Abstract
The newly described severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for a pandemic (coronavirus disease 2019 [COVID-19]). It is now well established that certain comorbidities define high-risk patients. They include hypertension, diabetes, and coronary artery disease. In contrast, the context with bronchial asthma is controversial and shows marked regional differences. Because asthma is the most prevalent chronic inflammatory lung disease worldwide and SARS-CoV-2 primarily affects the upper and lower airways leading to marked inflammation, the question arises about the possible clinical and pathophysiological association between asthma and SARS-CoV-2/COVID-19. Here, we analyze the global epidemiology of asthma among patients with COVID-19 and propose the concept that patients suffering from different asthma endotypes (type 2 asthma vs non–type 2 asthma) present with a different risk profile in terms of SARS-CoV-2 infection, development of COVID-19, and progression to severe COVID-19 outcomes. This concept may have important implications for future COVID-19 diagnostics and immune-based therapy developments.
Key words: SARS-CoV-2, COVID-19, asthma, endotypes, type 2 asthma, non–type 2 asthma
Abbreviations used: ACE-2, Angiotensin-converting enzyme-2; COVID-19, Coronavirus disease 2019; HI, Heterologous immunity; ICS, Inhaled corticosteroid; TMPRSS2, Transmembrane proteases serine 2
Global epidemiology
The susceptibility of patients with asthma to COVID-19
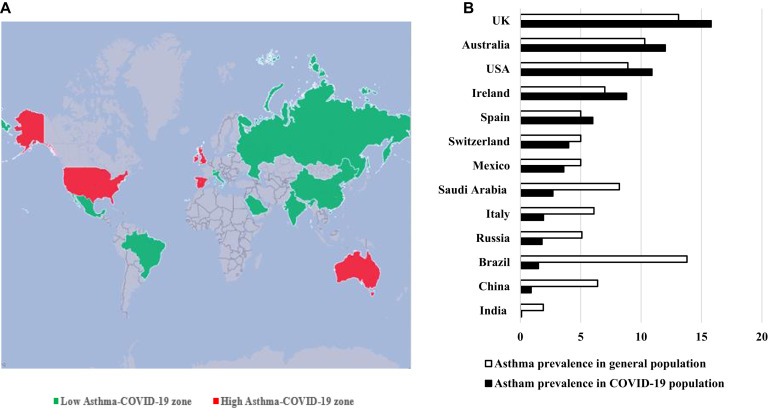
First epidemiologic studies of the coronavirus disease 2019 (COVID-19) pandemic in China included 72,314 case records, of which 44,672 were classified as confirmed cases of COVID-19 (diagnosis based on positive viral nucleic acid test result on throat swab samples) and did not identify asthma as a risk factor of severe COVID-19.1 A total of 548 patients with COVID-19 admitted to Tongji Hospital were retrospectively analyzed and 5 of 548 patients had asthma (0.9%).2 Zhongnan Hospital of Wuhan University retrospectively analyzed 140 hospitalized patients with COVID-19 and also concluded that allergic disease or asthma is not a risk factor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.3 After 3 months, they had analyzed 290 hospitalized patients with COVID-19 and identified only 1 patient with asthma.4 A retrospective study of 191 patients with COVID-19 (135 from Jinyintan Hospital and 56 from Wuhan Pulmonary Hospital) did not report on asthma among comorbidities.5 The prevalence of asthma in the adult population of Wuhan is 6.4%. The prevalence of asthma among patients with COVID-19 was subsequently reported in several other countries and regions. Studies from Russia, Saudi Arabia, and Brazil confirmed the lower rates of asthma among patients with COVID-19 (1.8%, 2.7%, and 1.5% respectively).6, 7, 8 The prevalence of asthma among patients with COVID-19 in Mexico (3.6%),9 which like Brazil is also in Latin America, was relatively high compared with that in Brazil. Asthma was not mentioned among the comorbidity list of COVID-19 in a retrospective Indian epidemiological study.10 In Europe, the prevalence of asthma varied from country to country, with Swedish and Italian cohort studies reporting relatively low rates of asthma, 1.8% and 2.6% (Sweden) and 1.96% and 1.92% (Italy)11 , 12 (see Table E1 in this article’s Online Repository at www.jacionline.org). Another retrospective case series of 1591 patients hospitalized with laboratory-confirmed COVID-19 in the Lombardy region of Italy did not mention patients with asthma.13 However, the prevalence of asthma among patients with COVID-19 was higher in Spain, Catalonia, and Ireland (5.2%, 6.8%, and 8.8%, respectively).14, 15, 16 The prevalence of asthma in the general population in Italy, Spain, and Ireland is 6.0%, 5.0%, and 7.0%, respectively12 , 17 , 18 (Fig 1 , A and B).19, 20, 21, 22, 23, 24, 25, 26
Fig 1.
