Abstract
The vaccines industry has not changed appreciably in decades regarding technology, and has struggled to remain viable, with large companies withdrawing from production. Meanwhile, there has been no let-up in outbreaks of viral disease, at a time when the biopharmaceuticals industry is discussing downsizing. The distributed manufacturing model aligns well with this, and the advent of synthetic biology promises much in terms of vaccine design. Biofoundries separate design from manufacturing, a hallmark of modern engineering. Once designed in a biofoundry, digital code can be transferred to a small-scale manufacturing facility close to the point of care, rather than physically transferring cold-chain-dependent vaccine. Thus, biofoundries and distributed manufacturing have the potential to open up a new era of biomanufacturing, one based on digital biology and information systems. This seems a better model for tackling future outbreaks and pandemics.
Keywords: vaccines, synthetic biology, distributed manufacturing, biofoundry, point of care
The Vaccine Production Model Needs to Change
The COVID-19 crisis has cast the vaccines industry into scrutiny [1]; an industry that has been silently beleaguered for decades (https://www.who.int/immunization/programmes_systems/procurement/mi4a/platform/module2/2019_Global_Vaccine_Market_Report.pdf?ua=1). A United States National Academy of Sciences study from 2003 [2] concluded that the amount that the nation spent on vaccines ‘appears to be insignificant compared with that spent on other medical and social interventions that may have lesser social benefits.’ Large company numbers that supply vaccines have steadily reduced [3]: some 80% of vaccines arise from five multinationals (https://www.who.int/immunization/programmes_systems/procurement/market/global_supply/en/). At the heart of the problem is a tension between relatively poor financial returns to the vaccine industry and the high costs in production and R&D [4].
Compared to small molecule pharmaceuticals, centralised vaccine production facilities are capital intensive [5]. Fixed costs are high [6], especially for new vaccines. For example, the need for eggs for production is at least 70 years old, is expensive and time-consuming, but difficult to replace [7]. In past high-income countries have paid a higher price until the fixed costs are amortised. Then lower-income countries have adopted vaccines as the price has dropped after paying the fixed costs. However, there is now tremendous pressure for a COVID-19 vaccine to be available for all who need it, effectively everyone.
Distributed Manufacturing, a More-Sustainable Vaccine Model
The long distribution chains of centralised vaccine production are patchy, resulting in incomplete geographical coverage [8] (Box 1 ). Even if there is no overall shortage, there may be where they are needed most. Centralisation of labour and production has been the norm in many industries, but in 2015 the World Economic Forum (WEF, see Glossary) put distributed manufacturing in its top ten emerging technologies for the year (https://www.weforum.org/agenda/2015/03/top-10-emerging-technologies-of-2015-2/). In essence, production is done close to the final customer, and much of the material supply chain is replaced by information [9]. It appears unsuited to high-production-volume industries, such as commodity chemicals and the automotive industry. However, vaccine production, already suboptimal for economies of scale [10], needs a different model, and distributed manufacturing is a good fit.
Box 1. Scaled-Down Manufacture and Supply Chain Close to Points of Care.
Although revenues from vaccine sales are higher in developed countries, greater volume of sales occur in low- and middle-income countries. Nevertheless, vaccine manufacturers in developing countries already supply over half of the vaccines in their immunisation programmes [50,51], so this initiative is far from needing to be started from scratch.
Given the large number of doses that are possible from small volumes of product, final production could happen in a variety of locations if they comply with regulations: adapted university laboratories; laboratories at university teaching hospitals; and small company facilities at science parks and medical campuses. As cold chain operations are especially challenging in remote locations, it may even be possible to manufacture in mobile laboratories (Figure I), extending their capabilities of early warning, reconnaissance, on-site investigation, verification and response that already exist [52]. German company CureVac is developing a mobile production unit, the RNA Printer (https://www.curevac.com/en/technology/production/) that will be deployable to hospitals, potentially even into mobile laboratories. A joint patent application has been filed between CureVac and Tesla, the electric vehicle manufacturer, for this ‘Bioreactor for RNA In Vitro Transcription’ [53].
