Abstract
Severe COVID-19 (coronavirus disease 2019) is associated with elevated inflammatory markers, consistent with cytokine release syndrome (CRS). Tocilizumab is an interleukin-6 (IL-6) inhibitor effective in treating CRS secondary to chimeric antigen receptor T-cell (CAR T-cell) therapy. The efficacy of tocilizumab in treating COVID-19 is unknown. This was a retrospective cohort study conducted at two hospitals in northern New Jersey (USA). All patients treated with tocilizumab for confirmed or suspected COVID-19 between 10 March 2020 and 9 April 2020 at the study sites were included. The primary endpoint was clinical improvement on Day 7 after treatment as assessed by respiratory status. Univariate analysis compared data between those who improved and those who did not. A total of 45 severe and critically ill patients treated with tocilizumab for COVID-19 were evaluated. Of the 45 patients, 11 (24.4%), 22 (48.9%) and 12 (26.7%) patients improved, had no change or worsened by Day 7 after treatment, respectively. Lower white blood cell count and lactate dehydrogenase at the time of drug administration as well as shorter time from supplemental oxygen initiation to dosing were significantly associated with clinical improvement in the univariate analysis. In conclusion, tocilizumab administration was associated with a low rate of clinical improvement within 7 days in this cohort of severe and critically ill patients with COVID-19.
Keywords: COVID-19, SARS-CoV-2, Tocilizumab, IL-6 inhibitor, Immunomodulatory agents
1. Introduction
COVID-19 (coronavirus disease 2019), a disease caused by the novel coronavirus SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), has swept across the globe unlike any disease seen in decades. According to the World Health Organization (WHO), nearly 47 million cases and 1.2 million deaths have been reported worldwide as of 3 November 2020 [1]. The rapid spread and considerable mortality has clinicians desperate for therapeutic options to improve patient outcomes.
Evidence has suggested that severe viral infections may be associated with cytokine release syndrome (CRS), a hyperinflammatory state that can result in multiorgan failure. Retrospective reports from China have demonstrated that severe COVID-19 is associated with elevated inflammatory markers, similar to CRS [2]. In particular, elevated interleukin-6 (IL-6), d-dimer, C-reactive protein, cardiac troponin and procalcitonin have been identified as predictors of mortality in COVID-19 [3], [4], [5], [6]. This has led to speculation that treatment with immunomodulatory agents may yield positive outcomes by lessening hyperinflammation and minimising acute and long-term end-organ damage [7].
Tocilizumab is a monoclonal antibody that functions as an IL-6 receptor antagonist. Initially approved for the treatment of autoimmune disorders, it was subsequently found to be effective for the treatment of CRS secondary to chimeric antigen receptor T-cell (CAR T-cell) therapy [8]. Given its efficacy for this indication as well as the apparent role of IL-6 in COVID-19, interest in its role for severe manifestations of COVID-19 has grown.
A variety of observational studies and randomised controlled trials (RCTs) have investigated this question. Two meta-analyses of observational studies came to conflicting conclusions regarding the efficacy of tocilizumab [9,10]. Aziz et al. evaluated 23 studies with 6279 patients and found that tocilizumab use was associated with lower mortality [risk difference (RD), –0.06; 95% confidence interval (CI) –0.12 to –0.01] and decreased need for mechanical ventilation (RD, –0.11; 95% CI –0.19 to –0.02) [9]. However, Lan et al. assessed seven studies with 592 patients and found no difference in mortality [risk ratio (RR), 0.62; 95% CI 0.31–1.22] or need for mechanical ventilation (RR, 0.82; 95% CI 0.14–4.94) between those treated with tocilizumab and the control group [10]. More recently, Gupta et al. conducted a multicentre cohort study of nearly 4000 patients and found that those treated with tocilizumab had a lower adjusted risk of death compared with patients not treated with tocilizumab [hazard ratio (HR), 0.71; 95% CI 0.56–0.92] [11].