Asthma prevalence. A, Countries with high prevalence of asthma among COVID-19 comorbidities in comparison with asthma prevalence in the general population (high asthma-COVID-19 zone) are marked in red color. Countries with low/equal prevalence of asthma among COVID-19 comorbidities in comparison with asthma prevalence in the general population (low asthma-COVID-19 zone) are marked in green. B, Asthma prevalence (%) among patients with COVID-19 in comparison with the general population in various countries.12,17,19, 20, 21, 22, 23, 24, 25, 26
However, recent studies from the United States and the United Kingdom indicated high rates of asthma in patients with COVID-19. The prevalence of asthma among patients with COVID-19 was 14.4% versus the national asthma prevalence of 8% to 9% in the United States. Asthma and inhaled corticosteroids (ICSs) were not associated with risk of hospitalization due to COVID-19.27 A huge retrospective study included 11 acute care hospitals of a public hospital system and described demographic and clinical characteristics and outcomes of patients tested for COVID-19. Of the 22,254 patients who were tested, 13,442 patients tested positive, 6,248 required hospitalization, and 1,724 died. Asthma prevalence rate was 7% among positive patients and 11% among negative patients.28 A case series of 5700 patients with confirmed COVID-19 in the New York City area provides characteristics of hospitalized patients; asthma prevalence was determined as 9%.29 The International Severe Acute Respiratory and emerging Infection Consortium (ISARIC) study based on 16,749 patients with COVID-19 in the United Kingdom reported about 14% asthma prevalence.30 A population-based prospective cohort study analyzed data from the UK Biobank and reported about 17.9% prevalence of asthma in patients with COVID-19.31 Another study that analyzed UK Biobank data reported an asthma rate of about 13% and added that adults with asthma had a higher risk of severe COVID-19.32 An Australian pediatric study retrospectively included all pediatric patients (aged 0-18 years) who presented to the emergency department of the newly established Respiratory Infection Clinic who tested for SARS-CoV-2 from the day of the first positive confirmed case. One in 4 COVID-19–positive patients had a comorbidity, which was asthma (1 of 4 [25%]).33 In the group of SARS-negative subjects, asthma was the most common comorbidity, with a prevalence of 11%.33 The “usual” prevalence of asthma among young Australians aged 0 to 17 years was 10.3% in the period 2017 to 2018.34
Overall, in most countries around the world, patients with asthma were not reported with higher rates of COVID-19 infection compared with the general population in the corresponding area. There is a big difference in the incidence of COVID-19 in patients with asthma among different areas and countries, with some of them reporting low rates of COVID-19 with asthma, probably due to the multiple factors including the rigorous self-protection awareness and low proportion of non–type 2 phenotypes.
Severity of COVID-19 in patients with asthma
Except for the susceptibility of patients with asthma to COVID-19, another important issue is whether there is a shift in severity and mortality among COVID-19 patients with asthma compared with those without. Among the 548 patients with COVID-19 admitted to Tongji Hospital, there were 5 with asthma, including 2 of 279 with nonsevere asthma (0.7%) and 3 of 269 with severe asthma (1.1%). There was no significant difference in asthma prevalence between patients with severe and nonsevere COVID-19.2 Saudi Arabia reported that the prevalence of asthma was 3 (2.9%), 0 (0%), and 1 (7.7%) in patients with mild, moderate, and severe COVID-19, respectively.7 In the Brazilian retrospective study including 51,770 COVID-19 cases, the prevalence of “moderate to severe asthma” is 1.5%.8 Asthma was identified in 5.2% of patients with COVID-19 in a Spanish study, with a lower prevalence rate of 3.7% in fatal cases and 5.5% in live discharge case.14 An Italian study of 355 lethal patients’ cases with COVID-19 reported that comorbidities were associated with an increased mortality risk, but asthma was not mentioned on the list of comorbidities.35 A study conducted in Switzerland included 200 patients with COVID-19 hospitalized in the Lausanne University Hospital and reported 4.0% asthma prevalence, 1 of 37 (2.7%) with asthma among those patients who required mechanical ventilation and 7 of 163 (4.3%) with asthma among those patients who did not require mechanical ventilation.36 Lieberman-Cribbin et al37 describe results from the Mount Sinai Health System and found that there was no statistically significant association between asthma status and mortality among patients with COVID-19. Therefore, we provisionally conclude that asthma is not associated with a higher risk of mortality among patients with COVID-19 who have a history of asthma. So far, there is no clear evidence that patients with asthma are more likely to be infected with SARS-CoV-2 or to become severely ill.
The phenotype of asthma patients with COVID-19
Although there is yet little information about asthma phenotypes in patients with COVID-19, we may speculate that patients with asthma with different phenotype hold various susceptibility and severity of COVID-19. The study by the UK Biobank reported that adults with asthma had a higher risk of severe COVID-19, which was driven by the increased risk in patients with nonallergic asthma.32 In contrast, the risk of severe COVID-19 was not significantly elevated in patients with allergic asthma.
Potential lower risk of patients with type 2 asthma to develop COVID-19
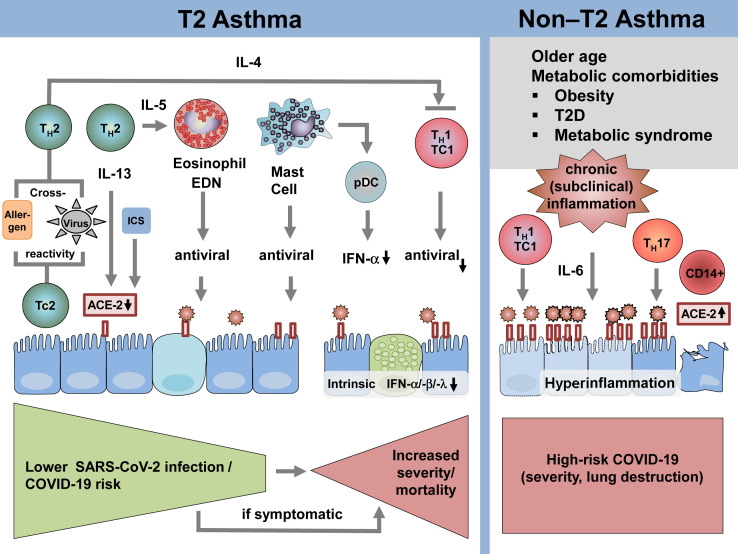
Type 2 asthma is characterized by TH2-driven airway inflammation with increased levels of IL-4, IL-5, and IL-13 production, blood and airway eosinophilia, and (in the case of allergic asthma) increased levels of total and allergen-specific IgE antibodies associated with mast cell activation. Lymphopenia, particularly due to the reduction of T cells, is a well-established marker for COVID-19 severity, and because patients with asthma with COVID-19 have increased numbers and activation-level T cells and show a less severe course of the disease, it is suggested that both CD4+ and CD8+ T cells reduce the destructive power of SARS-CoV-2.38 Angiotensin-converting enzyme-2 (ACE-2) serves as a major receptor for SARS-CoV-2 to enter host cells via its structural spike glycoprotein (Fig 2 ). ACE-2 is predominantly expressed on nasal epithelium, lung, heart, kidney, and intestine, but it is rarely expressed on immune cells.39 , 40 Markedly reduced levels of ACE-2 transcripts have been detected in nasal and bronchial epithelial cells of allergy sufferers, and this is associated with allergen exposure, allergen sensitization, and high IgE levels,41 with lowest levels among patients with both high levels of allergic sensitization and asthma. Conversely, nonatopic asthma was not associated with reduced ACE-2 expression.41 This is observed in both children and adults with asthma. However, data on the protein level are still lacking.41 Furthermore, ACE-2 gene expression levels correlate inversely with type 2 biomarkers42 , 43 and a functional contribution of these cytokines is indicated because IL-13 reduces ACE-2 gene expression in both the nasal and bronchial epithelium.41 In addition, ICSs, the first-line anti-inflammatory drug in type 2 asthma, also lower ACE-2 gene expression in sputum.44
Fig 2.