In one scenario, the vaccine sequence could be sent electronically across a telecommunications network from a biofoundry that has designed the sequence to another biofoundry that is the manufacturing facility. The second biofoundry then produces the liquid vaccine preparation for transportation. Further transportation could then be achieved using a mobile vaccination facility built into a mobile truck unit specially kitted out for this purpose. A recent example of this has been achieved by the Dutch company, Lamboo Mobile Medical, in partnership with Phillips (https://www.mobile-medical.eu/projects/mobile-primary-care-truck-unit-delivered-south-africa). A similar facility could be designed for local vaccine transportation to the point of immunisation. Any attempt to use mobile laboratories or production plants in multiple locations during a pandemic would require manufacturing best practices, like those outlined in good manufacturing practice (GMP), harmonised standard operating procedures and certification [52]. Training of staff in the correct use of a mobile biosafety level 3 (BSL-3) laboratory has in past been sponsored by the WHO (https://www.afro.who.int/news/who-supports-mobile-bsl-3-lab-re-qualification-and-training-experts).
The thermal stability of vaccines for worldwide distribution is a major problem, particularly in developing countries and outlying geographical regions. The ideal situation is for a vaccine to be stable for long periods at ambient temperatures. However, in reality, estimates vary widely for different types of vaccines regarding storage and transport temperatures. A big disadvantage of RNA vaccines is that their stability is lower than traditional vaccines [54]. Thus, their production close to the end user is especially pertinent.
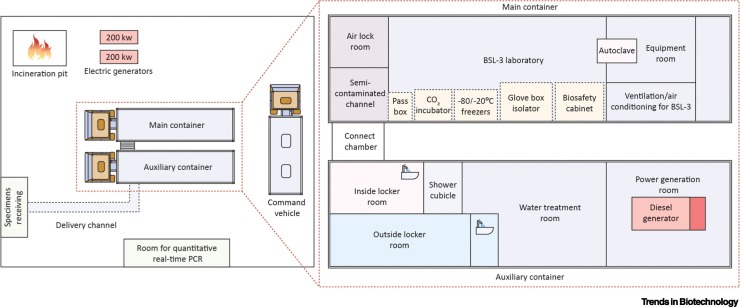
Figure I.
Mobile Laboratory Configuration for the Diagnosis of Ebola Virus Disease.
In 2014, a mobile Biosafety Level-3 (BSL-3) laboratory was sent from China to Sierra Leone during an Ebola virus disease outbreak to rapidly diagnose the disease using quantitative real-time PCR. In a 2-week period, 1635 patients were evaluated and none of the laboratory staff members became infected. Communication, ventilation, and electricity and gas supply formed part of this infrastructure, comprising three vehicles. This infrastructure could include distributed manufacturing functions. Image adapted from [55].
Alt-text: Box 1
Nucleic acid vaccines would be produced in kilogrammes rather than tonnes. An mRNA vaccine may have a low-cost, cell-free, egg-free production process [11] that is highly scalable and is easier to build and run than a facility handling virus particles. The actual production process could be relatively simple, consisting of a small number of standardisable molecular biology steps, purification by HPLC and final drug formulation [12]. Upon delivery to the patient, the process is completed there, with translation of the message to immunogenic protein in ribosomes, which then encounters the host’s immune system. While an oversimplification, compare this with standard vaccine production processes that might involve hundreds of complicated steps.
