is an odd-electron ion due to the loss of a (methanesulfinyl)oxidanyl radical (•CH3SO2), and an ion with m/z 413 due to the loss of propene (C3H6). The most abundant product ions are due to a cleavage of the amide bond with charge retention on the piperazine moiety, leading to the ion with m/z 221; the complementary ion with m/z 237 is also observed. Some additional fragments are outlined in the fragmentation scheme (Fig. 2). Delavirdine is currently hardly used as antiviral drug anymore.
4.3. Doravirine
The major product ions of doravirine ([M+H]+ with m/z 426) result from the cleavage of the C–N bond between the pyridin-2(1H)-one moiety and the (4-methyl-5-oxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)methylene group and charge retention on either side, resulting in the complementary product ions with m/z 315 and 112, which have also been used in SRM [53]. The ion with m/z 315 shows subsequent losses of hydrogen fluoride (HF) to an ion with m/z 295 and a chlorine radical (•Cl) to an ion with m/z 260. The fragmentation scheme (Fig. 2) has been confirmed by accurate-m/z data, acquired in the course of an impurity profiling study [63].
4.4. Efavirenz
The fragmentation of efavirenz ([M+H]+ with m/z 316) has been studied in detail in the context of a stability study [64]. Extensive fragmentation is observed, started from the opening of the 1,3-oxazinan-2-one ring by the loss of either water or carbon dioxide (CO2). The loss of water to an ion with m/z 298 is followed by the losses of hydrogen fluoride (HF), carbon monoxide (CO) or both to the ions with m/z 278, 270, and 250. The loss of CO2 to an ion with m/z 272 is followed by a variety of losses, of which those of HF to m/z 252, ethene (C2H4) from the cyclopropyl moiety to m/z 244, of Cl• and HCl to m/z 237 and 236, respectively, and of cyclopropene (C3H4) to m/z 232 lead to the more abundant product ions (Fig. 3 ) [64].
Fig. 3.
Structure and fragmentation scheme of the non-nucleoside reverse transcriptase inhibitor efavirenz in both positive-ion and negative-ion mode.
In negative-ion mode, efavirenz ([M−H]– with m/z 314) shows the loss of trifluoromethane (CHF3) to a product ion with m/z 244; the trifluoromethanide ion ([CF3]–) with m/z 69 is observed as well. The ion with m/z 250 is due to the loss of vinylidenecyclopropene (C5H4) from the C 4-substituent of the benzoxazinone cyclic structure (Fig. 3) [36].
4.5. Etravirine
The fragmentation of etravirine is somewhat difficult to understand, because widely different MS–MS spectra have been reported (cf. [14,58]) and accurate-m/z data for etravirine itself were not found; they were only given for the metabolites [65]. Etravirine ([M+H]+ with m/z 435) shows product ions with m/z 144.056 ([C8H5N3]+), that is the protonated (4-cyanophenyl)cyanamide, the odd-electron ion with m/z 288.996 ([C11H8BrN5]+•) due to the loss of the (4-cyano-2,6-dimethylphenyl)oxidanyl radical (C9H8NO•), the ion with m/z 162.950 ([C3H4BrN2O]+), the ion with m/z 305.998 ([C11H9BrN5O]+) due to the loss of C9H7N, and the ion with m/z 303.983 ([C11H7BrN5O]+) due to the loss of C9H9N (Fig. 2). This interpretation is based on metabolism studies [65,66]. The observation of the product ion with m/z 304 for the [D8]-labelled analogue (with labels on the 4-cyano-2,6-dimethylphenyl moiety) [67] and for the [13C6]-labelled analogues (with labels at the phenyl ring in the 4-cyanophenyl moiety) [21] also supports part of the interpretation.
4.6. Nevirapine
In positive-ion mode, nevirapine ([M+H]+ with m/z 267) shows the loss of either cyclopropene (C3H4) to an ion with m/z 227 or the cyclopropyl radical (•C3H5) to an odd-electron ion with m/z 226, which shows subsequent loss of carbon monoxide (CO) to an ion with m/z 198 ([C11H10N4]+•) (Fig. 2). At higher collision energy, a cleavage in the 1,4-diazepinone ring results in the amino-4-methylpyridinylium ion ([C6H7N2]+) with m/z 107 with the amino substituent and the charge on either the 2- or 3-position. In principle, the ion with m/z 80 can be interpreted as protonated pyridine ([C5H6N]+), but the +3-Da shift of this product ion in the spectrum of the [D3]-labelled analogue (labelling of the 4-methyl substituent) [20] indicates that the ion with m/z 80 is more likely the 3-methyl-1H-pyrrol-2-ylium ion (loss of hydrogen cyanide (HCN) from the ion with m/z 107) (Fig. 2).
4.7. Rilpivirine
Rilpivirine ([M+H]+ with m/z 367) shows product ions with m/z 350 due to an unexpected loss/extrusion of ammonia (NH3), with m/z 340 due to the loss of hydrogen cyanide (HCN), with m/z 224 due to a cleavage in the pyrimidine ring and the loss of (4-cyanophenyl)cyanamide (C8H5N3), and with m/z 195 due to another cleavage in the pyrimidine ring and the loss of (e.g.) 4-[(1,3-diazet-2-yl)amino]benzonitrile (C9H6N4) (Fig. 2). The [D6]-labelled analogue (labeling at the two methyl substituents) results in an ion with m/z 201 [57], confirming this interpretation. Alternative structure proposals have been given elsewhere [68], but also based on tandem quadrupole data. The odd-electron product ion with m/z 128, due to a homolytic cleavage of the C–N bond at the dimethylphenyl moiety followed by the loss of HCN, has been used in SRM as well [59]. Extensive fragmentation of rilpivirine is observed by others [69]. Unfortunately, no accurate-m/z data have been found.
5. Protein inhibitors (PIs)
Protease inhibitors (PIs) are antiviral drugs that prevent viral replication by selectively binding to viral proteases and thereby blocking the proteolytic cleavage of protein precursors needed for the replication of the virus. Antiretroviral HIV-related PIs have the suffix -navir. Because of the high inter- and intra-individual variability in the effectiveness of these drugs, therapeutic drug monitoring is required for these drugs. As a result, multi-analyte LC–MS methods for the bioanalysis PIs have been reported [[12], [13], [14],[17], [18], [19], [20], [21], [22], [23],54,70] and reviewed [6,7]. The protease inhibitors are generally analyzed in positive-ion mode. Their fragmentation mostly involves cleavages of C–N bonds in the amine and amide moieties. The position of the cleavage and the charge retention on the fragments is not always easy to predict. Characteristic SRM transitions used in the bioanalysis of HIV-related protease inhibitors are summarized in Table 4 .
Table 4.
SRM transitions used in the bioanalysis of HIV-related protein inhibitor antiviral drugs in positive-ion mode.
| Compound | m/z of [M+H]+ | Formula | m/z of SRM product-ion | Formula | Literature |
|---|---|---|---|---|---|
| amprenavir (AMP) | 506.232 | [C25H36N3O6S]+ | 245.165 | [C15H21N2O]+ | [11,13,14,16,18,70] |
| 156.011 | [C6H6NO2S]+ | [17,54] | |||
| 418.180 | [C21H28N3O4S]+ | [70] | |||
| atazanavir | 705.397 | [C38H3N6O7]+ | 168.081 | [C12H10N]+ | [14,16,54,55,71] |
| 335.197 | [C18H27N2O4]+ | [17,23,70] | |||
| 534.307 | [C30H40N5O4]+ | [18,55,70] | |||
| 144.102 | [C7H14NO2]+ | [19] | |||
| cobicistat | 776.397 | [C38H53N6O7]+ | 98.006 | [C4H4NS]+ | [19] |
| 606.274 | [C32H40N5O5S]+ | [19,23] | |||
| indinavir (IDV) | 614.370 | [C36H48N5O4]+ | 465.286 | [C27H37N4O3]+ | [70] |
| 421.236 | [C25H31N3O3]+• | [11,13,14,16,18,54] | |||
| 364.202 | [C22H26N3O2]+ | [21] | |||
| 97.076 | [C5H9N2]+ | [19] | |||
| lopinavir (LPV) | 629.370 | [C37H49N4O5]+ | 183.113 | [C9H15N2O2]+ | [19,54] |
| 155.118 | [C8H15N2O]+ | [17,55] | |||
| 429.254 | [C28H33N2O2]+ | [17,55] | |||
| 447.264 | [C28H35N2O3]+ | [11,13,14,16,18,55,70] | |||
| nelfinavir (NFV) | 568.320 | [C32H46N3O4S]+ | 467.236 | [C27H35N2O3S]+ | [70] |
| 330.116 | [C18H20NO3S]+ | [11,13,14,70] | |||
| 135.044 | [C8H7O2]+ | [19] | |||
| nelfinavir-M8 | 584.315 | [C32H46N3O5S]+ | 330.116 | [C18H20NO3S]+ | [13,14] |
| ritonavir (RTV) | 721.320 | [C37H49N6O5S2]+ | 426.185 | [C23H28N3O3S]+ | [23,55,70] |
| 296.143 | [C14H22N3O2S]+ | [13,14,16,18,22,54,55,70] | |||
| 268.148 | [C13H22N2OS]+ | [11,17,22,55,70] | |||
| saquinavir | 671.392 | [C38H51N6O5]+ | 570.307 | [C33H40N5O4]+ | [11,13,14,16,55,70] |
| 433.187 | [C24H25N4O4]+ | [55,70] | |||
| 416.160 | [C24H22N3O4]+ | [55] | |||
| 242.092 | [C13H12N3O2]+ | [18] | |||
| 225.066 | [C13H9N2O2]+ | [17,54] | |||
| 128.049 | [C9H6N]+ | [19] |
5.1. Amprenavir
Amprenavir ([C25H36N3O6S]+, [M+H]+ with m/z 506) shows a product ion with m/z 156, that is the 4-aminobenzenesulfonyl ion ([C6H6NO2S]+) (Fig. 4 ). Other product ions are the ion with m/z 418 due to the loss of oxolan-3-ol (C4H8O2), with m/z 392 due to the loss of C5H6O3, e.g., 5,6-dihydro-2H-furo[2,3-d] [,1,3]dioxole or protonated 4-benzyl-1-(2-methylpropyl)-3,4-dihydropyrimidin-2(1H)-one, with m/z 245 ([C15H21N2O]+), which is due to the losses of water and 4-(dioxo-λ6-sulfanylidene)cyclohexa-2,5-dien-1-imine (C6H5NO2S) from the ion with m/z 418; a structure involving a ring closure is proposed (Fig. 4). The fragmentation is confirmed by accurate-m/z data [72].