Results from RCTs have been similarly inconsistent. Stone et al. conducted a randomised, double-blind, placebo-controlled study and found no benefit of tocilizumab with respect to intubation or death (HR, 0.83; 95% CI 0.38–1.81) or disease worsening (HR, 1.11; 95% CI 0.59–2.10) [12]. An open-label RCT conducted by Salvarani et al. also found no differences in outcomes with tocilizumab compared with placebo, with a rate ratio for clinical worsening at 14 days of 1.05 (95% CI 0.59–1.86) [13]. Hermine et al. found no difference in respiratory status at Day 4 [median posterior absolute risk difference, −9.0%; 90% credible interval (CrI) −21.0 to 3.1] but a reduction in death or need for ventilation with tocilizumab at Day 14 (median posterior HR, 0.58; 90% CrI 0.33–1.00) [14].
The availability of some RCT results prior to peer-reviewed publication has further clouded this issue. Two such randomised, double-blind, placebo-controlled studies of tocilizumab have reported conflicting results. The COVACTA trial in patients with severe or critical COVID-19 found no difference in clinical status at Day 28 (odds ratio, 1.19; 95% CI 0.81–1.76) [15]. The EMPACTA study conducted in patients with severe COVID-19 only found reduced death or need for mechanical ventilation at Day 28 (HR, 0.56, 95% CI 0.32–0.97) [16].
In the setting of unclear evidence and increasing cases of COVID-19 worldwide, interest in the role of tocilizumab as a therapeutic option persists. The purpose of this study was to assess the characteristics and clinical outcomes of patients treated with tocilizumab for COVID-19.
2. Materials and methods
This was a retrospective cohort study conducted at two community medical centres located in northern New Jersey (USA). Both hospitals are members of a health system that shared identical institutional guidance regarding COVID-19 treatment recommendations during the study period. This guidance recommended consideration of tocilizumab as a treatment option for severe COVID-19 based on elevated inflammatory markers, increasing oxygen requirement and elevated temperature without specific thresholds. The recommended dose was 8 mg/kg (maximum 800 mg) once. This dose could be repeated for patients who did not respond within 8–12 h of the initial dose. The treatment and dosing strategy for tocilizumab, as well as use of other antivirals and corticosteroids, were based upon provider discretion. All patients treated with tocilizumab for confirmed COVID-19 between 12 March 2020 and 9 April 2020 at the study sites were included. COVID-19 was diagnosed by real-time PCR detection of SARS-CoV-2 from nasopharyngeal swabs.
The primary endpoint was clinical improvement on Day 7 after treatment as assessed by respiratory status. Respiratory status was assessed at the end of the day according to the following ordinal scale recommended by the WHO R&D Blueprint Expert Group [17]: death (7); hospitalised on invasive mechanical ventilation (6); hospitalised on non-invasive ventilation or high-flow oxygen device (5); hospitalised requiring supplemental oxygen (4); hospitalised not requiring supplemental oxygen (3); not hospitalised, limitation on activities (2); and not hospitalised, no limitations on activities (1). Clinical improvement was defined as any decrease in score on Day 7 compared with the day of treatment initiation. Data were collected from admission until 7 days following tocilizumab administration. Data on baseline demographics, medical history, oxygen requirements and COVID-19 therapy were collected. Additionally, vital signs and laboratory data immediately prior to tocilizumab therapy were collected. Data were compared between those who improved and those who did not. Differences between patients who improved and did not were assessed using the Wilcoxon rank-sum test for continuous variables and the χ2 test or Fisher's exact test for categorical variables as appropriate. This study was approved by the central Institutional Review Board.
3. Results
A total of 46 patients received tocilizumab at the study sites during the 4-week study period. One patient was transferred to an outside hospital prior to the Day 7 assessment and was excluded from the analysis. Table 1 outlines the baseline and clinical characteristics of the 45 patients included for analysis as well as the results from the univariate analysis comparing characteristics of those who improved and those who did not. Overall, 29 patients (64.4%) were male, the median patient age was 55.0 years [interquartile range (IQR) 48.0–63.0 years] and the median body mass index (BMI) was 30.0 kg/m2 (IQR 26.6–36.7 kg/m2). The most common co-morbidities were hypertension (51.1%) and type 2 diabetes mellitus (31.1%).
Table 1.