Economically, locating production close to the end user has the highest potential to capture value, particularly for emergency preparedness and response [13]. The need for a cold chain, prone to logistics malfunctions and temperature excursions (https://pelibiothermal.com/resource/active-versus-passive-shipping-containers-making-optimum-choice) [14], is almost completely removed. Most importantly, a small production facility situated close to an outbreak site can, potentially, fill the gap between routine production and precipitous need at the onset of outbreak creating a stockpile in a short timeframe [15]. The design and manufacture of mRNA vaccines also offers the critical advantage of being a fully synthetic platform process [16] for multiple targets [12]. This will be an important aspect of creating a future industry that is economically viable. It may then be a quicker process to optimise the product for the next virus outbreak.
The Synthetic Biology Approach Lends Itself to a Distributed Vaccine Industry
Vaccine technology is decades old and in need of modernising (https://www.youtube.com/watch?v=LVhnQ1sqTQU). One of the problems is that, while many nonbiological drugs are produced using standardised chemical engineering processes, vaccine production, especially with whole cells, has been less amenable to standardisation and is thus less predictable. To break the economies-of-scale model for vaccine manufacturing, cost savings have to be made through strain engineering and molecular design [17]. This is fertile breeding ground for the emerging synthetic biology industry [18].
Vaccine production would benefit from the systematic workflow approach of synthetic biology [19]. The technology of mRNA is amenable to optimisation through almost limitless combinations of derivatives [12]. Likewise, the biofoundry is an obvious vehicle for generation of these combinations. Systematic workflow increasingly involves the use of biofoundries. Biofoundries are highly automated facilities that comprise the extensive and coordinated use of laboratory robots that are programmed to perform specific tasks according to a workflow [20]. Typically, different platforms within the biofoundries perform different tasks; for example, liquid handling, genetic assembly, characterisation functions. Biofoundries are based on information infrastructures that allow the robots and other equipment within the biofoundries to be programmed to follow detailed, complex workflows [21].
The combination of biodesign tools (BioCAD) and biofoundries is rapidly producing a new type of biology – digital biology – that could revolutionise the production of vaccines and many other areas of biomedicine. The approach chimes with the distributed supply chains and distributed manufacturing that could be transformative in dealing with COVID-19 and future pandemics throughout the world. The potential of the approach was demonstrated by Crone and co-workers [22] who showed that an automated SARS-CoV-2 clinical diagnostics platform designed and developed in a biofoundry can be quickly deployed and scaled.
Software allows the simultaneous investigation of a range of experimental factors or variables to rapidly optimise design of an industrial production. The recently formed Global Biofoundries Alliance [23] has publicly funded biofoundries in North America, Europe, Asia, and Australia, which could be brought to bear for the design of new vaccines, as needed. As pointed out by Crone and co-workers [22], the protocols and workflows they developed can be quickly incorporated and modified as necessary by members of the Global Biofoundry Alliance. Box 2 shows front-running approaches to vaccine production that are highly amenable to the synthetic biology approach. They are all ripe for public–private cooperation.
Box 2. Vaccines Amenable to the Synthetic Biology/Distributed Manufacturing Approach.
RNA vaccines work by introducing a biodesigned mRNA sequence into muscle or other cells. The mRNA is specifically designed to produce the exact antigens required to counteract the target virus. There are a number of advantages of this approach. Production/manufacture can be achieved directly in the laboratory, cell free and egg free. One litre of SARS-CoV-2 vaccine could be enough to produce at least 1 million doses. The big potential advantage of the method is that it is amenable to synthetic-biology-based biodesign, where knowing the genome of the virus allows the direct design of an RNA vaccine. Ulmer and co-workers [56] described a proof of concept for the production of a self-amplifying mRNA influenza vaccine, from gene synthesis to formulation and release, in 13 days, which they anticipated could be reduced to 5 days.
DNA vaccines are also amenable to synthetic biology design but production is more complex than RNA vaccines if there is a dependence on amplification of plasmids in bacterial or cell cultures. Both DNA and RNA platforms use similar established production processes and facilities. An entirely synthetic, in vitro process for mRNA vaccines is inherently simpler and easier to monitor. However, the arrival of enzymatically produced linear DNA provides some key advantages, not least being speed of production free of bacterial sequence [57], and may herald new opportunities for DNA vaccines.