Fig. 4.
Structures and fragmentation scheme of the protein inhibitor (PI) antiviral drugs amprenavir, atazanavir, cobicistat, and indinavir in positive-ion mode.
5.2. Atanazavir
Atanazavir ([C38H3N6O7]+, [M+H]+ with m/z 705) shows product ions with m/z 612 due to the loss of water and methyl carbamate (C2H5NO2), with m/z 534 due to the cleavage of an amide bond with charge retention on the amine side (the loss of C8H13NO3), with m/z 335 due to the cleavage of the C–N bond to the formohydrazide moiety, with m/z 168, that is the [4-(pyridin-2-yl)benzyl ion ([C12H10N]+), and with m/z 144, which is the 1-[(methoxycarbonyl)amino]-2,2-dimethylprop-1-ylium ion ([C7H14NO2]+) (Fig. 4) [73,74]. The identity of the product ions is confirmed by accurate-m/z data, obtained in the course of the identification of forced degradation products, where also additional minor product ions have been discussed [75].
5.3. Cobicistat
Cobicistat is used as pharmacokinetic enhancer of atanazavir and darunavir. Cobicistat ([M+H]+ with m/z 776) shows a major product ion with m/z 606 due to the loss of N-methyl-1-[2-(propan-2-yl)-1,3-thiazol-4-yl]methanamine (C8H14N2S) (Fig. 4). The loss of morpholine (C4H9NO) leads to a product ion with m/z 689. In addition, product ions are observed with m/z 98, that is the (1,3-thiazol-5-yl)methylium ion ([C4H4NS]+), and with m/z 140, that is the [2-(propan-2-yl)-1,3-thiazol-4-yl]methylium ion ([C7H10NS]+). Cleavage in the urea moiety leads to product ions with m/z 197 and, after water loss, m/z 562, and to product ions with m/z 171 and 606. Secondary fragmentation of the ion with m/z 606 leads to product ions with m/z 491 due to the loss of (1,3-thiazol-5-yl)methanol (C4H5NOS), with m/z 448 due to the subsequent loss of hydrogen isocyanate (HNCO). The loss of morpholine from the ion with m/z 448 leads to the ion with m/z 361, whereas the cleavage of the amide bond in the ion with m/z 448 leads to an ion with m/z 250 (Fig. 4). The identity of the product ions is confirmed by accurate-m/z data acquired in the course of a metabolite study [76] and of identification of forced degradation products [75].
5.4. Indinavir
The fragmentation of indinavir ([M+H]+ with m/z 614) has been described in the context of several metabolite studies [[77], [78], [79], [80]] and a forced degradation study [81]. The product ion with m/z 513 is due to the loss of N-tert-butylformamide (C5H11NO) (Fig. 4). Subsequent losses of the (pyridin-3-yl)methyl radical (•C6H6N) or of 2-amino-indan-1-ol (C9H11NO) leads to the product ions with m/z 421 and 364, respectively. The product ion with m/z 465 results from the cleavage of an amide bond and the loss of 2-amino-indan-1-ol. Cleavages of the N–C bonds on either side of the piperazine moiety leads to the odd-electron ion with m/z 421 ([C25H31N3O3]+•), and the ion with m/z 92, that is the (pyridin-3-yl)methylium ion ([C6H6N]+), and the product ions with m/z 277 ([C15H25N4O]+) and m/z 338 ([C21H24NO3]+) (Fig. 4). Finally, the 1-methylidenepiperazinium (iminium) ion with m/z 97 ([C5H9N2]+) and the 5-benzyl-6-oxopiperidin-2-ylium ion (assuming ring closure) ([C12H14NO]+) with m/z 188 are observed as well (Fig. 4). Water losses are observed for [M+H]+ and the ions with m/z 421 and 338.
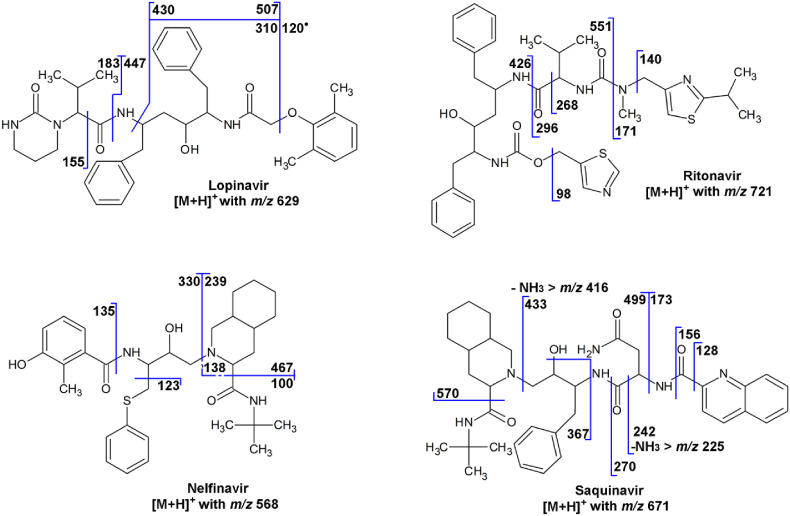
5.5. Lopinavir
Lopinavir ([M+H]+ with m/z 629) shows product ions resulting from the cleavage of one of the two amide bonds, resulting in two complementary ions, i.e., the ion with m/z 447 due the loss of 3-methyl-2-(2-oxo-1,3-diazinan-1-yl)but-2-enal (C9H14N2O2) and the ion with m/z 183 ([C9H15N2O2]+) (Fig. 5 ). Loss of carbon monoxide (CO) from the ion with m/z 183 results in the ion with m/z 155. Loss of 3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide (C9H17N3O2) and 2-methyl-6-methylidenecyclohexa-2,4-dien-1-one (C8H8O) leads to the ion with m/z 310. An odd-electron ion with m/z 120 ([C8H8O]+•) is also observed [82] (Fig. 5). This interpretation in accordance with accurate-m/z data [83]. Note that, unlike the structure proposal for the ion with m/z 310 given elsewhere [83], the structure proposal given here is in accordance with the nitrogen rule (even m/z, thus odd number of N in an even-electron ion).
Fig. 5.
Structures and fragmentation scheme of the protein inhibitor (PI) antiviral drugs lopinavir, nelfinavir, ritonavir, and saquinavir in positive-ion mode.
Lopinavir may be administered as a prodrug, e.g., a succinic acid ester ([M+H]+ with m/z 729; product ion in SRM with m/z 447), a glutaric acid ester ([M+H]+ with m/z 743; product ion with m/z 429), or a diglycolic acid ester ([M+H]+ with m/z 745; product ion with m/z 563) [84].
5.6. Nelfinavir
The fragmentation of nelfinavir ([C32H46N3O4S]+, [M+H]+ with m/z 568) has been discussed in relation to the identification of degradation products [85] and in a drug metabolite study [86]. The major product ions are the ion with m/z 467 due to the loss of N-tert-butylformamide (C5H11NO), the ion with m/z 330 due to the subsequent loss of octahydroisoquinoline (C9H15N), the ion with m/z 239, which is complementary to the ion with m/z 330, the ion with m/z 135, which is the (3-hydroxy-2-methylphenyl)acylium ion ([C8H7O2]+), and the ion with m/z 123, which is the (phenylsulfanyl)methylium ion ([C7H7S]+) (Fig. 5). In addition, numerous minor product ions have been observed. Next to nelfinavir, therapeutic drug monitoring often involves the pharmacologically active M8 metabolite, involving hydroxylation at the tert-butyl moiety [13].
5.7. Ritonavir
Ritonavir ([M+H]+ with m/z 721) shows a product ion with m/z 296 due to the cleavage of an amide bond, resulting in the 3-methyl-2-[(methyl{[2-(propan-2-yl)-1,3-thiazol-4-yl]methyl}carbamoyl)amino]-1-oxobut-1-ylium ion ([C14H22N3O2S]+) and its complementary ion with m/z 426, and well as an ion with m/z 268 due to the loss of carbon monoxide (CO) from the ion with m/z 296 (Fig. 5). Product ions with m/z 140, that is the [2-(propan-2-yl)-1,3-thiazol-4-yl]methylium ion ([C7H10NS]+), and with m/z 98, that is the (1,3-thiazol-5-yl)methylium ion ([C4H4NS]+), are also observed. The ions with m/z 171 and 551 are complementary ions resulting from another amide bond cleavage (Fig. 5) [79]. The interpretation is confirmed by accurate-m/z data [87]. The interpretation for the ion with m/z 296 ([C14H22N3O2S]+; m/z 296.143) differs from the one given elsewhere [13], as that one is not in agreement with the accurate-m/z data ([C18H25N2O]+; m/z 285.196).
5.8. Saquinavir
Saquinavir ([M+H]+ with m/z 671) shows product ions with m/z 570 due to the loss of N-tert-butylformamide (C5H11NO), the subsequent loss of octahydroisoquinoline (C9H15N) to the ion with m/z 433, which can show loss of ammonia (NH3) to m/z 416 (Fig. 5). Cleavage of one of the amide bonds leads to an acylium ion with m/z 270, which shows the subsequent losses of carbon monoxide (CO) and ammonia to the ions with m/z 242 and 225, whereas cleavage of the other amide bond leads to an acylium ion with m/z 156, which shows the subsequent loss of carbon monoxide to the ion with m/z 128, that is the quinolin-2-ylium ion ([C9H6N]+). Some other product ions are indicated in the fragmentation scheme (Fig. 5). The identities of the product ions have been confirmed from accurate-m/z data from a forced degradation study [88].
6. Non-peptide protein inhibitors (npPIs)
Protein inhibitors like ritonavir (Section 5.7) are based on the peptide structure and contain a number of amide (peptide) bonds. As such, they resemble the protein that is the substrate of the HIV protease. Thus, the protease inhibition is based on competition with the substrate for the active site of the protease. However, not all protease inhibitors have this structure similarity to the substrate. This is the case for non-peptide protease inhibitors (npPIs) like the ones discussed in this section. The npPIs are generally administered in combination with a peptide protein inhibitor [10]. Characteristic SRM transitions used in the bioanalysis of HIV-related npPIs are summarized in Table 5a,b .