The impact of asthma endotypes on infection of airway epithelium with SARS-CoV-2, development and progression of COVID-19. The 2 major asthma endotypes, type 2 asthma and non–type 2 asthma, are defined by unique inflammatory patterns on the level of adaptive immunity and effector cell responses. Type 2 asthma is characterized by the presence of TH2 cells secreting IL-4, IL-5, and IL-13. These cytokines have a strong impact on the regulation of helper-cell subsets, airway epithelial function, and regulation of effector cell responses, including eosinophils and mast cells. In contrast, non–type 2 asthma is defined by the presence of TH1 cells and/or TH17 cells among other effector T-cell responses. The subset of these patients shows vascular and metabolic comorbidities, which are underlined by the presence of subclinical chronic inflammatory responses (eg, activation of IL-1 signaling pathways). EDN, Eosinophil-derived neurotoxin; pDC, plasmacytoid dendritic cell; T2D, type 2 diabetes.
However, there is an increasing amount of data providing evidence that ICSs are associated with reduction in ACE-2 and transmembrane proteases serine 2 (TMPRSS2) gene expression.44 This presumably occurs by a mechanism independent of any ICS-mediated suppression of TH2 inflammation. In addition, there is evidence to support that taking ICSs may be beneficial in dealing with coronavirus infections, based on in vitro studies with coronavirus application and cytokine production as an outcome.45
Host TMPRSS2 cleaves a viral spike protein and facilitates virus fusion to the cellular membrane.46 , 47 In contrast to ACE-2, TMPRSS2 gene expression is upregulated under TH2 conditions.48 However, there are very few cells coexpressing ACE-2 and TMPRSS2 simultaneously. Therefore, it is questionable whether this increased TMPRSS2 expression favors SARS-CoV-2 infection in patients with allergic asthma.48
Blood eosinophilia is an established biomarker for type 2 inflammation,49 , 50 and eosinophils have important antiviral properties. This includes single-stranded RNA activating eosinophils via Toll-like receptor-7/myeloid differentiation primary response 88-dependent mechanisms,51 and eosinophil-derived neurotoxin, which serves as a ribonuclease.52 Conversely, eosinopenia has been observed in patients with severe COVID-19, and blood cell counts normalize following lopinavir treatment, suggesting that they may serve as a marker for improvement.3 , 53 , 54 In a Russian retrospective study, absolute blood eosinophil counts were below 0.02 × 109/L in 85.7% of patients with asthma, and no patient showed blood eosinophilia.6
Infections with respiratory viruses including rhinovirus, respiratory syncyntial virus, and influenza virus are major triggers for asthma exacerbation, particularly in children.55, 56, 57, 58 Moreover, asthma has consistently been 1 of the most frequent comorbidities among patients hospitalized because of influenza. In contrast, this is not always the case for SARS-CoV-2. We hypothesize that the main reason for this is a high prevalence of type 2 airway signatures, even in nonatopic children.48 This could be a major reason why the prevalence of COVID-19 is relatively low in this age group, particularly because allergic, eosinophilic asthma is the major asthma endotype among young patients with asthma. Mast cells also contribute to combat viral infections and, in particular, SARS-CoV-2, because they are a major source of IFN.59 , 60
Potential role of allergen-induced cross-reactive T-cell responses to SARS-CoV-2 among patients with asthma
T-cell responses against SARS-CoV-2 are first detectable approximately 1 week following symptom onset and remain in convalescence, while numbers of virus-specific T cells correlate with neutralization antibody titers.61 Patients who recovered from SARS-CoV infection developed long-lived virus-specific T memory cells, detectable up to 2 years following infection resolution.62 , 63
Heterologous immunity (HI) has been originally described as a consequence of previous infections, which alter the immune response to a subsequent infection with a different pathogen.64 This mechanism may occur between closely related or completely unrelated antigens. HI may ultimately alter the outcome of infections due to cross-reactive recognition and immune protection or due to induction of immunopathology.65 Cellular-mediated HI may be elicited by means of T-cell receptor cross-reactivity, recognizing similar but distinct antigens or by cytokine-induced nonspecific activation of T cells.66
We have previously reported on influenza-induced heterologous immune responses against allergens, which mediated protection from experimental allergic asthma.67 We recently hypothesized that SARS-CoV-2 may show protein sequence homology to allergens, which may generate cross-reactive T-cell epitopes. We thus applied 2 independent but complementary bioinformatic approaches to identify potentially cross-reactive allergen T-cell and SARS-CoV-2 T-cell epitopes. Our in silico analysis revealed numerous candidate epitope pairs, including previously published as well as predicted peptides, and highlighted an important role of MHC class I aeroallergens in this regard68 (Fig 2). In hosts, who are sensitized to 1 of the predicted allergens, the identified similarities with the SARS-CoV-2 proteome may be protective or harmful. Allergen-specific T cells may develop a memory-type response against heterologous SARS-CoV-2 epitopes, which is by definition faster and more efficient. Quite recently, the role of SARS-CoV-2–specific T cells in exposed and nonexposed individuals has been discussed, which further underlines the potential importance of HI in COVID-19 outcome.69 , 70 Specifically, it was suggested that cross-reactive CD4+ T cells in some populations may be recruited into an amplified primary SARS-CoV-2–specific response. Therefore, patients with allergic asthma may carry potential SARS-CoV-2 cross-reactive T cells in their T-cell repertoire if they are sensitized to the respective cross-reactive allergens. This would provide a significant advantage for patients with allergic asthma over other patients with asthma in combating SARS-CoV-2 infections. Further experimental studies are underway to provide supporting functional data and confirm this concept.