Programmable and modular synthetic gene circuits can be introduced into adenoviral vectors for immunotherapeutic purposes [58]. The COVID-19 vaccine under development at the University of Oxford is an adenovirus vaccine vector (ChAdOx1). The Oxford group had previously developed a successful vaccine for SARS. The strategy was that, as SARS is another form of coronavirus, it should be possible to modify that vaccine for COVID-19. The advantage of the methodology is that conventional production techniques should be readily modified for the production of the new vaccine.
A virus-like particle (VLP) is an engineered viral structure with the immunoprotective traits of a native virus but is noninfectious. When synthetic biology techniques are applied to the rational design of VLPs greater precision and predictability is achievable. It is now possible to screen for pathogen-specific antigens with high immunogenic potential for inclusion in the design [59].
Rapidly multiplying a little-known plant virus (cowpea mosaic virus, CPMV) in plants is a potential solution to vaccine production [60]. CPMV is used to produce a noninfectious viral shell VLP. In the case of influenza, genetic information from the human virus decorates the shell with influenza surface proteins. In relation to COVID-19, the Canadian biopharmaceutical company Medicago, having produced the appropriate VLPs for coronavirus, began testing its plant-based coronavirus vaccine in an early-stage clinical trial in July 2020. The production ‘factory’ is a plant related to the tobacco plant. Using plants as the production factory could prove more rapid and scalable than conventional methods. Medicago recently announced the construction of a facility to grow plants with a capacity for 40–50 million planned doses of flu vaccine per year [61].
Alt-text: Box 2
Improvements in synthetic biology are currently increasing exponentially, and data from the design, build, test, learn (DBTL) cycle are being used to train machine learning algorithms to reduce human interventions [24] – a hallmark of modern engineering.
Downstream of Synthetic Biology
The large number of doses of mRNA vaccines that can be made from small volumes (Box 2) is suited to small, distributed operations. If a bioprocess is still required, then the size and footprint of that process can be decreased through cell culture process intensification, such as fed batch [14] and, in the future, continuous manufacturing [25]. Smaller footprint processes would benefit from the use of single-use disposable culture systems that reduce fixed costs dramatically and can be established more quickly than hard-pipe facilities [26]. In terms of chemical engineering, many of the required tools for bioprocess intensification are already available, including scaled-down equipment for rapid process development and process integration [27]. That said, process standardisation and robustness are absolutely essential to guarantee the quality and consistency of the product. Inherent to standardisation of the products will be the interoperability of the hardware and software in different biofoundries/distributed manufacturing units (Box 3 ).
Box 3. Robustness, Standardisation and Quality.
It is essential that the product is standardised, no matter where it is produced. Robustness contributes to ensuring the safety, efficacy, and quality of a product [62,63]. Regulatory authorities review manufacturing process robustness and its continuous verification. Robustness is influenced by variations in environment, equipment, reagents, and operator skills. All of these influences need to be standardised and measured to show compliance with the manufacturing limits documented in the regulatory dossier. Automation will make processes at each manufacturing site more comparable. However, safe-by-design synthetic biology [64] holds the promise of incorporating robustness into the factors for the automated DBTL engineering cycle. Safe-by-design synthetic biology is consistent with the Quality-by-Design initiative outlined by the US Food and Drug Administration (US FDA) [65]. This is a systematic process control approach with predefined objectives to ensure safety, efficacy, and quality are built into the final drug product.
Inherent to standardisation of the products will be interoperability of the hardware and software in different biofoundries. Currently this is done on a rather ad hoc basis. However, a similar situation existed 25 years ago when hospital equipment like scanners used different data formats. This was overcome by the development of data standards. In the field of synthetic biology there are now two standards for interoperability, which are in operation in different biofoundries, but are also going through formal review. The standards are called SBOL (https://sbolstandard.org/) and DICOM-SB [66].