Table 5a.
SRM transitions for three other classes of HIV-related antiviral drugs in positive-ion mode: non-peptide protein inhibitors (npPIs), integrase inhibitors (INIs), and C–C chemokine receptor type 5 inhibitors (CCR5).
| Compound | Class | m/z of [M+H]+ | Formula | m/z of SRM product-ion | Formula | Literature |
|---|---|---|---|---|---|---|
| Non-peptide protein inhibitors (npPIs) | ||||||
| darunavir | npPI | 548.242 | [C27H38N3O7S]+ | 436.190 | [C21H30N3O5S]+ | [22] |
| 392.200 | [C20H30N3O3S]+ | [14,16,18,22,23,54,56] | ||||
| 156.011 | [C6H6NO2S]+ | [17] | ||||
| 69.033 | [C4H5O]+ | [19] | ||||
| tipranavir | npPI | 603.214 | [C31H34F3N2O5S]+ | 411.062 | [C18H14F3N2O5S]+ | [14,16] |
| 201.082 | [C12H10FN2]+ ? | [18] | ||||
| 172.039 | [C10H6NO2]+ | [54] | ||||
| Integrase inhibitors (INIs) | ||||||
| bictegravir | INI | 450.127 | [C21H19F3N3O5]+ | 289.082 | [C14H13N2O5]+ | [23,89,90] |
| 145.026 | [C7H4F3]+ | [90] | ||||
| cabotegravir | INI | 406.121 | [C19H18F2N3O5]+ | 263.066 | [C12H11N2O5]+ | [91,92] |
| dolutegravir (DTG) | INI | 420.137 | [C20H20F2N3O5]+ | 277.082 | [C13H13N2O5]+ | [19,22,42,89,93] |
| 127.052 | [C7H5F2]+ | [19,22,23] | ||||
| 136.003 | [C6H2NO3]+ | [93] | ||||
| 126.055 | [C6H8NO2]+ | [42] | ||||
| elvitegravir | INI | 448.132 | [C23H24ClFNO5]+ | 430.122 | [C23H22ClFNO4]+ | [19,22] |
| 344.048 | [C18H12ClFNO3]+ | [18,22,23,42] | ||||
| 143.006 | [C7H5ClF]+ | [42] | ||||
| raltegravir | INI | 445.163 | [C20H22FN6O5]+ | 361.131 | [C17H18FN4O4]+ | [22,42,[40], [55],94] |
| 109.045 | [C7H6F]+ | [16,18,22,23,42,55,94] | ||||
| 193.077 | [C10H10FN2O]+ | [55] | ||||
| C–C chemokine receptor type 5 inhibitors (CCR5) | ||||||
| maraviroc (MVC) | CCR5 | 514.335 | [C29H42F2N5O]+ | 389.240 | [C23H31F2N2O]+ | [17,55,95] |
| 280.151 | [C16H20F2NO]+ | [[54], [55], [56]] | ||||
| 117.070 | [C9H9]+ | [18,55] | ||||
Table 5b.
SRM transitions for three other classes of HIV-related antiviral drugs in negative-ion mode.
6.1. Darunavir
The major product ion of darunavir ([M+H]+ with m/z 548) is the ion with m/z 392. The identity of this ion is not completely clear. Based on the accurate-m/z initially reported [98], using an AB-Sciex QSTAR XL quadrupole–time-of-flight hybrid (Q–TOF) instrument, it should be interpreted as an even-electron ion ([C20H30N3O3S]+; m/z 392.200), due to the losses of tetrahydrofuro[2,3-b]furan (C6H8O2) and carbon dioxide (CO2). However, according to accurate-m/z data in a later report [99], using an Agilent Model 6510 Q–TOF instrument, it should be interpreted as an odd-electron ion ([C21H32N2O5]+•; m/z 392.231) due to the loss of the (4-aminophenyl)(dioxo)-λ6-sulfanyl radical (C6H6NO2S•). Both possibilities, which can be considered to be expected losses, are indicated in Fig. 6 . Note that the radical loss is also observed for tipranavir, also having a sulfonamide moiety. On the other hand, such radical losses are not commonly observed for sulfonamides [5].
Fig. 6.
Structures and fragmentation scheme of the non-peptide protein inhibitor (npPI) antiviral drugs darunavir and tipranavir in positive-ion mode.
Next to the ion with m/z 392, product ions are observed with m/z 436 due to the loss of tetrahydrofuro[2,3-b]furan (C6H8O2), with m/z 241 due to the subsequent loss of 2-amino-3-phenylpropan-1-ol (C9H13NO), with m/z 156, which is the 4-aminobenzenesulfonyl ion ([C6H6NO2S]+), and with m/z 113, which is the hexahydrofuro[2,3-b]furan-3-ylium ion ([C6H9O2]+), which in turn fragments to an ion with m/z 69 ([C4H5O]+) (Fig. 6). The identity of these product ions is confirmed by accurate-m/z data [98,99].
6.2. Tipranavir
Tipranavir ([M+H]+ with m/z 603) shows a product ion with m/z 585 due to the loss of water, and with 521 due to the subsequent extrusion of sulfur dioxide (SO2). The ion with m/z 411 has been explained as an odd-electron ion ([C19H16F3NO4S]+•; m/z 411.075) being due to the loss of a propyl radical (•C3H7) and extrusion of the C3H7-substituted aniline (as C9H9N) from the ion with m/z 585, thus involving a rearrangement of the dioxo[5-(trifluoromethyl)pyridin-2-yl]-λ6-sulfanyl moiety [73]. However, accurate-m/z data indicate an even-electron ion with m/z 411.062 ([C18H14F3N2O5S]+), thus involving a cleavage of the 6-hydroxy-2,3-dihydro-4H-pyran-4-one moiety [100] (see Fig. 6 for both alternatives). The ion with m/z 375 ([C25H29NO2]+•) is due to the loss of water and the dioxo[5-(trifluoromethyl)pyridin-2-yl]-λ6-sulfanyl radical (C6H3F3NO2S•). Subsequent loss of another water results in the ion with m/z 357, whereas the loss of propene (C3H6) results in the ion with m/z 333 ([C22H23NO2]+•; m/z 333.172), which seems more likely than the suggestion [100] of the loss of water and CO2 ([C24H31N]+•; m/z 333.245). The ion with m/z 201 has been suggested to be the 4-oxo-2-(2-phenylethyl)-3,4-dihydro-2H-pyran-3-ylium ion ([C13H13O2]+ with m/z 201.091) [73], but accurate-m/z data indicate an odd-electron ion with m/z 201.078 ([C12H11NO2]+•) (Li et al., 2010) (see Fig. 6 for both alternatives). The most likely elemental composition for the ion with m/z 172.041 [100] seems to be [C10H6NO2]+, which would be consistent with the effective loss of an ethyl radical (•C2H5) from the ion with m/z 201. The structures proposed for the ions with m/z 201 and 172 are based on this feature. The interpretation is confirmed by accurate-m/z data [24,100].
7. Integrase inhibitors (INI)
Integrase inhibitors (INIs) are antiretroviral drugs that block the action of the viral integrase enzyme, which inserts the viral genome into the DNA of the host cell. Blocking the integrase enzyme stops the retroviral replication. The first FDA approved INI is raltegravir. Since then, other INIs have been developed or are under development. In general, limited MS data are available for this class of antiviral drugs. They are mainly used in combination therapies, e.g., in combination with tenofovir alafenamide (TAF) and emtricitabine (FTC) [10].
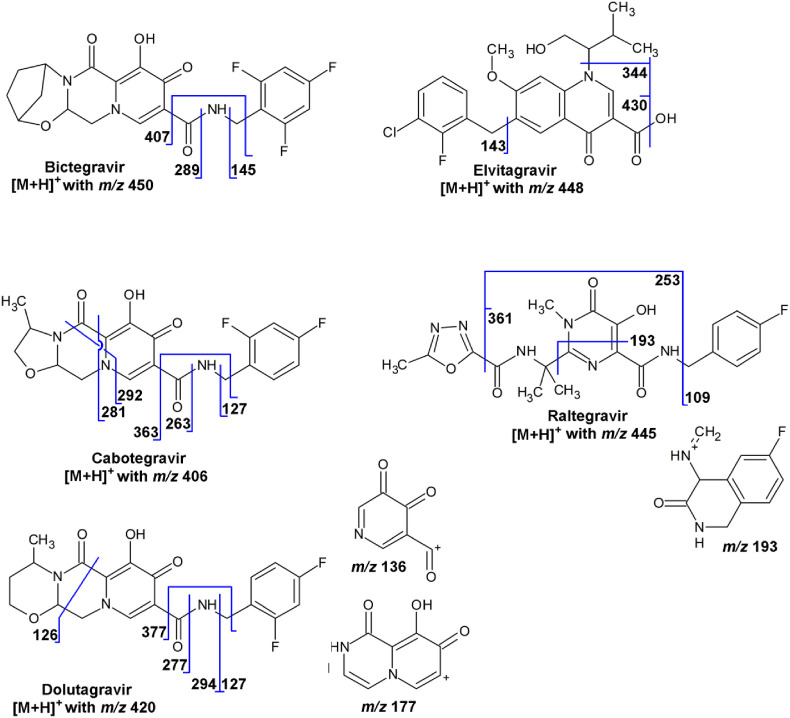
For the carbamoyl pyridone INIs bictegravir, cabotegravir, and dolutegravir, a common neutral loss of 43 Da is observed, consistent with the loss of hydrogen isocyanate (HNCO). In principle, the loss of HNCO could arise from the piperazin-2-one moiety, but this would require the cleavage of a substantial number of bonds. An alternative proposal would be the extrusion of HNCO from the amide bond, requiring the rearrangement of the trifluorobenzyl (with bictegravir) or the difluorobenzyl moiety (with cabotegravir and dolutegravir). Rearrangements of benzyl groups have been observed more often for various compound classes [5]. In the fragmentation schemes in Fig. 7 , the latter explanation is assumed to be correct. Further fragmentation of the [M+H-43]+-ions as precursor ion would be required to confirm the rearrangement.
Fig. 7.