Progression of COVID-19 in patients with type 2 asthma
We hypothesize that if SARS-CoV-2 succeeds to establish clinical manifestations in patients with allergic asthma, the risk for disease progression is higher as compared with that in patients with nonallergic asthma with COVID-19. This may occur because of several reasons: (1) TH2 inflammation counteracts TH1 immunity and limits the production of proinflammatory cytokines (eg, IL-1β, TNF-α, IL-6, and IL-12), which are required to combat viral infections; (2) an impaired production of type I and type III IFNs (IFN-α, IFN-β, IFN-λ) by airway epithelial cells has been described in patients with asthma in response to viral infections71, 72, 73, 74; and (3) plasmocytoid dendritic cells represent the primary source of IFN-α to defend against viral infections and IgE negatively regulates IFN-α production through inhibition of TLR signaling in these cells.75, 76, 77, 78
COVID-19 in patients with non–type 2 asthma
There is circumstantial evidence that patients suffering from non–type 2 asthma are at a higher risk for progression to severe COVID-19. Non–type 2 is defined as patients with asthma with other inflammatory profile such as TH1- or TH17-dominated inflammation including patients with chronic obstructive pulmonary disease and asthma. A recently published nationwide South Korean study reported that among patients with asthma, particularly nonallergic asthma, there is a greater risk of susceptibility to SARS-CoV-2 infection and severe clinical outcomes of COVID-19.79 Elderly patients with asthma are at a higher risk for morbidity and mortality than younger patients with asthma. In many of these individuals, the inflammatory response is non–type 2-mediated,80 and the inflammatory endotype is dominated by type 1 and/or type 17 T-cell responses. A molecular phenotype is characterized by inflammasome-associated and metabolic/mitrochrondrial pathways.81 Many of these patients with asthma suffer from comorbidities including obesity, type 2 diabetes, and hypertension as part of the metabolic syndrome.82 This endotype is particularly prevalent in inner-city adults83 and among African Americans.84
CD147 activation suppresses several T-cell functions through the inhibition of nuclear factor of activated T-cell pathways.85 , 86 CD147 expression correlates positively with body mass index in patients with COVID-19, and is also upregulated by high glycose concentrations.87 IL-6 serves as a biomarker for systemic inflammation and metabolic dysfunction as well as for severity in these patients with asthma.88 This subset of patients with asthma may be particularly susceptible to develop severe COVID-19. It is striking that increased cytokine levels with particularly high levels of IL-6 are characteristic for patients with severe COVID-19 as well.89 It is well established that patients with COVID-19 with these comorbidities are at a higher risk for severity and mortality (L. Qin, C. Zhang, J. Yue, X. Min, unpublished data, 2020). The increased expression of ACE-2 detected in subgroups41 with type 2 diabetes, chronic obstructive pulmonary disease, African Americans, smokers, and males44 further contributes to this.44 , 90 This may be regulated through IL-17 and supported because of the positive correlation between ACE-2 expression and TH17 gene signatures.42 The latter are more frequent among females and based on known age- and sex-induced influences on immune responses, it is likely that such skewing could also take place independently of the presence of asthma.
In contrast to patients with allergic asthma, patients with asthma with this endotype have a low number of blood eosinophils42 and therefore lack the contribution of these effector cells in anti–SARS-COV-2 defense. CD147, also termed basigin, acts as a receptor for SARS-CoV-2 in T cells and epithelial cells, and humanized anti-CD147 antibody treatment has been shown to reduce inflammation and to improve patients with severe COVID-19.91, 92, 93
Conclusions
This model may provide a comprehensive explanation for regional differences in asthma prevalence among patients with COVID-19 in North America, Europe, Russia, China, and other nations around the world.
However, to further validate this novel concept, more data and studies are required. Many of the so far published studies are retrospective and are nondiscriminating regarding asthma phenotypes. There is a considerable lack of additional clinical and immunologic parameters. Deep endotyping of patients with COVID-19 and asthma would be required to get a better understanding about the immunologic and metabolic association between these 2 entities. Also, on the level of virus-host interactions with regard to the cellular entry mechanism used by the virus, more data on the transcriptional and translational level of receptor regulation is certainly needed. In addition, there is a lack of longitudinal prospective studies.
A high proportion of patients with type 2 (allergic, eosinophilic) asthma in the population may help to limit SARS-CoV-2 dissemination. However, if patients with allergic asthma develop COVID-19, they may have a higher risk of disease progression. This is mainly due to diminished intrinsic IFN signaling pathways. This might be in contrast to regions with a relatively high population of patients with non–type 2 asthma, which are in particular elderly patients with metabolic comorbidities such as obesity, metabolic syndrome, and glucose dysregulation. This group of patients with asthma has a different inflammatory profile, and due to the chronic subclinical inflammation associated with the metabolic dysregulation, there is circumstantial evidence that the immune system is already (pre-) programmed to develop hyperinflammation in the context of a cytokine storm in association with COVID-19. In both situations, patients with asthma with metabolic dysregulation and patients with COVID-19 with associated hyperinflammation, the IL-6 signaling pathways contribute to the disease among other proinflammatory cytokines.