Baseline and clinical characteristics of 45 patients included in the analysis, and results of the univariate analysis comparing characteristics of those who improved and those who did not.
| Variable | Missing data | Group | Entire cohort (n = 45) | Not improved (n = 34) | Improved (n = 11) | P-value |
|---|---|---|---|---|---|---|
| Site | >0.999 | |||||
| Site 1 | 14 (31.1) | 11 (32.4) | 3 (27.3) | |||
| Site 2 | 31 (68.9) | 23 (67.6) | 8 (72.7) | |||
| Demographics | ||||||
| Age (years) | 55.0 (48.0–63.0) | 57.0 (49.8–63.8) | 53.0 (44.5–56.0) | 0.149 | ||
| BMI (kg/m2) | 30.0 (26.6–36.7) | 29.5 (26.5–36.1) | 35.0 (28.6–37.7) | 0.457 | ||
| Sex | 0.067 | |||||
| F | 16 (35.6) | 15 (44.1) | 1 (9.1) | |||
| M | 29 (64.4) | 19 (55.9) | 10 (90.9) | |||
| Co-morbidities | ||||||
| ESRD | >0.999 | |||||
| No | 44 (97.8) | 33 (97.1) | 11 (100.0) | |||
| Yes | 1 (2.2) | 1 (2.9) | 0 (0) | |||
| Malignancy | >0.999 | |||||
| No | 43 (95.6) | 32 (94.1) | 11 (100.0) | |||
| Yes | 2 (4.4) | 2 (5.9) | 0 (0) | |||
| Hypertension | 0.141 | |||||
| No | 22 (48.9) | 14 (41.2) | 8 (72.7) | |||
| Yes | 23 (51.1) | 20 (58.8) | 3 (27.3) | |||
| Diabetes mellitus type 2 | 0.132 | |||||
| No | 31 (68.9) | 21 (61.8) | 10 (90.9) | |||
| Yes | 14 (31.1) | 13 (38.2) | 1 (9.1) | |||
| Heart disease | 0.558 | |||||
| No | 41 (91.1) | 30 (88.2) | 11 (100.0) | |||
| Yes | 4 (8.9) | 4 (11.8) | 0 (0) | |||
| COPD | >0.999 | |||||
| No | 44 (97.8) | 33 (97.1) | 11 (100.0) | |||
| Yes | 1 (2.2) | 1 (2.9) | 0 (0) | |||
| Asthma | 0.143 | |||||
| No | 42 (93.3) | 33 (97.1) | 9 (81.8) | |||
| Yes | 3 (6.7) | 1 (2.9) | 2 (18.2) | |||
| Medication use | ||||||
| Cumulative tocilizumab dose (mg/kg) | 7.3 (5.5–7.9) | 7.3 (6.2–8.3) | 7.1 (4.7–7.8) | 0.552 | ||
| Re-dose | >0.999 | |||||
| No | 37 (82.2) | 28 (82.4) | 9 (81.8) | |||
| Yes | 8 (17.8) | 6 (17.6) | 2 (18.2) | |||
| Corticosteroid use | 0.304 | |||||
| No | 18 (40.0) | 12 (35.3) | 6 (54.5) | |||
| Yes | 27 (60.0) | 22 (64.7) | 5 (45.5) | |||
| Vasopressor use (pre-dose a) | 0.482 | |||||
| No | 27 (60.0) | 19 (55.9) | 8 (72.7) | |||
| Yes | 18 (40.0) | 15 (44.1) | 3 (27.3) | |||
| Vital signs | ||||||
| Respiratory rate (pre-dose a, breaths/min) | 25.0 (22.0–31.0) | 25.5 (22.0–31.8) | 25.0 (22.5–29.5) | 0.905 | ||
| Heart rate (pre-dose a, beats/min) | 110.0 (98.0–122.0) | 114.0 (104.2–123.8) | 107.0 (92.0–115.5) | 0.110 | ||
| Temperature (pre-dose a,°F) | 100.9 (100.2–102.2) | 100.9 (100.0–102.2) | 100.7 (100.4–102.2) | 0.989 | ||
| Laboratory parameters | ||||||
| Neutrophils (admission, × 103/mm3) | 5.6 (4.2–8.2) | 5.5 (4.4–7.6) | 7.4 (4.0–9.8) | 0.905 | ||