Alt-text: Box 3
Then, potentially the longest step in the process is regulatory compliance to ensure product safety (Box 4 ).
Box 4. Responsive Regulation.
Various countries have drug manufacturing regulatory systems with procedures to expedite the availability of a drug, particularly if there is a serious condition with an unmet need [67,68]. For example, the US Food and Drug Administration (US FDA) can expedite a drug using fast track, breakthrough therapy, accelerated approval and priority review procedures (https://www.fda.gov/patients/learn-about-drug-and-device-approvals/fast-track-breakthrough-therapy-accelerated-approval-priority-review). Even so, an optimum amount of data showing that a drug will be safe and effective needs to be collected: expediting a drug still takes time. The synthetic biology approach can save time in the development of a vaccine. In times of emergency, the regulatory regime should enable a streamlined process to save more time whilst still delivering a safe product. Thus, National Regulatory Authorities have to remain flexible and follow the evolving science to develop regulatory requirements during public health emergencies [69]. The nucleic acid and subunit vaccines are already considered inherently safer than those depending on attenuated virus particles (https://www.niaid.nih.gov/research/vaccine-types).
The distributed approach described here will need greater regulatory harmonisation between countries [70,71]. The International Council on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is the current resource for global harmonisation activities, quality, safety and efficacy guidelines and standards. Safe-by-design synthetic biology, like quality-by-design, should be placed up-front in design and build, rather than after manufacture.
Alt-text: Box 4
Sustainability
Final manufacturing in scaled-down production plants could occur in a large number of countries, giving global coverage to bring manufacturing closer to the point of care. The United Nations Sustainable Development Goals (SDGs) make explicit reference to the need for affordable vaccines (SDG target 3.8) (https://unstats.un.org/sdgs/metadata/). In terms of environmental footprint, there is evidence that the pharmaceutical industry is significantly more emissions-intensive than the automotive industry [28]. The biofoundries, where design and initial vaccine selection and build (prototype) is performed, can be anywhere, and certainly separated from manufacturing. In this case it is not vaccine that is transported, but information (Figure 1 , Key Figure). Replacing material transfer by information transfer saves money and emissions, lowers risk due to cold chain failures and speeds the innovation process (https://www.economist.com/science-and-technology/2017/04/01/managing-supplies-of-vaccines-is-a-huge-problem).
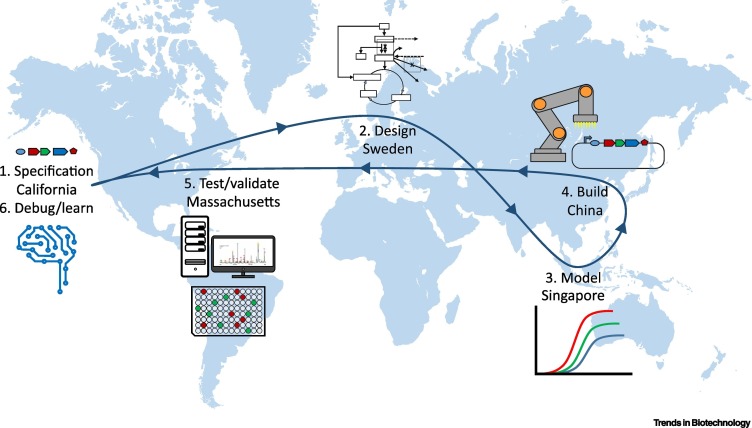
Figure 1.
Key Figure. The Design–Build–Test-Learn Cycle (DBTL) in Synthetic Biology on a Global Scale Interconnected by the Transfer of Information.
The diagram is hypothetical, based on current areas of expertise in different countries.