This concept may illustrate that different asthma endotypes have a differential impact on the infection of airway epithelial cells with SARS-CoV-2, and the development and the progression of COVID-19. This concept requires further clinical and experimental evidence, and we propose to include immunologic endotyping in SARS-CoV-2–infected patients with regard to the presence (or absence) of underlying inflammatory profiles on the level of both innate and adaptive immunity. This could have further implications for immunomodulatory therapies in patients with COVID-19 with asthma comorbidity.
New data are emerging rapidly and deepening our understanding about COVID-19 in patients with asthma/allergy. From the present perspective, current recommendations for treatment of asthma in the context of COVID-19 should not be changed. Patients should receive guideline-based pharmacological treatment including ICSs and biological therapies if needed.
Footnotes
H.R. is funded by the Universities Giessen Marburg Lung Center (UGMLC) and the German Center for Lung Disease (DZL German Lung Center, no. 82DZL00502) for UGMLC. C.S. is funded by UGMLC and the German Center for Lung Research, University Hospital Gießen and Marburg research funding according to article 2, section 3 cooperation agreement, the Deutsche Forschungsgemeinschaft (DFG)-funded Sonderforschungsbereich (SFB), Collaborative Research Center (CRC) (grant no. 1021 [C04]), the DFG-funded KFO (Klinische Forschungsgruppe), Germany (grant no. 309 [P10]), and SK 317/1-1 (project number 428518790) as well as by the Foundation for Pathobiochemistry and Molecular Diagnostics.
Disclosure of potential conflict of interest: C. Skevaki received consultancy fees and research funding from Hycor Biomedical and Thermo Fisher Scientific, research funding from Mead Johnson Nutrition, and consultancy fees from Bencard Allergie. The rest of the authors declare that they have no relevant conflicts of interest.
Supplementary data
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention [published online ahead of print 2020]. JAMA. https://doi.org/10.1001/jama.2020.2648. [DOI] [PubMed]
- 2.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J.-J., Dong X., Cao Y.-Y., Yuan Y.-D., Yang Y.-B., Yan Y.-Q. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J.-J., Cao Y.-Y., Dong X., Wang B.-C., Liao M.-Y., Lin J. Distinct characteristics of COVID-19 patients with initial rRT-PCR-positive and rRT-PCR-negative results for SARS-CoV-2. Allergy. 2020;75:1809–1812. doi: 10.1111/all.14316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avdeev S, Moiseev S, Brovko M, Yavorovskiy A, Umbetova K, Akulkina L, et al. Low prevalence of bronchial asthma and chronic obstructive lung disease among intensive care unit patients with COVID-19 [published online ahead of print 2020]. Allergy. https://doi.org/10.1111/all.14420. [DOI] [PMC free article] [PubMed]
- 7.Shabrawishi M, Al-Gethamy MM, Naser AY, Ghazawi MA, Alsharif GF, Obaid EF, et al. Clinical, radiological and therapeutic characteristics of patients with COVID-19 in Saudi Arabia [published online ahead of print 2020]. Medrxiv. https://doi.org/10.1101/2020.05.07.20094169. Available at: http://medrxiv.org/cgi/content/short/2020.05.07.20094169. [DOI] [PMC free article] [PubMed]
- 8.Rezende L.F.M., Thome B., Schveitzer M.C., Souza-Júnior P.R.B.d., Szwarcwald C.L. Adults at high-risk of severe coronavirus disease-2019 (Covid-19) in Brazil. Revista Saude Publica. 2020;54:50. doi: 10.11606/s1518-8787.2020054002596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solís P, Carreňo H. COVID-19 fatality and comorbidity risk factors among confirmed patients in Mexico [published online ahead of print 2020]. medRxiv. https://doi.org/10.1101/2020.04.21.20074591. Available at: http://medrxiv.org/content/early/2020/04/25/2020.04.21.20074591.abstract.
- 10.Aggarwal A., Shrivastava A., Kumar A., Ali A. Clinical and epidemiological features of SARS-CoV-2 patients in SARI ward of a tertiary care centre in New Delhi. J Assoc Phys India. 2020;68:19–26. [PubMed] [Google Scholar]
- 11.Gémes K., Talbäck M., Modig K., Ahlbom A., Berglund A., Feychting M. Burden and prevalence of prognostic factors for severe COVID-19 in Sweden. Eur J Epidemiol. 2020;35:401–409. doi: 10.1007/s10654-020-00646-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caminati M., Lombardi C., Micheletto C., Roca E., Bigni B., Furci F. Asthmatic patients in COVID-19 outbreak: few cases despite many cases. J Allergy Clin Immunol. 2020;146:541–542. doi: 10.1016/j.jaci.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borobia A.M., Carcas A.J., Arnalich F., Álvarez-Sala R., Monserrat-Villatoro J., Quintana M. A cohort of patients with COVID-19 in a major teaching hospital in Europe. J Clin Med. 2020;9:E1733. doi: 10.3390/jcm9061733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prieto-Albhambra D, Ballo E, Coma-Redon E, Mora N, Aragon M, Prats-Uribe A, et al. Hospitalization and 30-day fatality in 121,263 COVID-19 outpatient cases [published online ahead of print 2020]. medRxiv. https://doi.org/10.1101/2020.05.04.20090050.