Structures and fragmentation scheme of the integrase inhibitor (INI) antiviral drugs in positive-ion mode.
7.1. Bictegravir
The major product ion of bictegravir ([M+H]+ with m/z 450) is the ion with m/z 289 due to cleavage of the amide bond and the formation of an acylium ion, thus involving the loss of 1-(2,4,6-trifluorophenyl)methanamine (C7H6F3N). Other product ions are the ions with m/z 432 due to the loss of water, the ion with m/z 407 due to the loss of hydrogen isocyanate (HNCO) and the rearrangement of the trifluorobenzyl moiety, and the ion with m/z 145, which is the 2,4,6-trifluorobenzyl ion ([C7H4F3]+) (Fig. 7).
7.2. Cabotegravir
Cabotegravir ([M+H]+ with m/z 406) is an investigational new INI with a carbamoyl pyridone structure. The product ion with m/z 263, which is due to cleavage of the amide bond and the formation of an acylium ion, thus involving the loss of 1-(2,4-difluorophenyl)methanamine (C7H7F2N), is used in SRM [91,92]. The ion with m/z 363 is due to the loss of hydrogen isocyanate (HNCO) with the rearrangement of the difluorobenzyl moiety (m/z 363.115; [C18H17F2N2O4]+). Other product ions are the ions with m/z 292 and 281, which are possibly due to a ring-through cleavages of the piperazin-2-one moiety (m/z 292.042; [C14H8F2NO4]+ and m/z 281.073; [C13H11F2N2O3]+), and the ion with m/z 127 which is the 2,4-difluorobenzyl ion ([C7H5F2]+) (Fig. 7). Accurate-m/z data for cabotegravir were not found.
7.3. Dolutegravir
Dolutegravir ([C27H38N3O7S]+, [M+H]+ with m/z 420) shows product ions with m/z 127, which is the 2,4-difluorobenzyl ion ([C7H5F2]+), and with m/z 277, which is due to the loss of 1-(2,4-difluorophenyl)methanamine (C7H7F2N) (Fig. 7) [89,93]. Other product ions are the ions with m/z 402 due to the loss of water, with m/z 377, due to the loss of hydrogen isocyanate (HNCO) with the rearrangement of the difluorobenzyl moiety, with m/z 177, possibly [C8H5N2O3]+ (m/z 177.029), and the ion with m/z 136, possibly [C6H2NO3]+ (m/z 136.003) (Fig. 7). The identity of the product ions is partly confirmed by accurate-m/z data for metabolites of dolutegravir [101] and a study into its degradation products [102].
7.4. Elvitegravir
Elvitegravir ([C23H24ClFNO5]+, [M+H]+ with m/z 448) shows product ions with m/z 430 due to the loss of water and with m/z 344 due to the subsequent loss of C5H10O, e.g., 3-methylbut-2-en-1-ol. The product ion with m/z 143 is the 3-chloro-2-fluorobenzyl ion ([C7H5ClF]+) (Fig. 7). No accurate-m/z data were found.
7.5. Raltegravir
Raltegravir ([C20H22FN6O5]+, [M+H]+ with m/z 445) shows product ions with m/z 361 due to the loss of 2-methyl-1,3,4-oxadiazole (C3H4N2O) and with m/z 109, which is the 4-fluorobenzyl ion ([C7H6F]+) (Fig. 7). The loss of both these groups (an effective loss of C10H9FN2O) leads to the ion with m/z 253. The loss of the 5-methyl-N-(prop-1-en-2-yl)-1,3,4-oxadiazole-2-carboxamide (C7H9N3O2) and the cleavage of the ring with loss of N-methylidene-2-oxoacetamide (C3H3NO2) leads to the ion with m/z 193 ([C10H10FN2O]+), for which a structure is proposed (6-fluoro-4-(methylideneamino)-1,4-dihydroisoquinolin-3(2H)-one), based on the assumption of ring closure (Fig. 7). This fragmentation scheme was reported in a comparison between LC–MS in SRM mode and accurate-m/z mass spectrometry imaging of raltegravir in tissue [103].
The analysis of raltegravir ([M−H]– with m/z 443) in negative-ion mode has also been reported, using the product-ions with m/z 316 ([C16H15FN3O3]– with m/z 316.110), which is due to the loss of 5-methyl-1,3,4-oxadiazole-2-carboxamide (C4H5N3O2). In addition, in negative-ion mode, product ions are observed with m/z 276 ([C13H11FN3O3]– with m/z 276.079) due to the loss of 5-methyl-N-(prop-1-en-2-yl)-1,3,4-oxadiazole-2-carboxamide (C7H9N3O2) and with m/z 165, possibly an ion with m/z 165.067 ([C8H9N2O2]–) [96,97]. No accurate-m/z data were found.
8. C–C chemokine receptor type 5 inhibitors
The C–C motif chemokine receptor CCR5 is involved in the process by which HIV enters the cell. Compounds like maraviroc are CCR5 antagonist, that in effect inhibit the viral entry into human cells. Other CCR5 inhibitors are aplaviroc, nifeviroc, vicriviroc and cenicriviroc; very limited MS–MS data are available for these compounds (except for maraviroc).
8.1. Maraviroc
Maraviroc ([C29H42F2N5O]+, [M+H]+ with m/z 514) shows product ions with m/z 389 due to the loss of 3-methyl-5-(propan-2-yl)-4H-1,2,4-triazole (C6H11N3), with m/z 280 due to the loss of 3-[3-methyl-5-(propan-2-yl)-4H-1,2,4-triazol-4-yl]-8-azabicyclo[3.2.1]octane (C13H22H4), and with m/z 252 due to a cleavage at the tertiary carbon atom (Fig. 8 ). The ion with m/z 117 is [C9H9]+ (m/z 117.070) due to the loss of 4,4-difluorocyclohexane-1-carboxamide (C7H11F2NO) from the ion with m/z 280, whereas the ion with m/z 106 is the amino(phenyl)methylium ion ([C7H8N]+) (Fig. 8). The fragmentation is partly based on data from a metabolite study [104] and confirmed by accurate-m/z data [105].
Fig. 8.
Structures and fragmentation scheme of the C–C motif chemokine receptor CCR5 inhibitor antiviral drugs in positive-ion mode.
8.2. Vicriviroc
The major product ion of vicriviroc ([C28H39F3N5O2]+, [M+H]+ with m/z 534) is the ion with m/z 303 ([C15H22F3N2O]+ with m/z 303.168) which is due to the cleavage of the C–N4 bond of the piperazine moiety; the complementary ion with m/z 232 ([C13H18N3O]+ with m/z 232.144) is also observed. Subsequent loss of methanol (CH3OH) from the ion with m/z 303 results in the ion with m/z 271 ([C14H19F3N2]+ with m/z 272.150). The ion with m/z 135 results from the cleavage of the amide bond and the formation of the (4,6-dimethylpyrimidin-5-yl)(oxo)methylium ion ([C5H7N2O]+ with m/z 135.055), whereas the ion with m/z 101 is the protonated 2-methylpiperazine ([C5H13N2]+ with m/z 101.107) (Fig. 8) [106].
8.3. Other CCR5 inhibitors
The pharmacokinetic analysis of nifeviroc ([C33H43N4O6]+; [M+H]+ with m/z 591) was described [107], using the product ion with m/z 332 ([C17H22N3O4]+; m/z 332.160) in SRM, which is due to the cleavage of a tertiary C–C bond at the pyrrolidine moiety and the formation of a 4-substituted 1-methylidenepiperidin-1-ium ion (Fig. 8). Its development as a drug has most likely been discontinued.
The analysis of cenicriviroc ([C41H3N4O4S]+; [M+H] with m/z 697) was recently reported [108]. The product ion with m/z 574 was used in SRM. Based on the fact that the same product ion is observed for the [D7]-labelled internal standard (with labels at the propyl substituent of the imidazole moiety), the fragmentation should involve the loss of the (1-propyl-1H-imidazole-5-yl)methyl group (C7H10N2; 123.092) to an odd-electron product ion ([C34H42N2O4S]+•; m/z 574.286) (Fig. 8).
9. General aspects of the fragmentation of HIV-related antivirals
The fragmentation of even-electron ions, like the protonated molecules [M+H]+ or deprotonated molecules [M−H]– of antiviral agents, as studied here, usually complies with the even-electron rule [5,109,110], which means that only even-electron product ions are generated. The primary fragmentation of a protonated molecule is a backbone cleavage via either an inductive cleavage or a four-center H-rearrangement, involving the cleavage of a C–heteroatom (N, O, S) bond. This may lead to two product ions with charge retention of either site of the cleavage, where the relative abundance of the two product ions is determined by Field’s rule [111] as well as the relative stability of the product ions. A characteristic feature of these complementary product ions is that the sum of their m/z-values equals m/z ([M+H]+1). In the current data set, this behavior is for instance observed for delavirdine ([M+H]+ with m/z 457; the ions with m/z 221 and 237; Fig. 2), for indinavir ([M+H]+ with m/z 614; the ions with m/z 277 and 338; Fig. 4), and for ritonavir ([M+H]+ with m/z 721; the ions with m/z 426 and 296 as well as the ions with m/z 551 and 171; Fig. 5). However, often only one of the two expected ions is actually observed, e.g., when the two ions show widely different proton affinities, such as for most of the NRTIs (Fig. 1), or when one of the two ions readily shows secondary fragmentation. The latter is often difficult to predict. Numerous other product ions are due to cleavages of a C–heteroatom (N, O, S) bond, with or without H-rearrangement.
The formation of the two complementary ions relies on the ability to perform a H-rearrangement. In some cases, the H-rearrangement is hindered by structural features, such as with di-aromatic ethers or amines. In such cases, often a homolytic cleavage of one of the two C–heteroatom bonds is observed, thus resulting in an odd-electron product ions and the loss of a radical. This is for instance observed for etravirine (the ion with m/z 289; Fig. 2) and rilpivirine (the ion with m/z 128; Fig. 2).