Vigilance
In the 20 years of this century, there have been several major outbreaks of viral diseases in humans, and we have been unprepared for all of them. Funding became available to combat each outbreak but as the immediate danger receded, so did the financial interest [29]. Speed is of the essence when an outbreak occurs. Predictive tools such as computational epidemiology (https://cordis.europa.eu/article/id/415792-using-prediction-models-to-manage-the-coronavirus-outbreak) to provide accurate predictions of the spread of future epidemics need to be developed further. Real-time tracking of the evolution and spread of a virus in an open access platform aids public health decision making [30].
Challenges
Here, we address some of the readily identifiable barriers to the described approach and what can be done about them.
Economic Viability: How Do Such Small, Decentralised Facilities Survive during Periods of Low Demand for Vaccines?
In the next 10 to 20 years, it is estimated that biomolecules and biosystems innovation could directly impact the healthcare market to the tune of $500 billion to $1.2 trillion [31]. Roughly 40% of products in clinical development globally are biopharmaceuticals [32]. Of these, a growing number of nucleic acid therapies is gradually entering the market, and estimates predict strong compound annual growth rate (CAGR) in the next few years. Nucleic acid therapies have a promising future in various healthcare areas, including antitoxins, infectious diseases, rare diseases, and oncology [33]. In particular, mRNA therapeutics encoding known cancer-specific shared antigens hold the promise of personalised cancer treatments [34]. This does not include the recently announced BioNtech [35] and Moderna COVID-19 vaccines. Tew [36] reviewed applications of synthetic biology in healthcare recently.
The 3Rs is a global regulatory initiative to replace, reduce, and refine animal testing without compromising drug safety and effectiveness [37]. As molecular biology understanding develops it increasingly contributes to the 3Rs and knowledge about in vivo toxicology pathways. The in vitro toxicology testing market is expected to reach $14.9 billion by 2025 at a CAGR of 10.3% [38]. Biofoundries could help develop in vitro and in silico services to support toxicology testing before a drug molecule is tested in humans.
Cyberbiosecurity
As digital biology develops, there are various places in a bioproduction process susceptible to cyberattack, succinctly summarised by Peccoud and co-workers [39], and representing a new threat landscape [40]. Companies are elevating cybersecurity to a strategic imperative [41]. Blockchain technology is the obvious solution. It can record transactions between two parties with secure traceability and provides enhanced security of data in clinical trials. Public policy efforts to enhance cybersecurity could be recognised through, for example, voluntary standards, regulations and information-sharing frameworks [42]. In its most serious form, when state sponsorship is suspected, there may be a case for a ‘voluntary, non-binding norm for responsible state behaviour in the use of information and communications technology’ [43].
Talent and Education
The convergence of artificial intelligence, machine learning, and robotics means that there is a growing need for a new kind of graduate. Biologists are required with greater knowledge of computer science and IT systems as the biofoundries take over the laborious liquid handling functions. The need for a specially trained workforce for the synthetic biology sector is considered by many in industry to be a key pinch point for the industrial development of the area. The industrial view, regarding personnel, is that for every engineer, mathematician, and computer scientist there are at least 30 PhDs in biology and biochemistry available. What is crucial for the development of the synthetic biology industry is the training of many more engineers, mathematicians and computer scientists with an understanding of biology.
It is entirely feasible that apprenticeships and day-release education will play a prominent part in developing this workforce. Higher education is rising to the challenges with a range of solutions from technician training, undergraduate degrees, Masters and interdisciplinary PhD programmes, massive open online courses (MOOCs) and business management courses [44,45].
Concluding Remarks
In the midst of and in the aftermath of COVID-19, we call for a radical departure from the conventional approaches to outbreak prediction, monitoring, protection and cure (see Outstanding Questions). It will require much international public and private funding (https://www.gatesnotes.com/Health/What-you-need-to-know-about-the-COVID-19-vaccine). Radical departures may appear expensive and complex to deliver, more so if vaccine production is to be global and democratised, preventing vaccine nationalism [46,47], putting a common strategy ahead of national self-interest [48]. However, with the huge human toll of COVID-19, the World Economic Forum predicting $1 trillion of damage to the global economy in 2020 alone (https://www.weforum.org/agenda/2020/03/coronavirus-covid-19-cost-economy-2020-un-trade-economics-pandemic/), and the United Nations predicting that 130 million more people will enter extreme poverty [49], it will now not seem expensive at all.