- 16.Butler MW, O’Reilly A, Dunican EM, Mallon P, Feeney ER, Keane MP, et al. Prevalence of comorbid asthma in COVID-19 patients [published online ahead of print 2020]. J Allergy Clin Immunol. https://doi.org/10.1016/j.jaci.2020.04.061. [DOI] [PMC free article] [PubMed]
- 17.ClinicalTrials.gov Identifier: NCT03137043 Prevalence of severe asthma in Spanish hospitals—full text view. https://clinicaltrials.gov/ct2/show/NCT03137043 Available at:
- 18.GOV.IE - From Healthy Ireland, Department of Health. https://www.gov.ie/en/campaigns/healthy-ireland/?referrer=/accessibility/healthy-ireland-survey/#publications Available at:
- 19.Wang X.D., Zheng M., Lou H.F., Wang C.S., Zhang Y., Bo M.Y. An increased prevalence of self-reported allergic rhinitis in major Chinese cities from 2005 to 2011. Allergy. 2016;71:1170–1180. doi: 10.1111/all.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang K., Yang T., Xu J., Yang L., Zhao J., Zhang X. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet (London, England) 2019;394:407–418. doi: 10.1016/S0140-6736(19)31147-X. [DOI] [PubMed] [Google Scholar]
- 21.Lin J., Wang W., Chen P., Zhou X., Wan H., Yin K. Prevalence and risk factors of asthma in mainland China. The CARE study. Respir Med. 2018;137:48–54. doi: 10.1016/j.rmed.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Chuchalin A.G., Khaltaev N., Antonov N.S., Galkin D.V., Manakov L.G., Antonini P. Chronic respiratory diseases and risk factors in 12 regions of the Russian Federation. Int J Chronic Obstr Pulmon Dis. 2014;9:963–974. doi: 10.2147/COPD.S67283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agrawal S., Pearce N., Millett C., Subramanian S.V., Ebrahim S. Occupations with an increased prevalence of self-reported asthma in Indian adults. J Asthma. 2014;51:814–824. doi: 10.3109/02770903.2014.913619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del-Rio-Navarro B.E., Navarrete-Rodríguez E.M., Berber A., Reyes-Noriega N., García-Marcos Álvarez L. The burden of asthma in an inner-city area: a historical review 10 years after Isaac. World Allergy Organ J. 2020;13:100092. doi: 10.1016/j.waojou.2019.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliveira TB, Persigo ALK, Ferrazza CC, Ferreira ENN, Veiga ABG. Prevalence of asthma, allergic rhinitis and pollinosis in a city of Brazil: a monitoring study [published online ahead of print 2020]. Allergol Immunopathol. https://doi.org/10.1016/j.aller.2020.03.010. [DOI] [PubMed]
- 26.Musharrafieh U., Tamim H., Houry R., AlBuhairan F. A nationwide study of asthma correlates among adolescents in Saudi Arabia. Asthma Res Pract. 2020;6:3. doi: 10.1186/s40733-020-00056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention Asthma prevalence. https://www.cdc.gov/asthma/data-visualizations/prevalence.htm Available at:
- 28.Kalyanaraman Marcello R, Dolle J, Grami S, Adule R, Li Z, Tatem K, et al. Characteristics and outcomes of COVID-19 patients in New York City’s public hospital system [published online ahead of print 2020]. medRxiv. https://doi.org/10.1101/2020.05.29.20086645. [DOI] [PMC free article] [PubMed]
- 29.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol [published online ahead of print 2020]. medRxiv. 10.1101/2020.04.23.20076042. [DOI]
- 31.Khawaja AP, Warwick AN, Hysi PG, Kastner A, Dick A, Khaw PT, et al. Associations with covid-19 hospitalisation amongst 406,793 adults. The UK Biobank prospective cohort study [published online ahead of print 2020]. medRxiv. https://doi.org/10.1101/2020.05.06.20092957. Available at: https://www.medrxiv.org/content/10.1101/2020.05.06.20092957v1.
- 32.Zhu Z, Hasegawa K, Ma B, Fujiogi M, Camargo CA, Liang L. Association of asthma and its genetic predisposition with the risk of severe COVID-19 [published online ahead of print 2020]. J Allergy Clin Immunol. https://doi.org/10.1016/j.jaci.2020.06.001. [DOI] [PMC free article] [PubMed]
- 33.Ibrahim L.F., Tosif S., McNab S., Hall S., Lee H.J., Lewena S. SARS-CoV-2 testing and outcomes in the first 30 days after the first case of COVID-19 at an Australian children’s hospital. Emerg Med Australas. 2020;32:801–808. doi: 10.1111/1742-6723.13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrie S. COVID-19, Australia. Epidemiol Rep. 2020;15 [Google Scholar]
- 35.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 36.Regina J, Papadimitriou-Olivgeris M, Burger R, Filippidis P, Tschopp J, Desgranges F, et al. Epidemiology, risk factors and clinical course of SARS-CoV-2 infected patients in a Swiss university hospital: an observational retrospective study [published online ahead of print 2020]. medRxiv. https://doi.org/10.1101/2020.05.11.20097741. [DOI] [PMC free article] [PubMed]
- 37.Lieberman-Cribbin W, Rapp J, Alpert N, Tuminello S, Taioli E. The impact of asthma on mortality in patients with COVID-19 [published online ahead of print 2020]. Chest. https://doi.org/10.1016/j.chest.2020.05.575. [DOI] [PMC free article] [PubMed]
- 38.Shi W, Gao Z, Ding Y, Zhu T, Zhang W, Xu Y. Clinical characteristics of COVID-19 patients combined with allergy [published online ahead of print 2020]. Allergy. https://doi.org/10.1111/ALL.14434. [DOI] [PMC free article] [PubMed]
- 39.Wu C., Zheng M. Single-cell RNA expression profiling shows that ACE2, the putative receptor of Wuhan 2019-nCoV, has significant expression in the nasal, mouth, lung and colon tissues, and tends to be co-expressed with HLA-DRB1 in the four tissues. Res Square, Preprint. 2020 [Google Scholar]
- 40.Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–1035.e19. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson D.J., Busse W.W., Bacharier L.B., Kattan M., O’Connor G.T., Wood R.A. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol. 2020;146:203–206.e3. doi: 10.1016/j.jaci.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bradding P., Richardson M., Hinks T.S.C., Howarth P.H., Choy D.F., Arron J.R. ACE2, TMPRSS2, and furin gene expression in the airways of people with asthma—implications for COVID-19. J Allergy Clin Immunol. 2020;146:208–211. doi: 10.1016/j.jaci.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peters M.C., Sajuthi S., DeFord P., Christenson S., Rios C.L., Montgomery M.T. COVID-19 related genes in sputum cells in asthma: relationship to demographic features and corticosteroids. Am J Respir Crit Care Med. 2020;1:83–90. doi: 10.1164/rccm.202003-0821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamaya M., Nishimura H., Deng X., Sugawara M., Watanabe O., Nomura K. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir Investig. 2020;58:155–168. doi: 10.1016/j.resinv.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894–904.e9. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sajuthi SP, DeFord P, Jackson ND, Montgomery MT, Everman JL, Rios CL, et al. Type 2 and interferon inflammation strongly regulate SARS-CoV-2 related gene expression in the airway epithelium [published online ahead of print 2020]. bioRxiv. 2020 Apr 10;2020.04.09.034454.