Another violation of the even-electron rule involves the loss of relatively stable radicals, like halogens (•Cl and •Br), nitrosyl (•NO), nitryl (•NO2), or methyl(dioxo)-λ6-sulfanyl (•SO2CH3) radicals as well as losses of methyl (•CH3) or methoxy (•OCH3) radicals from aromatic methoxy compounds [5,110,112,113]. In the current data set, this is observed for delavirdine (loss of •SO2CH3) and doravirine (secondary loss of •Cl) (Fig. 2), and for efavirenz (secondary loss of •Cl) (Fig. 3). Such radical losses are in competition with the loss of the corresponding neutral, e.g., the ions with m/z 237 (loss of •Cl) and m/z 236 (loss of HCl) for efavirenz (Fig. 3) or the ions with m/z 226 (loss of the cyclopropyl radical, •C3H5) and m/z 227 (loss of cyclopropene, C3H4). The loss of the (4-aminophenyl)(dioxo)-λ6-sulfanyl radical (C6H6NO2S•) from darunavir and of the dioxo[5-(trifluoromethyl)pyridin-2-yl]-λ6-sulfanyl radical (C6H3F3NO2S•) from tipranavir is probably comparable to the loss of the methyl(dioxo)-λ6-sulfanyl radical (•SO2CH3).
One of the most fascinating fragmentation reactions is a skeletal rearrangement [5], involving an extrusion reaction in combination with the rearrangement of a bulky group. In the current data set, this is most likely occurring for the carbamoyl pyridone INIs bictegravir, cabotegravir, and dolutegravir, where extrusion of hydrogen isocyanate in combination with a benzyl rearrangement is observed. In those cases, accurate-m/z data are needed to ascertain the loss and MSn experiments to elucidate the structure of the resulting product ion. The MSn data are not available for the INIs.
10. Conclusion
Antiviral agents are important in the treatment of HIV infection. Often, combination therapies are applied. Therapeutic drug monitoring is an important practice in this field. For this, LC–MS in SRM mode is the method of choice. In this study, the various product ions applied in SRM of some 40 antiviral drugs as well as, when applicable, their phosphorylated anabolites have been collected and identified. In addition, available information on the major product ions of these HIV-related antiviral drugs has been collected and reviewed. In some cases, proposed structures of product ions can be corrected, based on accurate-m/z data.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijms.2020.116370.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Baltimore D. Expression of animal virus genomes. Bacteriol Rev Sep. 1971;35(3):235–241. doi: 10.1128/MMBR.35.3.235-241.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niessen W.M.A. Analysis of antibiotics by liquid chromatography - mass spectrometry. J. Chromatogr. A. 1998;812:53–76. doi: 10.1016/s0021-9673(98)00281-7. [DOI] [PubMed] [Google Scholar]
- 3.Niessen W.M.A. Fragmentation of toxicologically relevant drugs in positive-ion liquid chromatography–tandem mass spectrometry. Mass Spectrom. Rev. 2011;30:626–663. doi: 10.1002/mas.20332. [DOI] [PubMed] [Google Scholar]
- 4.Falck D., Kool J., Honing M., Niessen W.M.A. Tandem mass spectrometry study of p38a kinase inhibitors and related substances. J. Mass Spectrom. 2013;48:718–731. doi: 10.1002/jms.3219. [DOI] [PubMed] [Google Scholar]
- 5.Niessen W.M.A., Correa R.A., C. Interpretation of MS-MS Mass Spectra of Drugs and Pesticides (ISBN 978-1-118-50018-7) Wiley Blackburn; 2017. [Google Scholar]
- 6.Müller D.M., Rentsch K.M. Therapeutic drug monitoring by LC–MS–MS with special focus on anti-infective drugs. Anal. Bioanal. Chem. 2010;398:2573–2594. doi: 10.1007/s00216-010-3986-z. [DOI] [PubMed] [Google Scholar]
- 7.Nováková L., Pavlík J., Chrenková L., Martinec O., Červený L. Current antiviral drugs and their analysis in biological materials - Part II: antivirals against hepatitis and HIV viruses. J. Pharmaceut. Biomed. Anal. 2018;147:378–399. doi: 10.1016/j.jpba.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 8.DiFrancesco R., Maduke G., Patel R., Taylor C.R., Morse G.D. Antiretroviral bioanalysis methods of tissues and body biofluids. Bioanalysis. 2013;5:351–368. doi: 10.4155/bio.12.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Punyawudho B., Singkham N., Thammajaruk N., Dalodom T., Kerr S.J., Burger D.M., Ruxrungtham K. Therapeutic drug monitoring of antiretroviral drugs in HIV-infected patients. Expert Rev Clin Pharmacol Dec. 2016;9(12):1583–1595. doi: 10.1080/17512433.2016.1235972. [DOI] [PubMed] [Google Scholar]
- 10.Kwong J. New drug treatment options for HIV antiretroviral therapy. Nurs. Pract. 2020;45(3):28–38. doi: 10.1097/01.NPR.0000653948.44968.cc. [DOI] [PubMed] [Google Scholar]
- 11.Volosov A., Alexander C., Ting L., Soldin S.J. Simple rapid method for quantification of antiretrovirals by liquid chromatography-tandem mass-spectrometry. Clin. Biochem. 2002;35:99–103. doi: 10.1016/s0009-9120(02)00286-2. [DOI] [PubMed] [Google Scholar]
- 12.Rentsch K.M. Sensitive and specific determination of eight antiretroviral agents in plasma by high-performance liquid chromatography-mass spectrometry. J. Chromatogr. B. 2003;788:339–350. doi: 10.1016/s1570-0232(03)00039-4. [DOI] [PubMed] [Google Scholar]
- 13.Crommentuyn K.M., Rosing H., Nan-Offeringa L.G., Hillebrand M.J., Huitema A.D., Beijnen J.H. Rapid quantification of HIV protease inhibitors in human plasma by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2003;38:157–166. doi: 10.1002/jms.425. [DOI] [PubMed] [Google Scholar]
- 14.ter Heine R., Alderden-Los C.G., Rosing H., Hillebrand M.J., van Gorp E.C., Huitema A.D., Beijnen J.H. Fast and simultaneous determination of darunavir and eleven other antiretroviral drugs for therapeutic drug monitoring: method development and validation for the determination of all currently approved HIV protease inhibitors and non-nucleoside reverse transcriptase inhibitors in human plasma by liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007;21:2505–2514. doi: 10.1002/rcm.3119. [DOI] [PubMed] [Google Scholar]
- 15.Elens L., Veriter S., Yombi J.C., Di Fazio V., Vanbinst R., Lison D., Wallemacq P., Vandercam B., Haufroid V. Validation and clinical application of a high performance liquid chromatography tandem mass spectrometry (LC-MS/MS) method for the quantitative determination of 10 antiretrovirals in human peripheral blood mononuclear cells. J. Chromatogr. B. 2009;877:1805–1814. doi: 10.1016/j.jchromb.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 16.Quaranta S., Woloch C., Paccou A., Giocanti M., Solas C., Lacarelle B. Validation of an electrospray ionization LC-MS/MS method for quantitative analysis of raltegravir, etravirine, and 9 other antiretroviral agents in human plasma samples. Ther. Drug Monit. 2009;31:695–702. doi: 10.1097/FTD.0b013e3181c05adf. [DOI] [PubMed] [Google Scholar]
- 17.Else L., Watson V., Tjia J., Hughes A., Siccardi M., Khoo S., Back D. Validation of a rapid and sensitive high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) assay for the simultaneous determination of existing and new antiretroviral compounds. J. Chromatogr. B. 2010;878:1455–1465. doi: 10.1016/j.jchromb.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 18.Djerada Z., Feliu C., Tournois C., Vautier D., Binet L., Robinet A., Marty H., Gozalo C., Lamiable D., Millart H. Validation of a fast method for quantitative analysis of elvitegravir, raltegravir, maraviroc, etravirine, tenofovir, boceprevir and 10 other antiretroviral agents in human plasma samples with a new UPLC-MS/MS technology. J. Pharmaceut. Biomed. Anal. 2013;86:100–111. doi: 10.1016/j.jpba.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Simiele M., Ariaudo A., De Nicolò A., Favata F., Ferrante M., Carcieri C., Bonora S., Di Perri G., De Avolio A. UPLC-MS/MS method for the simultaneous quantification of three new antiretroviral drugs, dolutegravir, elvitegravir and rilpivirine, and other thirteen antiretroviral agents plus cobicistat and ritonavir boosters in human plasma. J. Pharmaceut. Biomed. Anal. 2017;138:223–230. doi: 10.1016/j.jpba.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y., Yang J., Duan C., Chu L., Chen S., Qiao S., Li X., Deng H. Simultaneous determination of antiretroviral drugs in human hair with liquid chromatography-electrospray ionization-tandem mass spectrometry. J. Chromatogr. B. 2018;1083:209–221. doi: 10.1016/j.jchromb.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daskapan A., van Hateren K., Stienstra Y., Kosterink J., van der Werf T., Touw D., Alffenaar J.W. Development and validation of a bioanalytical method for the simultaneous determination of 14 antiretroviral drugs using liquid chromatography-tandem mass spectrometry. J Appl Bioanal. 2018;4:37–50. doi: 10.17145/jab.18.007. [DOI] [Google Scholar]
- 22.Zheng Y., Aboura R., Boujaafar S., Lui G., Hirt D., Bouazza N., Foissac F., Treluyer J.M., Benaboud S., Gana I. HPLC-MS/MS method for the simultaneous quantification of dolutegravir, elvitegravir, rilpivirine, darunavir, ritonavir, raltegravir and raltegravir-β-d-glucuronide in human plasma. J. Pharmaceut. Biomed. Anal. 2020;182 doi: 10.1016/j.jpba.2020.113119. [DOI] [PubMed] [Google Scholar]
- 23.Gouget H., Noé G., Barrail-Tran A., Furlan V. UPLC-MS/MS method for the simultaneous quantification of bictegravir and 13 others antiretroviral drugs plus cobicistat and ritonavir boosters in human plasma. J. Pharmaceut. Biomed. Anal. 2020;181 doi: 10.1016/j.jpba.2019.113057. [DOI] [PubMed] [Google Scholar]