Outstanding Questions.
What are the technical barriers to bringing mRNA vaccines to the market? Some known barriers include understanding the fundamentals of the molecular mode of action, sequence optimisation, improving mRNA stability, and optimisation of the carrier system.
How feasible is the production of vaccines in very small production plants? How robust is this process? Batches from different locations absolutely must behave in the same way. There are essential roles for regulatory bodies to ensure the safety of the process. If a product is not affected by economies of scale, small-scale production plants are more likely to work economically. As long as the process is cell free and egg free, the barriers to small-scale production are much lower.
What are the key attributes of a biofoundry for this model? The small number of public and private biofoundries currently in existence are demonstrating success in products for different sectors. The ability to synthesise many candidates, and the rapid iteration of the DBTL cycle with limited human intervention speeds innovation. Biofoundries can communicate rapidly via digital information in very different locations. Using several biofoundries can be used for quality control and to debug processes.
Can this model be truly economically viable? There is a need to integrate various capabilities to maximise the benefits. Pre-emptive surveillance with computational, real-time tracking of the evolution and spread of a virus, rapid sequence acquisition, integrated with the described model is key. The economic analysis should incorporate the cost of not acting: the human and economic consequences of COVID-19 are highlighting this very point. Product pipelines will be necessary when vaccine needs are lower.
Alt-text: Outstanding Questions
Acknowledgments
Acknowledgements
The work for this manuscript was partly funded by SynbiCITE grants from the Engineering and Physical Science Research Council (EPSRC), UK, grant numbers EP/M006700/1 and EP/S001859/2 IKC-Phase 2.
Disclaimer Statement
The views expressed are those of the authors and not necessarily those of the OECD or of the governments of its member countries. The views expressed are those of the authors and not necessarily those of International Pharmaceutical Quality (IPQ).
Glossary
- BioCAD
CAD stands for computer-aided design, a well-established process that uses software and hardware for the design and redesign of all manner of goods and materials. BioCAD, thus pertains to computer-aided designs in biology, particularly molecular biology.
- Biosafety level 3 (BSL-3)
biosafety level refers to a set of biocontainment procedures and precautions to isolate hazardous biological agents in a laboratory facility. There are four levels, 1 being the lowest hazard, and 4 the highest, for work with agents that can cause severe to fatal disease in humans by airborne transmission for which there are no available vaccines or treatments. BSL-3 refers to appropriate containment for work involving microbes that can cause serious and potentially lethal disease. Thus the series 1–4 involves increasing levels of containment to minimise risk to staff.
- Blockchain
this can be viewed as a series of checks in a transaction, all linked by cryptography. The data in each block is protected from modification as each change in a block requires changes in all subsequent blocks. In practical terms a block chain forms a verifiable and permanent record of a transaction that is highly tamper-proof.
- Compound annual growth rate (CAGR)
a number that describes the rate at which an investment would have grown if it had grown at the same rate every year and the profits were reinvested at the end of each year. It is a common way to calculate returns for anything that can rise or fall in value over time. Expressed as a percentage, a high CAGR indicates a high growth rate.
- Design–build–test–learn (DBTL) cycle
the DBTL cycle of engineering or synthetic biology. It is intended that iterations of the cycle optimise and control a given genetic construct in a host, or chassis strain that is to be used in a bioprocess to make a product.
- Good manufacturing practice (GMP)
standard guidelines that outline practices required to conform to the agency recommendations that control the authorisation and licensing of the manufacture of pharmaceutical products.
- International Council on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH)
an international nonprofit organisation set up to bring together the regulatory authorities and pharmaceutical industry to discuss scientific and technical aspects of drug registration, with a view to harmonisation of guidelines.