| Lymphocytes (admission, × 103/mm3) | 0.8 (0.5–1.1) | 0.8 (0.5–1.2) | 0.8 (0.5–0.9) | 0.730 | ||
| Neutrophil-to-lymphocyte ratio (admission) | 8.0 (4.9–13.0) | 7.8 (5.0–12.1) | 8.2 (4.7–14.5) | 0.653 | ||
| WBC count (pre-dose a, × 103/mm3) | 9.4 (8.1–12.0) | 10.1 (8.3–12.3) | 8.1 (5.8–9.4) | 0.038 | ||
| Platelets (pre-dose a, × 103/mm3) | 250.0 (209.0–362.0) | 249.0 (210.2–358.5) | 325.0 (207.0–364.5) | 0.812 | ||
| Lymphocytes (pre-dose a, × 103/mm3) | 5 (11.1) | 0.8 (0.5–1.2) | 0.8 (0.5–1.4) | 0.7 (0.6–0.9) | 0.696 | |
| Ferritin (pre-dose a, mg/L) | 18 (40.0) | 1303.0 (763.8–1786.0) | 1303.0 (684.6–1777.0) | 1397.0 (1066.8–1922.8) | 0.622 | |
| Creatinine (pre-dose a, mg/dL) | 1.0 (0.7–1.4) | 1.0 (0.7–2.3) | 1.0 (0.9–1.0) | 0.682 | ||
| Bilirubin (pre-dose a, mg/dL) | 5 (11.1) | 0.6 (0.4–1.0) | 0.6 (0.4–1.1) | 0.5 (0.4–0.7) | 0.549 | |
| Lactate dehydrogenase (pre-dose a, units/L) | 21 (46.7) | 497.0 (369.8–660.8) | 599.0 (425.0–684.5) | 354.0 (320.0–394.0) | 0.015 | |
| C-reactive protein (pre-dose a, mg/dL) | 21 (46.7) | 16.0 (9.4–25.6) | 20.3 (8.1–27.5) | 12.4 (10.7–12.9) | 0.251 | |
| Respiratory status b | ||||||
| Baseline respiratory status | 6.0 (5.0–6.0) | 6.0 (6.0–6.0) | 6.0 (4.5–6.0) | 0.136 | ||
| Baseline respiratory status | 0.251 | |||||
| <6 | 13 (28.9) | 8 (23.5) | 5 (45.5) | |||
| 6 | 32 (71.1) | 26 (76.5) | 6 (54.5) | |||
| Timing variables | ||||||
| Time from symptom onset to dose (days) | 11.0 (7.0–15.0) | 10.5 (7.0–13.8) | 13.0 (8.5–15.5) | 0.218 | ||
| Time from admission to dose (h) | 112.2 (51.5–155.8) | 116.4 (85.6–170.8) | 82.2 (38.5–114.6) | 0.055 | ||
| Time from admission to intubation (h) | 10 (22.2) | 87.2 (37.8–121.8) | 87.8 (45.5–121.9) | 34.7 (23.4–89.6) | 0.146 | |
| Time from initiation of oxygen supplementation to dose (h) | 1 (2.2) | 113.6 (74.3–151.8) | 117.2 (88.4–177.3) | 80.7 (41.2–115.4) | 0.044 | |
| Time from initiation of high-flow oxygen to dose (h) | 6 (13.3) | 76.2 (26.3–112.8) | 93.8 (33.8–113.2) | 35.0 (28.9–55.7) | 0.123 | |
| Time from ARDS to dose (h) | 14 (31.1) | 75.8 (37.3–98.7) | 87.3 (40.0–107.6) | 49.5 (32.3–63.3) | 0.091 | |
| Time from intubation to dose (h) | 16 (35.6) | 51.1 (25.0–93.8) | 60.0 (28.6–93.8) | 24.6 (11.7–41.4) | 0.114 |
NOTE: Data are presented as n (%) or median (interquartile range).
BMI, body mass index; ESRD, end-stage renal disease; COPD, chronic obstructive pulmonary disease; WBC, white blood cell; ARDS, acute respiratory distress syndrome.
Pre-dose, collected within 24 h (vital signs, vasopressor use) or 48 h (laboratory values) prior to the dose.
Respiratory status was assessed at the end of the day according to the ordinal scale recommended by the WHO R&D Blueprint Expert Group [17].