- 24.Marzinke M.A., Breaud A., Parsons T.L., Cohen M.S., Piwowar-Manning E., Eshleman S.H., Clarke W. The development and validation of a method using high-resolution mass spectrometry (HRMS) for the qualitative detection of antiretroviral agents in human blood. Clin. Chim. Acta. 2014;433:157–168. doi: 10.1016/j.cca.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agilent Technologies Agilent forensic toxicology LC/MS personal compound database and library. 2016. https://www.agilent.com/cs/library/flyers/public/5991-7000EN_PCDL.pdf
- 26.Compain S., Schlemmer D., Levi M., Pruvost A., Goujard C., Grassi J., Benech H. Development and validation of a liquid chromatographic/tandem mass spectrometric assay for the quantitation of nucleoside HIV reverse transcriptase inhibitors in biological matrices. J. Mass Spectrom. 2005;40:9–18. doi: 10.1002/jms.752. [DOI] [PubMed] [Google Scholar]
- 27.Coulier L., Gerritsen H., van Kampen J.J., Reedijk M.L., Luider T.M., Osterhaus A.D., Gruters R.A., Brüll L. Comprehensive analysis of the intracellular metabolism of antiretroviral nucleosides and nucleotides using liquid chromatography–tandem mass spectrometry and method improvement by using ultra performance liquid chromatography. J. Chromatogr. B. 2011;879:2772–2782. doi: 10.1016/j.jchromb.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 28.Lai J., Wang J., Cai Z. Nucleoside reverse transcriptase inhibitors and their phosphorylated metabolites in human immunodeficiency virus-infected human matrices. J. Chromatogr. B. 2008;868:1–12. doi: 10.1016/j.jchromb.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Clark T.N., White C.A., Bartlett M.G. Determination of Abacavir in maternal plasma, amniotic fluid, fetal and placental tissues by a polarity switching liquid chromatography/tandem mass spectrometry method. Rapid Commun. Mass Spectrom. 2004;18:405–411. doi: 10.1002/rcm.1329. [DOI] [PubMed] [Google Scholar]
- 30.Bezy V., Morin P., Couerbe P., Leleu G., Agrofoglio L. Simultaneous analysis of several antiretroviral nucleosides in rat-plasma by high-performance liquid chromatography with UV using acetic acid/hydroxylamine buffer. Test of this new volatile medium-pH for HPLC-ESI-MS/MS. J. Chromatogr. B. 2005;821:132–143. doi: 10.1016/j.jchromb.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Robbins B.L., Poston P.A., Neal E.F., Slaughter C., Rodman J.H. Simultaneous measurement of intracellular triphosphate metabolites of zidovudine, lamivudine and abacavir (carbovir) in human peripheral blood mononuclear cells by combined anion exchange solid phase extraction and LC-MS/MS. J. Chromatogr. B. 2007;850:310–317. doi: 10.1016/j.jchromb.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Fromentin E., Gavegnano C., Obikhod A., Schinazi R.F. Simultaneous quantification of intracellular natural and antiretroviral nucleosides and nucleotides by liquid chromatography-tandem mass spectrometry. Anal. Chem. 2010;82:1982–1989. doi: 10.1021/ac902737j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pruvost A., Théodoro F., Agrofoglio L., Negredo E., Bénech H. Specificity enhancement with LC-positive ESI-MS/MS for the measurement of nucleotides: application to the quantitative determination of carbovir triphosphate, lamivudine triphosphate and tenofovir diphosphate in human peripheral blood mononuclear cells. J. Mass Spectrom. 2008;43:224–233. doi: 10.1002/jms.1294. [DOI] [PubMed] [Google Scholar]
- 34.Yan Z., Sun J., Wang J., Xu Y., Chang Y., Meng P., Zhu M., Fu Q., Sun Y., He Z. Simultaneous determination of didanosine and its amino acid prodrug, valdidanosine by hydrophilic interaction chromatography coupled with electrospray ionization tandem mass spectrometry: application to a pharmacokinetic study in rats. J. Chromatogr. B. 2010;878:466–470. doi: 10.1016/j.jchromb.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 35.Kuklenyik Z., Martin A., Pau C.P., Holder A., Youngpairoj A.S., Zheng Q., Cong M.E., Garcia-Lerma J.G., Heneine W., Pirkle J.L., Barr J.R. On-line coupling of anion exchange and ion-pair chromatography for measurement of intracellular triphosphate metabolites of reverse transcriptase inhibitors. J. Chromatogr. B. 2009;877:3659–3666. doi: 10.1016/j.jchromb.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Fan B., Bartlett M.G., Stewart J.T. Determination of lamivudine/stavudine/efavirenz in human serum using liquid chromatography/electrospray tandem mass spectrometry with ionization polarity switch. Biomed. Chromatogr. 2002;16:383–389. doi: 10.1002/bmc.169. [DOI] [PubMed] [Google Scholar]
- 37.Kenney K.B., Wring S.A., Carr R.M., Wells G.N., Dunn J.A. Simultaneous determination of zidovudine and lamivudine in human serum using HPLC with tandem mass spectrometry. J. Pharmaceut. Biomed. Anal. 2000;22:967–983. doi: 10.1016/s0731-7085(00)00248-x. [DOI] [PubMed] [Google Scholar]
- 38.Ntshangase S., Mdanda S., Naicker T., Kruger H.G., Baijnath S., Govender T. Spatial distribution of elvitegravir and tenofovir in rat brain tissue: application of matrix-assisted laser desorption/ionization mass spectrometry imaging and liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2019;33:1643–1651. doi: 10.1002/rcm.8510. [DOI] [PubMed] [Google Scholar]
- 39.Ouyang B., Zhou F., Zhen L., Peng Y., Sun J., Chen Q., Jin X., Wang G., Zhang J. Simultaneous determination of tenofovir alafenamide and its active metabolites tenofovir and tenofovir diphosphate in HBV-infected hepatocyte with a sensitive LC-MS/MS method. J. Pharmaceut. Biomed. Anal. 2017;146:147–153. doi: 10.1016/j.jpba.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 40.Ocque A.J., Hagler C.E., Morse G.D., Letendre S.L., Ma Q. Development and validation of an LC-MS/MS assay for tenofovir and tenofovir alafenamide in human plasma and cerebrospinal fluid. J. Pharmaceut. Biomed. Anal. 2018;156:163–169. doi: 10.1016/j.jpba.2018.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becher F., Pruvost A., Goujard C., Guerreiro C., Delfraissy J.F., Grassi J., Benech H. Improved method for the simultaneous determination of d4T, 3TC and ddl intracellular phosphorylated anabolites in human peripheral-blood mononuclear cells using high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2002;16:555–565. doi: 10.1002/rcm.605. [DOI] [PubMed] [Google Scholar]
- 42.Bollen P.D.J., de Graaff-Teulen M.J.A., Schalkwijk S., van Erp N.P., Burger D.M. Development and validation of an UPLC-MS/MS bioanalytical method for simultaneous quantification of the antiretroviral drugs dolutegravir, elvitegravir, raltegravir, nevirapine and etravirine in human plasma. J. Chromatogr. B. 2019;1105:76–84. doi: 10.1016/j.jchromb.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Bedse G., Kumar V., Singh S. Study of forced decomposition behavior of lamivudine using LC, LC-MS/TOF and MSn. J. Pharmaceut. Biomed. Anal. 2009;49:55–63. doi: 10.1016/j.jpba.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Rao R.N., Vali R.M., Ramachandra B., Raju S.S. Separation and characterization of forced degradation products of abacavir sulphate by LC-MS/MS. J. Pharmaceut. Biomed. Anal. 2011;54:279–285. doi: 10.1016/j.jpba.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 45.Ramesh T., Rao P.N., Rao R.N. LC-MS/MS method for the characterization of the forced degradation products of Entecavir. J. Separ. Sci. 2014;37:368–375. doi: 10.1002/jssc.201300959. [DOI] [PubMed] [Google Scholar]
- 46.Yang F.Q., Li D.Q., Feng K., Hu D.J., Li S.P. Determination of nucleotides, nucleosides and their transformation products in Cordyceps by ion-pairing reversed-phase liquid chromatography-mass spectrometry. J. Chromatogr. A. 2010;1217:5501–5510. doi: 10.1016/j.chroma.2010.06.062. [DOI] [PubMed] [Google Scholar]
- 47.Nirogi R., Bhyrapuneni G., Kandikere V., Mudigonda K., Komarneni P., Aleti R., Mukkanti K. Simultaneous quantification of a non-nucleoside reverse transcriptase inhibitor efavirenz, a nucleoside reverse transcriptase inhibitor emtricitabine and a nucleotide reverse transcriptase inhibitor tenofovir in plasma by liquid chromatography positive ion electrospray tandem mass spectrometry. Biomed. Chromatogr. 2009;23:371–381. doi: 10.1002/bmc.1125. [DOI] [PubMed] [Google Scholar]
- 48.Kurmi M., Golla V.M., Kumar S., Sahu A., Singh S. Stability behaviour of antiretroviral drugs and their combinations. 1: characterization of tenofovir disoproxil fumarate degradation products by mass spectrometry. RSC Adv. 2015;5:96117. 0.1039/c5ra17532a. [Google Scholar]
- 49.Golla V.M., Kurmi M., Shaik K., Singh S. Stability behaviour of antiretroviral drugs and their combinations. 4: characterization of degradation products of tenofovir alafenamide fumarate and comparison of its degradation and stability behaviour with tenofovir disoproxil fumarate. J. Pharmaceut. Biomed. Anal. 2016;131:146–155. doi: 10.1016/j.jpba.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 50.Niessen W.M.A., Tandem mass spectrometry of small-molecule antiviral drugs: 2. Hepatitis-related antivirals, Int. J. Mass Spectrom. (In press), doi:10.1016/j.ijms.2020.116371. [DOI] [PMC free article] [PubMed]