- 49.Morais-Almeida M., Pité H., Aguiar R., Ansotegui I., Bousquet J. Asthma and the coronavirus disease 2019 pandemic: a literature review. Int Arch Allergy Immunol. 2020;181:1–9. doi: 10.1159/000509057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woodruff P.G., Modrek B., Choy D.F., Jia G., Abbas A.R., Ellwanger A. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phipps S., Lam C.E., Mahalingam S., Newhouse M., Ramirez R., Rosenberg H.F. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110:1578–1586. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 52.Domachowske J.B., Dyer K.D., Bonville C.A., Rosenberg H.F. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis. 1998;177:1458–1464. doi: 10.1086/515322. [DOI] [PubMed] [Google Scholar]
- 53.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;395:497. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu F., Xu A., Zhang Y., Xuan W., Yan T., Pan K. Patients of COVID-19 may benefit from sustained Lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis. 2020;95:183–191. doi: 10.1016/j.ijid.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gibson P.G., Grootendor D.C., Henry R.L., Pin I., Rytila P.H., Wark P. Sputum induction in children. Eur Respir J. 2002;37:44s–46. doi: 10.1183/09031936.02.00004402. [DOI] [PubMed] [Google Scholar]
- 56.Peltola V., Jartti T., Putto-Laurila A., Mertsola J., Vainionpää R., Waris M. Rhinovirus infections in children: a retrospective and prospective hospital-based study. J Med Virol. 2009;81:1831–1838. doi: 10.1002/jmv.21590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nicholson K.G., Kent J., Ireland D.C. Respiratory viruses and exacerbations of asthma in adults. BMJ (Clin Res ed) 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnston S.L., Pattemore P.K., Sanderson G., Smith S., Lampe F., Josephs L. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ (Clin Res ed) 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marshall J.S., Portales-Cervantes L., Leong E. Mast cell responses to viruses and pathogen products. Int J Mol Sci. 2019;20:4241. doi: 10.3390/ijms20174241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mukai K., Tsai M., Saito H., Galli S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev. 2018;282:121–150. doi: 10.1111/imr.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ni L., Ye F., Cheng M.-L., Feng Y., Deng Y.-Q., Zhao H. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52:971–977.e3. doi: 10.1016/j.immuni.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Libraty D.H., O’Neil K.M., Baker L.M., Acosta L.P., Olveda R.M. Human CD4(+) memory T-lymphocyte responses to SARS coronavirus infection. Virology. 2007;368:317–321. doi: 10.1016/j.virol.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang L.-T., Peng H., Zhu Z.-L., Li G., Huang Z.-T., Zhao Z.-X. Long-lived effector/central memory T-cell responses to severe acute respiratory syndrome coronavirus (SARS-CoV) S antigen in recovered SARS patients. Clin Immunol (Orlando, Fla) 2006;120:171–178. doi: 10.1016/j.clim.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Welsh R.M., Selin L.K. No one is naive: the significance of heterologous T-cell immunity. Nat Rev Immunol. 2002;2:417–426. doi: 10.1038/nri820. [DOI] [PubMed] [Google Scholar]
- 65.Selin L.K., Varga S.M., Wong I.C., Welsh R.M. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J Exp Med. 1998;188:1705–1715. doi: 10.1084/jem.188.9.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Welsh R.M., Che J.W., Brehm M.A., Selin L.K. Heterologous immunity between viruses. Immunol Rev. 2010;235:244–266. doi: 10.1111/j.0105-2896.2010.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Skevaki C., Hudemann C., Matrosovich M., Möbs C., Paul S., Wachtendorf A. Influenza-derived peptides cross-react with allergens and provide asthma protection. J Allergy Clin Immunol. 2018;142:804–814. doi: 10.1016/j.jaci.2017.07.056. [DOI] [PubMed] [Google Scholar]
- 68.Balz K., Chen M., Kaushik A., Cemic F., Heger V., Renz H. Homologies between SARS-CoV-2 and allergen proteins may direct T cell-mediated heterologous immune responses. Res Sq [Preprint] 2020 Oct 6 doi: 10.1038/s41598-021-84320-8. rs.3.rs-86873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Braun J, Loyal L, Frentsch M, Wendisch D, Georg P, Kurth F, et al. Presence of SARS-CoV-2 reactive T cells in COVID-19 patients and healthy donors [published online ahead of print 2020]. Nature. https://doi.org/10.1038/s41586-020-2598-9. [DOI] [PubMed]