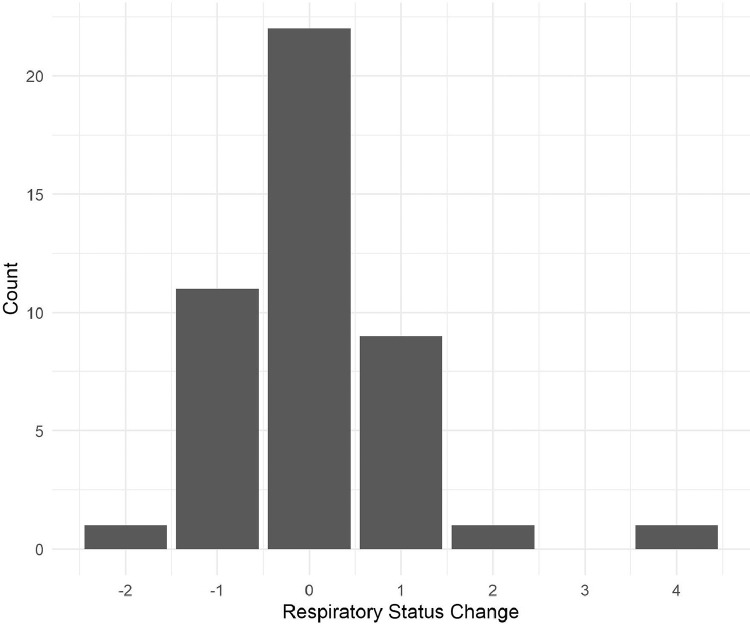
Patients received tocilizumab a median of 11.0 days (IQR 7.0–15.0 days) after symptom onset and 112.2 h (IQR 51.5–155.8 h) after admission. Of the 45 patients, 32 (71.1%) were intubated (score of 6 on the respiratory status scale) on the day of tocilizumab administration, and an additional three patients were intubated within the 7-day follow-up period. Moreover, 31 patients (68.9%) had documented acute respiratory distress syndrome (ARDS) at the time of dosing. All patients received hydroxychloroquine, two (4.4%) received lopinavir/ritonavir and 27 (60.0%) received corticosteroids. Of the 45 patients, 11 (24.4%) improved by Day 7 after treatment, 22 (48.9%) had no change and 12 (26.7%) worsened. Fig. 1 illustrates the respiratory status change at Day 7 for the entire cohort. Lower white blood cell (WBC) count (P = 0.038) and lactate dehydrogenase (LDH) (P = 0.015) at the time of drug administration as well as shorter time from initiation of supplemental oxygen to dose (P = 0.044) were significantly associated with clinical improvement.
Fig. 1.
Histogram plot of respiratory status change at Day 7. Negative values indicate clinical worsening, whereas positive values indicate improvement.
4. Discussion
In this small retrospective cohort study, we found low rates of early clinical improvement in patients with severe or critical COVID-19. In fact, worsening respiratory status was common and was observed in 27% of patients by Day 7 after treatment. These findings are similar to those reported by Salvarani et al. who found that 28.3% of patients randomised to tocilizumab experienced clinical worsening by Day 6 [13]. Results from Hermine et al., which found positive outcomes compared with standard of care at Day 14 but not Day 4, further suggest that any benefits with tocilizumab are uncommon within the first week after treatment [14].
Evidence made available after this study period has demonstrated the efficacy of corticosteroids in treating severe and critical COVID-19 [18,19]. Corticosteroid use was common but not universal in this cohort. Their use was not associated with clinical improvement and was numerically more common in those who did not improve (64.7% vs. 45.5%; P = 0.304). This is a question of particular importance given that the benefit of the incremental immunomodulatory impact of tocilizumab combined with corticosteroids may be different than the benefit of tocilizumab or corticosteroids alone. This is precisely the issue that Rodríguez-Baño et al. sought to answer in their multicentre cohort study [20]. Ultimately, those authors found that tocilizumab alone (HR, 0.32; 95% CI 0.22–0.47) and pulse-dose corticosteroids alone (HR, 0.61; 95% CI 0.43–0.86) were associated with decreased intubation and death but the combination of both was not (HR, 1.17; 95% CI 0.86–1.58) [20]. In contrast, the RCT conducted by Hermine et al. found a signal of potentially improved outcomes in the subgroup of patients who received corticosteroids (HR, 0.38; 90% CrI, 0.13–1.11), although this was not statistically significant [14]. The interaction of the effects of tocilizumab and corticosteroids in the management of COVID-19 will require careful examination in order to determine the optimal deployment of these therapies.