- 51.Seserko L.A., Emory J.F., Hendrix C.W., Marzinke M.A. The development and validation of an UHPLC-MS/MS method for the rapid quantification of the antiretroviral agent dapivirine in human plasma. Bioanalysis. 2013;5:2771–2783. doi: 10.4155/bio.13.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parsons T.L., Emory J.F., Seserko L.A., Aung W.S., Marzinke M.A. Dual quantification of dapivirine and maraviroc in cervicovaginal secretions from ophthalmic tear strips and polyester-based swabs via liquid chromatographic-tandem mass spectrometric (LC-MS/MS) analysis. J. Pharmaceut. Biomed. Anal. 2014;98:407–416. doi: 10.1016/j.jpba.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Courlet P., Alves Saldanha S., Cavassini M., Marzolini C., Choong E., Csajka C., Günthard H.F., André P., Buclin T., Desfontaine V., Decosterd L.A. Development and validation of a multiplex UHPLC-MS/MS assay with stable isotopic internal standards for the monitoring of the plasma concentrations of the antiretroviral drugs bictegravir, cabotegravir, doravirine, and rilpivirine in people living with HIV. J. Mass Spectrom. 2020;55 doi: 10.1002/jms.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin J., Deslandes G., Dailly E., Renaud C., Reliquet V., Raffi F., Jolliet P. A liquid chromatography-tandem mass spectrometry assay for quantification of nevirapine, indinavir, atazanavir, amprenavir, saquinavir, ritonavir, lopinavir, efavirenz, tipranavir, darunavir and maraviroc in the plasma of patients infected with HIV. J. Chromatogr. B. 2009;877:3072–3082. doi: 10.1016/j.jchromb.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 55.Abafe O.A., Späth J., Fick J., Jansson S., Buckley C., Stark A., Pietruschka B., Martincigh B.S. LC-MS/MS determination of antiretroviral drugs in influents and effluents from wastewater treatment plants in KwaZulu-Natal, South Africa. Chemosphere. 2018;200:660–670. doi: 10.1016/j.chemosphere.2018.02.105. [DOI] [PubMed] [Google Scholar]
- 56.Fayet A., Béguin A., Zanolari B., Cruchon S., Guignard N., Telenti A., Cavassini M., Günthard H.F., Buclin T., Biollaz J., Rochat B., Decosterd L.A. A LC-tandem MS assay for the simultaneous measurement of new antiretroviral agents: raltegravir, maraviroc, darunavir, and etravirine. J. Chromatogr. B. 2009;877:1057–1069. doi: 10.1016/j.jchromb.2009.02.057. [DOI] [PubMed] [Google Scholar]
- 57.Parsons T.L., Marzinke M.A. Development and validation of a liquid chromatographic-tandem mass spectrometric method for the multiplexed quantification of etravirine, maraviroc, raltegravir, and rilpivirine in human plasma and tissue. J. Pharmaceut. Biomed. Anal. 2016;131:333–344. doi: 10.1016/j.jpba.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 58.Abobo C.V., Wu L., John J., Joseph M.K., Bates T.R., Liang D. LC-MS/MS determination of etravirine in rat plasma and its application in pharmacokinetic studies. J. Chromatogr. B. 2010;878:3181–3186. doi: 10.1016/j.jchromb.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta A., Guttikar S., Patel Y., Shrivastav P.S., Sanyal M. Reliable LC-MS/MS assay for the estimation of rilpivirine in human plasma: application to a bioequivalence study and incurred sample reanalysis. Drug Test. Anal. 2015;7:290–299. doi: 10.1002/dta.1665. [DOI] [PubMed] [Google Scholar]
- 60.Chang M., Sood V.K., Kloosterman D.A., Hauer M.J., Fagerness P.E., Sanders P.E., Vrbanac J.J. Identification of the metabolites of the HIV-1 reverse transcriptase inhibitor delavirdine in monkeys. Drug Metab. Dispos. 1997;25:814–827. [PubMed] [Google Scholar]
- 61.Chang M., Sood V.K., Wilson G.J., Kloosterman D.A., Sanders P.E., Hauer M.J., Zhang W., Branstetter D.G. Metabolism of the HIV-1 reverse transcriptase inhibitor delavirdine in mice. Drug Metab. Dispos. 1997;25:828–839. [PubMed] [Google Scholar]
- 62.Voorman R.L., Maio S.M., Hauer M.J., Sanders P.E., Payne N.A., Ackland M.J. Metabolism of delavirdine, a human immunodeficiency virus type-1 reverse transcriptase inhibitor, by microsomal cytochrome P450 in humans, rats, and other species: probable involvement of CYP2D6 and CYP3A. Drug Metab. Dispos. 1998;26:631–639. [PubMed] [Google Scholar]
- 63.Zhang L.K., Yang R., Sheng H., Helmy R., Zheng J., Cao Y., Gauthier D.R., Jr. Characterization of impurities of HIV NNRTI Doravirine by UHPLC-high resolution MS and tandem MS analysis. J. Mass Spectrom. 2016;51:959–968. doi: 10.1002/jms.3807. [DOI] [PubMed] [Google Scholar]
- 64.Kurmi M., Sahu A., Singh D.K., Singh I.P., Singh S. Stability behaviour of antiretroviral drugs and their combinations. 8: characterization and in-silico toxicity prediction of degradation products of efavirenz. J. Pharmaceut. Biomed. Anal. 2018;148:170–181. doi: 10.1016/j.jpba.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 65.Godinho A.L.A., Martins I.L., Nunes J., Charneira C., Grilo J., Silva D.M., Pereira S.A., Soto K., Oliveira M.C., Marques M.M., Jacob C.C., Antunes A.M.M. High resolution mass spectrometry-based methodologies for identification of Etravirine bioactivation to reactive metabolites: in vitro and in vivo approaches. Eur. J. Pharmaceut. Sci. 2018;119:70–82. doi: 10.1016/j.ejps.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 66.Yanakakis L.J., Bumpus N.N. Biotransformation of the antiretroviral drug etravirine: metabolite identification, reaction phenotyping, and characterization of autoinduction of cytochrome P450-dependent metabolism. Drug Metab. Dispos. 2012;40:803–814. doi: 10.1124/dmd.111.044404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Belkhir L., De Laveleye M., Vandercam B., Zech F., Delongie K.A., Capron A., Yombi J., Vincent A., Elens L., Haufroid V. Quantification of darunavir and etravirine in human peripheral blood mononuclear cells using high performance liquid chromatography tandem mass spectrometry (LC-MS/MS), clinical application in a cohort of 110 HIV-1 infected patients and evidence of a potential drug-drug interaction. Clin. Biochem. 2016;49:580–586. doi: 10.1016/j.clinbiochem.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 68.Lade J.M., Avery L.B., Bumpus N.N. Human biotransformation of the nonnucleoside reverse transcriptase inhibitor rilpivirine and a cross-species metabolism comparison. Antimicrob. Agents Chemother. 2013;57:5067–5079. doi: 10.1128/AAC.01401-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burugula L., Pilli N.R., Makula A., Lodagala D.S., Kandhagatla R. Liquid chromatography-tandem mass spectrometric assay for the non-nucleoside reverse transcriptase inhibitor rilpivirine in human plasma. Biomed. Chromatogr. 2013;27:172–178. doi: 10.1002/bmc.2765. [DOI] [PubMed] [Google Scholar]
- 70.Dickinson L., Robinson L., Tjia J., Khoo S., Back D. Simultaneous determination of HIV protease inhibitors amprenavir, atazanavir, indinavir, lopinavir, nelfinavir, ritonavir and saquinavir in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B. 2005;829:82–90. doi: 10.1016/j.jchromb.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 71.Schuster A., Burzawa S., Jemal M., Loizillon E., Couerbe P., Whigan D. Quantitative determination of the HIV protease inhibitor atazanavir (BMS-232632) in human plasma by liquid chromatography-tandem mass spectrometry following automated solid-phase extraction. J. Chromatogr. B. 2003;788:377–386. doi: 10.1016/s1570-0232(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 72.Tréluyer J.M., Bowers G., Cazali N., Sonnier M., Rey E., Pons G., Cresteil T. Oxidative metabolism of amprenavir in the human liver. Effect of the CYP3A maturation. Drug Metab. Dispos. 2003;31:275–281. doi: 10.1124/dmd.31.3.275. [DOI] [PubMed] [Google Scholar]
- 73.Crommentuyn K.M., Rosing H., Hillebrand M.J., Huitema A.D., Beijnen J.H. Simultaneous quantification of the new HIV protease inhibitors atazanavir and tipranavir in human plasma by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J. Chromatogr. B. 2004;804:359–367. doi: 10.1016/j.jchromb.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 74.Cheng C., Gallegos R., Bridson G., Wu L., Harbeson S., Zelle R., Tung R. Identification and structural elucidation of in vitro metabolites of atazanavir by HPLC and tandem mass spectrometry. J. Mass Spectrom. 2013;48:640–650. doi: 10.1002/jms.3201. [DOI] [PubMed] [Google Scholar]
- 75.Baira S.M., Gunupudi P., Abbaraju V., Srinivas R., Kumar Talluri M.V.N. Study of forced degradation behaviour of cobicistat and atazanavir using LC/ESI/QTOF/MS; a combination of in-sourced and collision-induced dissociation for evaluation of the fragmentation patterns of degradation products. New J. Chem. 2018;42:19113–19128. doi: 10.1039/c8nj03418d. [DOI] [Google Scholar]
- 76.Wang P., Shehu A.I., Liu K., Lu J., Ma X. Biotransformation of cobicistat: metabolic pathways and enzymes. Drug Metabol. Lett. 2016;10:111–123. doi: 10.2174/1872312810666160303112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lopez L.L., Yu X., Cui D., Davis M.R. Identification of drug metabolites in biological matrices by intelligent automated liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 1998;12:1756–1760. doi: 10.1002/(SICI)1097-0231(19981130)12:22<1756::AID-RCM381>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 78.Yu X., Cui D., Davis M.R. Identification of in vitro metabolites of indinavir by "intelligent automated LC-MS/MS" (INTAMS) utilizing triple quadrupole tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 1999;10:175–183. doi: 10.1016/S1044-0305(98)00132-9. [DOI] [PubMed] [Google Scholar]
- 79.Gangl E., Utkin I., Gerber N., Vouros P. Structural elucidation of metabolites of ritonavir and indinavir by liquid chromatography-mass spectrometry. J. Chromatogr. A. 2002;974:91–101. doi: 10.1016/s0021-9673(02)01243-8. [DOI] [PubMed] [Google Scholar]
- 80.Anari M.R., Sanchez R.I., Bakhtiar R., Franklin R.B., Baillie T.A. Integration of knowledge-based metabolic predictions with liquid chromatography data-dependent tandem mass spectrometry for drug metabolism studies: application to studies on the biotransformation of indinavir. Anal. Chem. 2004;76:823–832. doi: 10.1021/ac034980s. [DOI] [PubMed] [Google Scholar]