- 71.Cakebread J.A., Xu Y., Grainge C., Kehagia V., Howarth P.H., Holgate S.T. Exogenous IFN-β has antiviral and anti-inflammatory properties in primary bronchial epithelial cells from asthmatic subjects exposed to rhinovirus. J Allergy Clin Immunol. 2011;127:1148–1154.e9. doi: 10.1016/j.jaci.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 72.Zhu J., Message S.D., Mallia P., Kebadze T., Contoli M., Ward C.K. Bronchial mucosal IFN-α/β and pattern recognition receptor expression in patients with experimental rhinovirus-induced asthma exacerbations. J Allergy Clin Immunol. 2019;143:114–125.e4. doi: 10.1016/j.jaci.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Contoli M., Message S.D., Laza-Stanca V., Edwards M.R., Wark P.A.B., Bartlett N.W. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 74.Gill M.A., Bajwa G., George T.A., Dong C.C., Dougherty, Jiang N. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol (Baltimore, Md 1950) 2010;184:5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carli G, Cecchi L, Stebbing J, Parronchi P, Farsi A. Is asthma protective against COVID-19? [published online ahead of print 2020]. Allergy. https://doi.org/10.1111/ALL.14426. [DOI] [PMC free article] [PubMed]
- 76.Gonzales-van Horn S.R., Farrar J.D. Interferon at the crossroads of allergy and viral infections. J Leukocyte Biol. 2015;98:185–194. doi: 10.1189/jlb.3RU0315-099R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lebre M.C., van Capel T.M.M., Bos J.D., Knol E.F., Kapsenberg M.L., Jong E.C. d.e. Aberrant function of peripheral blood myeloid and plasmacytoid dendritic cells in atopic dermatitis patients. J Allergy Clin Immunol. 2008;122:969–976.e5. doi: 10.1016/j.jaci.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 78.Maggi E., Parronchi P., Manetti R., Simonelli C., Piccinni M.P., Rugiu F.S. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol (Baltimore, Md 1950) 1992;148:2142–2147. [PubMed] [Google Scholar]
- 79.Yang JM, Koh HY, Moon SY, Yoo IK, Ha EK, You S, et al. Allergic disorders and susceptibility to and severity of COVID-19: a nationwide cohort study [published online ahead of print 2020]. J Allergy Clin Immunol. https://doi.org/10.1016/j.jaci.2020.08.008. [DOI] [PMC free article] [PubMed]
- 80.Dunn R.M., Busse P.J., Wechsler M.E. Asthma in the elderly and late-onset adult asthma. Allergy. 2018;73:284–294. doi: 10.1111/all.13258. [DOI] [PubMed] [Google Scholar]
- 81.Kuo C.-H.S., Pavlidis S., Loza M., Baribaud F., Rowe A., Pandis I. T-helper cell type 2 (Th2) and non-Th2 molecular phenotypes of asthma using sputum transcriptomics in U-BIOPRED. Eur Respir J. 2017;49:1602135. doi: 10.1183/13993003.02135-2016. [DOI] [PubMed] [Google Scholar]
- 82.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fitzpatrick S., Joks R., Silverberg J.I. Obesity is associated with increased asthma severity and exacerbations, and increased serum immunoglobulin E in inner-city adults. Clin Exp Allergy. 2012;42:747–759. doi: 10.1111/j.1365-2222.2011.03863.x. [DOI] [PubMed] [Google Scholar]
- 84.White S.R., Laxman B., Naureckas E.T., Hogarth D.K., Solway J., Sperling A.I. Evidence for an IL-6-high asthma phenotype in asthmatic patients of African ancestry. J Allergy Clin Immunol. 2019;144:304–306.e4. doi: 10.1016/j.jaci.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yao H., Teng Y., Sun Q., Xu J., Chen Y.-T., Hou N. Important functional roles of basigin in thymocyte development and T cell activation. Int J Biol Sci. 2013;10:43–52. doi: 10.7150/ijbs.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ruiz S., Castro-Castro A., Bustelo X.R. CD147 inhibits the nuclear factor of activated T-cells by impairing Vav1 and Rac1 downstream signaling. J Biol Chem. 2008;283:5554–5566. doi: 10.1074/jbc.M708566200. [DOI] [PubMed] [Google Scholar]
- 87.Bao W., Min D., Twigg S.M., Shackel N.A., Warner F.J., Yue D.K. Monocyte CD147 is induced by advanced glycation end products and high glucose concentration: possible role in diabetic complications. Am J Physiol Cell Physiol. 2010;299:C1212–C1219. doi: 10.1152/ajpcell.00228.2010. [DOI] [PubMed] [Google Scholar]
- 88.Peters M.C., McGrath K.W., Hawkins G.A., Hastie A.T., Levy B.D., Israel E. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med. 2016;4:574–584. doi: 10.1016/S2213-2600(16)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pedersen S.F., Ho Y.-C. SARS-CoV-2: a storm is raging. J Clin Investig. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leung J.M., Yang C.X., Tam A., Shaipanich T., Hackett T.-L., Singhera G.K. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55 doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang K., Chen W., Zhou Y.-S., Lian J.-Q., Zhang Z., Du P. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv. 2020 doi: 10.1038/s41392-020-00426-x. 2020.2003.2014.988345 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang X, Xu W, Hu G, Xia S, Sun Z, Liu Z, et al. SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion [published online ahead of print 2020]. Cell Mol Immunol. https://doi.org/10.1038/s41423-020-0424-9. [DOI] [PMC free article] [PubMed]
- 93.Bian H, Zheng Z-H, Wei D, Zhang Z, Kang W-Z, Hao C-Q, et al. Meplazumab treats COVID-19 pneumonia: an open-labelled, concurrent controlled add-on clinical trial [published online ahead of print 2020]. medRxiv. https://doi.org/10.1101/2020.03.21.20040691.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.