Importantly, the lack of a control group in our study prohibits an assessment of the efficacy of tocilizumab for COVID-19. This is better assessed with the aforementioned RCTs, which have thus far failed to yield consistent findings. In light of such conflicting data, it would seem that dramatic benefits in a broad range of patients with COVID-19 are unlikely with tocilizumab. However, glimpses of possible clinical benefits warrant critical evaluations of specific subpopulations or patient characteristics that may predict benefit.
Factors that have been independently associated with poor outcome in COVID-19 include older age, neutrophil-to-lymphocyte ratio, and organ and coagulation dysfunction [21], [22], [23]. Our analysis identified that only lower WBC count and LDH at the time of drug administration as well as shorter time from initiation of supplemental oxygen to dose were associated with clinical improvement. Zhou et al. previously identified that elevated WBC count (>10 × 109/L) and elevated LDH (>245 U/L) were associated with higher mortality among inpatients with COVID-19 [21]. However, neither of these associations were significant in their multivariable analysis. Salvarani et al. also evaluated outcomes in the subgroup of patients with baseline LDH < 250 U/L and found numerically lower clinical worsening among those who received tocilizumab (23.1% vs. 36.45%; RR, 0.6; 95% CI 0.2–2.2) [13]. This imbalance in our study may simply be a reflection of differences in illness severity or it may support that the role of tocilizumab is earlier in the disease course.
C-reactive protein (CRP) is another biomarker that has been subject to considerable attention with respect to tocilizumab. Martínez-Sanz et al. found lower mortality (adjusted HR, 0.34; 95% CI 0.17–0.71) with tocilizumab in the subgroup of patients with baseline CRP > 15 mg/dL in their multicentre cohort study [24]. Similarly, Biran et al. evaluated a subgroup of patients with baseline CRP > 15 mg/dL and found that tocilizumab was associated with decreased mortality (HR, 0.48; 95% CI 0.30–0.77) [25]. Salvarani et al. found numerically lower clinical worsening among those with baseline CRP > 15 mg/dL that was not statistically significant (RR, 0.4; 95% CI 0.1–1.9) [13]. We did not find differences in median baseline CRP among those who did and did not improve [12.4 (IQR 10.7–12.9) mg/dL vs. 20.3 (IQR 8.1–27.5) mg/dL; P = 0.251]. Biomarkers predictive of clinical response could be highly useful for clinicians considering the use of this medication. Further research into these and other laboratory values is required to resolve this question.
The association between response and drug timing relative to the initiation of supplemental oxygen could have important implications for the use of this drug. Given its mechanism, it is plausible that there may be a critical window where tocilizumab can blunt excessive inflammation and yield positive outcomes. It has been suggested that suppressing the immune response prior to an excessive immune response is unlikely to provide benefit and may be detrimental [26]. Our data further suggest that tocilizumab administration after a dramatic immune response has mounted may be too late to reverse end-organ damage. Similarly, Sciascia et al. found that tocilizumab administration within 6 days from admission in the hospital was associated with an increased likelihood of survival (HR, 2.2; 95% CI 1.3–6.7) [27]. Nevertheless, the published RCTs to date have enrolled patients early relative to hospitalisation with a median of 2 days [13] and 1 day [14] from admission to randomisation. The lack of consistent benefits demonstrated in these studies does not support routine use of tocilizumab in this time window. Further study is required to determine whether an optimal time window for drug administration exists.
Important limitations in our study include the design, which is subject to selection bias. Differences identified between those who improved and those who did not may be reflective of the natural disease course and unrelated to tocilizumab use. Additionally, the small sample size and short follow-up period limit the implications of these results. Other questions regarding tocilizumab for COVID-19, including assessments of long-term safety and efficacy, are beyond the scope of this study.
In conclusion, tocilizumab administration was associated with low rates of clinical improvement within 7 days in this cohort of severe and critically ill patients with COVID-19. Further study is required to determine the optimal role of tocilizumab in the management of COVID-19.
Acknowledgments
The authors would like to thank Alison Brophy, PharmD, Christopher Makosiej, PharmD, and Vasyl Zybrak, PharmD, for their contributions to data collection and analysis.
Funding: None.
Competing interests: None declared.
Ethical approval: This study was approved by the central Institutional Review Board [FWA # 00003433].