- 81.Rao R.N., Vali R.M., Raju S.S. Liquid chromatography tandem mass spectrometric studies of indinavir sulphate and its forced degradation products. J. Pharmaceut. Biomed. Anal. 2013;74:101–110. doi: 10.1016/j.jpba.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 82.Kumar G.N., Jayanti V., Lee R.D., Whittern D.N., Uchic J., Thomas S., Johnson P., Grabowski B., Sham H., Betebenner D., Kempf D.J., Denissen J.F. In vitro metabolism of the HIV-1 protease inhibitor ABT-378: species comparison and metabolite identification. Drug Metab. Dispos. 1999;27:86–91. [PubMed] [Google Scholar]
- 83.Li F., Lu J., Ma X. CYP3A4-mediated lopinavir bioactivation and its inhibition by ritonavir. Drug Metab. Dispos. 2012;40:18–24. doi: 10.1124/dmd.111.041400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang M., Halquist M.S., Zhang Y., Gerk P.M. Simultaneous determination of lopinavir and three ester prodrugs by LC-MS/MS in lysates of BeWo cells. J. Chromatogr. B. 2015;975:84–90. doi: 10.1016/j.jchromb.2014.10.032. [DOI] [PubMed] [Google Scholar]
- 85.Tiwari R.N., Bonde C.G. LC, LC-MS/TOF and MSn studies for the identification and characterization of degradation products of nelfinavir mesylate. J. Pharmaceut. Biomed. Anal. 2011;55:435–445. doi: 10.1016/j.jpba.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 86.Jhajra S., Singh S. Identification of stable and reactive metabolite(s) of nelfinavir in human liver microsomes and rCYP3A4. J. Pharmaceut. Biomed. Anal. 2016;118:214–227. doi: 10.1016/j.jpba.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 87.Rao R.N., Ramachandra B., Vali R.M., Raju S.S. LC-MS/MS studies of ritonavir and its forced degradation products. J. Pharmaceut. Biomed. Anal. 2010;53:833–842. doi: 10.1016/j.jpba.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 88.Thummar M., Patel P.N., Petkar A.L., Swain D., Srinivas R., Samanthula G. Identification of degradation products of saquinavir mesylate by ultra-high-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry and its application to quality control. Rapid Commun. Mass Spectrom. 2017;31:771–781. doi: 10.1002/rcm.7842. [DOI] [PubMed] [Google Scholar]
- 89.Prathipati P.K., Mandal S., Destache C.J. Development and validation of LC-MS/MS method for quantification of bictegravir in human plasma and its application to an intracellular uptake study. Biomed. Chromatogr. 2019;33:e4379. doi: 10.1002/bmc.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rigo-Bonnin R., Tiraboschi J.M., Álvarez-Álvarez M., Pérez-Fernández G.A., Sanjuás-Iglesias M., Scévola S., Niubó J., Videla S., Podzamczer D. Measurement of total and unbound bictegravir concentrations in plasma and cerebrospinal fluid by UHPLC-MS/MS. J. Pharmaceut. Biomed. Anal. 2020;185 doi: 10.1016/j.jpba.2020.113250. [DOI] [PubMed] [Google Scholar]
- 91.Bowers G.D., Culp A., Reese M.J., Tabolt G., Moss L., Piscitelli S., Huynh P., Wagner D., Ford S.L., Gould E.P., Pan R., Lou Y., Margolis D.A., Spreen W.R. Disposition and metabolism of cabotegravir: a comparison of biotransformation and excretion between different species and routes of administration in humans. Xenobiotica. 2016;46:147–162. doi: 10.3109/00498254.2015.1060372. [DOI] [PubMed] [Google Scholar]
- 92.Shaik J.S.B., Ford S.L., Lou Y., Zhang Z., Bakshi K.K., Tenorio A.R., Trezza C., Spreen W.R., Patel P. A Phase 1 study to evaluate the pharmacokinetics and safety of cabotegravir in patients with hepatic impairment and healthy matched controls. Clin Pharmacol Drug Dev. 2019;8:664–673. doi: 10.1002/cpdd.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bennetto-Hood C., Tabolt G., Savina P., Acosta E.P. A sensitive HPLC-MS/MS method for the determination of dolutegravir in human plasma. J. Chromatogr. B. 2014;945–946:225–232. doi: 10.1016/j.jchromb.2013.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Long M.C., Bennetto-Hood C., Acosta E.P. A sensitive HPLC-MS-MS method for the determination of raltegravir in human plasma. J. Chromatogr. B. 2008;867:165–171. doi: 10.1016/j.jchromb.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 95.Emory J.F., Seserko L.A., Marzinke M.A. Development and bioanalytical validation of a liquid chromatographic-tandem mass spectrometric (LC-MS/MS) method for the quantification of the CCR5 antagonist maraviroc in human plasma. Clin. Chim. Acta. 2014;431:198–205. doi: 10.1016/j.cca.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun L., Li H., Willson K., Breidinger S., Rizk M.L., Wenning L., Woolf E.J. Ultrasensitive liquid chromatography-tandem mass spectrometric methodologies for quantification of five HIV-1 integrase inhibitors in plasma for a microdose clinical trial. Anal. Chem. 2012;84:8614–8621. doi: 10.1021/ac301581h. [DOI] [PubMed] [Google Scholar]
- 97.Gupta A., Guttikar S., Shah P.A., Solanki G., Shrivastav P.S., Sanyal M. Selective and rapid determination of raltegravir in human plasma by liquid chromatography-tandem mass spectrometry in the negative ionization mode. J Pharm Anal. 2015;5:101–109. doi: 10.1016/j.jpha.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rao R.N., Ramachandra B., Sravan B., Khalid S. LC-MS/MS structural characterization of stress degradation products including the development of a stability indicating assay of Darunavir: an anti-HIV drug. J. Pharmaceut. Biomed. Anal. 2014;89:28–33. doi: 10.1016/j.jpba.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 99.Rao R.N., Prasad K.G. LC-Q-TOF-MS/MS determination of darunavir and its metabolites in rat serum and urine: application to pharmacokinetics. J. Pharmaceut. Biomed. Anal. 2014;94:92–98. doi: 10.1016/j.jpba.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 100.Li F., Wang L., Guo G.L., Ma X. Metabolism-mediated drug interactions associated with ritonavir-boosted tipranavir in mice. Drug Metab. Dispos. 2010;38:871–878. doi: 10.1124/dmd.109.030817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu J., Wang P., Li F., Lu J., Shehu A.I., Xie W., McMahon D., Ma X. CYP1A1 and 1B1-mediated metabolic pathways of dolutegravir, an HIV integrase inhibitor. Biochem. Pharmacol. 2018;158:174–184. doi: 10.1016/j.bcp.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kalariya P.D., Patel P.N., Srinivas R., Kumar Talluri M.V.N. Quality by design based development of a selective stability-indicating UPLC method of dolutegravir and characterization of its degradation products by UPLC-QTOF-MS/MS. New J. Chem. 2015;39:6303–6314. doi: 10.1039/C5NJ00698H. [DOI] [Google Scholar]
- 103.Barry J.A., Robichaud G., Bokhart M.T., Thompson C., Sykes C., Kashuba A.D., Muddiman D.C. Mapping antiretroviral drugs in tissue by IR-MALDESI MSI coupled to the Q Exactive and comparison with LC-MS/MS SRM assay. J. Am. Soc. Mass Spectrom. 2014;25:2038–2047. doi: 10.1007/s13361-014-0884-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lu Y., Hendrix C.W., Bumpus N.N. Cytochrome P450 3A5 plays a prominent role in the oxidative metabolism of the anti-human immunodeficiency virus drug maraviroc. Drug Metab. Dispos. 2012;40:2221–2230. doi: 10.1124/dmd.112.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tseng E., Fate G.D., Walker G.S., Goosen T.C., Obach R.S. Biosynthesis and identification of metabolites of maraviroc and their use in experiments to delineate the relative contributions of cytochrome P4503A4 versus 3A5. Drug Metab. Dispos. 2018;46:493–502. doi: 10.1124/dmd.117.079855. [DOI] [PubMed] [Google Scholar]
- 106.Ghosal A., Ramanathan R., Yuan Y., Hapangama N., Chowdhury S.K., Kishnani N.S., Alton K.B. Identification of human liver cytochrome P450 enzymes involved in biotransformation of vicriviroc, a CCR5 receptor antagonist. Drug Metab. Dispos. 2007;35:2186–2195. doi: 10.1124/dmd.107.017517. [DOI] [PubMed] [Google Scholar]
- 107.Wu W., Xie D., Cheng Z., Liu Z., Ran L., Gu Z., Yu P. Determination of nifeviroc, a novel CCR5 antagonist: application to a pharmacokinetic study. J. Pharmaceut. Biomed. Anal. 2011;56:637–640. doi: 10.1016/j.jpba.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 108.Dilly Penchala S., Alagaratnam J., Challenger E., Amara A., Else L., Winston A., Khoo S. The development and validation of a novel LC-MS/MS method for the quantification of cenicriviroc in human plasma and cerebrospinal fluid. Biomed. Chromatogr. 2020;34 doi: 10.1002/bmc.4711. [DOI] [PubMed] [Google Scholar]
- 109.Karni M., Mandelbaum A. The ‘even-electron’ rule. Org. Mass Spectrom. 1980;15:53–64. doi: 10.1002/oms.1210150202. [DOI] [Google Scholar]
- 110.Levsen K., Schiebel H.M., Terlouw J.K., Jobst K.J., Elend M., Preiss A., Thiele H., Ingendoh A. Even-electron ions: a systematic study of the neutral species lost in the dissociation of quasi-molecular ions. J. Mass Spectrom. 2007;42:1024–1044. doi: 10.1002/jms.1234. [DOI] [PubMed] [Google Scholar]
- 111.Field F.H. In: Mass Spectrometry. Maccoll A., editor. Butterworths & Co Publishers Ltd; London: 1972. Chemical ionization mass spectrometry; pp. 133–181. [Google Scholar]
- 112.Thurman E.M., Ferrer I., Pozo O.J., Sancho J.V., Hernández F. The even-electron rule in electrospray mass spectra of pesticides. Rapid Commun. Mass Spectrom. 2007;21:3855–3868. doi: 10.1002/rcm.3271. [DOI] [PubMed] [Google Scholar]
- 113.Holčapek M., Jirasko R., Lisa M. Basic rules for the interpretation of atmospheric pressure ionization mass spectra of small molecules. J. Chromatogr. A. 2010;1217:3908–3921. doi: 10.1016/j.chroma.2010.02.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.