Editor: J. Gray
References
- 1.World Health Organization (WHO). WHO coronavirus disease (COVID-19) dashboard. https://covid19.who.int/ [accessed 3 November 2020].
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chilimuri S., Sun H., Alemam A., Mantri N., Shehi E., Tejada J. Predictors of mortality in adults admitted with COVID-19: retrospective cohort study from New York City. West J Emerg Med. 2020;21:779–784. doi: 10.5811/westjem.2020.6.47919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian W., Jiang W., Yao J., Nicholson C.J., Li R.H., Sigurslid H.H. Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2020;92:1875–1883. doi: 10.1002/jmv.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shang Y., Liu T., Wei Y., Li J., Shao L., Liu M. Scoring systems for predicting mortality for severe patients with COVID-19. EClinicalMedicine. 2020;24 doi: 10.1016/j.eclinm.2020.100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le R.Q., Li L., Yuan W., Shord S.S., Nie L., Habtemariam B.A. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23:943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aziz M., Haghbin H., Sitta E.A., Nawras Y., Fatima R., Sharma S. Efficacy of tocilizumab in COVID-19: a systematic review and meta-analysis. J Med Virol. 2020 Sep 12 doi: 10.1002/jmv.26509. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Lan S.H., Lai C.C., Huang H.T., Chang S.P., Lu L.C., Hsueh P.R. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta S., Wang W., Hayek S.S., Chan L., Mathews K.S., Melamed M.L. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2020 Oct 20 doi: 10.1001/jamainternmed.2020.6252. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salvarani C., Dolci G., Massari M., Merlo D.F., Cavuto S., Savoldi L. Effect of tocilizumab vs standard of care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2020 Oct 20 doi: 10.1001/jamainternmed.2020.6615. [Epub ahead of [print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermine O., Mariette X., Tharaux P.-L., Resche-Rigon M., Porcher R., Ravaud P., CORIMUNO-19 Collaborative Group Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2020 Oct 20 doi: 10.1001/jamainternmed.2020.6820. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.F. Hoffman-La Roche Ltd . Roche; Basel, Switzerland: 29 July 2020. Roche provides an update on the phase III COVACTA trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 associated pneumonia. https://www.roche.com/investors/updates/inv-update-2020-07-29.htm [accessed 25 October 2020] [Google Scholar]
- 16.F. Hoffman-La Roche Ltd . Roche; Basel, Switzerland: 18 September 2020. Roche's phase III EMPACTA study showed Actemra/RoActemra reduced the likelihood of needing mechanical ventilation in hospitalised patients with COVID-19 associated pneumonia. https://www.roche.com/investors/updates/inv-update-2020-09-18.htm [accessed 25 October 2020] [Google Scholar]
- 17.World Health Organization (WHO) WHO; Geneva, Switzerland: 2020. Coronavirus disease (COVID-2019) R&D blueprint. http://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus/en/ [accessed 15 December 2020] [Google Scholar]
- 18.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020 Jul 17 doi: 10.1056/NEJMoa2021436. [Epub ahead of print] [DOI] [Google Scholar]
- 19.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., Angus D.C. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodríguez-Baño J., Pachón J., Carratalà J., Ryan P., Jarrín I., Yllescas M. Treatment with tocilizumab or corticosteroids for COVID-19 patients with hyperinflammatory state: a multicentre cohort study (SAM-COVID-19) Clin Microbiol Infect. 2020 Aug 27 doi: 10.1016/j.cmi.2020.08.010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou F., Yu T., Du R., Fan G., Liu Y., Ciang J. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y., Du X., Chen J., Jin Y., Peng L., Wang H.H.X. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;81:e6–12. doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martínez-Sanz J., Muriel A., Ron R., Herrera S., Pérez-Molina J.A., Moreno S. Effects of tocilizumab on mortality in hospitalized patients with COVID-19: a multicenter cohort study. Clin Microbiol Infect. 2020 Sep 23 doi: 10.1016/j.cmi.2020.09.021. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biran N., Ip A., Ahn J., Go R.C., Wang S., Mathura S. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;2:e603–e612. doi: 10.1016/S2665-9913(20)30277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sciascia S., Aprà F., Baffa A., Baldovino S., Boaro D., Boero R. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol. 2020;38:529–532. [PubMed] [Google Scholar]