- Massive open online course (MOOC)
an online course aimed at unlimited participation and open access via the web. As well as filmed traditional lectures, tutorials, etc., a MOOC can contain other more interactive elements, such as quizzes and social media discussions.
- National Regulatory Authorities (NRAs)
in public health terms, these are national regulatory agencies responsible for ensuring that pharmaceuticals and biologicals like vaccines are evaluated properly and meet international standards of quality and safety.
- Quality by design (QbD)
in the pharmaceutical industry, QbD provides guidance to facilitate design of products and processes that maximises a product’s quality, safety and efficacy while also enabling manufacturing.
- Robustness
ability of an engineered pharmaceutical manufacturing process to maintain established quality and performance limits in variable environments.
- Sustainable Development Goals (SDGs) of the UN
there are 17 of these that are meant to be interlinked, and when enacted would result in a more sustainable future for humans and the planet.
- United States Food and Drug Administration (US FDA)
federal agency of the USA that is responsible for protecting and promoting public health.
- World Economic Forum (WEF)
an international nongovernmental organisation based in Switzerland that addresses major global problems by bringing together business leaders, political leaders, scientists, celebrities, journalists, and other societal leaders.
References
- 1.Mullard A. COVID-19 vaccine development pipeline gears up. Lancet. 2020;395:1751–1752. doi: 10.1016/S0140-6736(20)31252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Academy of Sciences . National Academies Press; 2003. Financing Vaccines in the 21st Century: Assuring Access and Availability. [PubMed] [Google Scholar]
- 3.Offit P.A. Why are pharmaceutical companies gradually abandoning vaccines? Health Aff. 2005;24:622–630. doi: 10.1377/hlthaff.24.3.622. [DOI] [PubMed] [Google Scholar]
- 4.Plotkin S., et al. The complexity and cost of vaccine manufacturing – an overview. Vaccine. 2017;35:4064–4071. doi: 10.1016/j.vaccine.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen A., Schwalbe N. Apples and oranges? Can second generation vaccines become as low cost as generic medicines? Vaccine. 2019;37:2910–2914. doi: 10.1016/j.vaccine.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 6.Danzon P.M., Pereira N.S. NBER Work. Pap. Ser. No. 17205. 2011. Vaccine supply: effects of regulation and competition. [Google Scholar]
- 7.Trombetta C.M., et al. Challenges in the development of egg-independent vaccines for influenza. Expert Rev. Vaccines. 2019;18:737–750. doi: 10.1080/14760584.2019.1639503. [DOI] [PubMed] [Google Scholar]
- 8.Utazi C.E., et al. Mapping vaccination coverage to explore the effects of delivery mechanisms and inform vaccination strategies. Nat. Commun. 2019;10:1633. doi: 10.1038/s41467-019-09611-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srai J.S., et al. Distributed manufacturing: scope, challenges and opportunities. Int. J. Prod. 2016;54:6917–6935. [Google Scholar]
- 10.Garcia-del-Barrio P. External economies of scale, government purchasing commitment and welfare improvements in the vaccines industry. Eur. J. Gov. Econ. 2012;1:163–179. [Google Scholar]
- 11.Maruggi G., et al. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol. Ther. 2019;27:757–772. doi: 10.1016/j.ymthe.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson N.A.C., et al. The promise of mRNA vaccines: a biotech and industrial perspective. npj Vaccines. 2020;5:11. doi: 10.1038/s41541-020-0159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison R.P., et al. Decentralised manufacturing of cell and gene therapy products: Learning from other healthcare sectors. Biotechnol. Adv. 2018;36:345–357. doi: 10.1016/j.biotechadv.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Xu J., et al. Development of an intensified fed-batch production platform with doubled titers using N-1 perfusion seed for cell culture manufacturing. Bioresour. Bioprocess. 2020;7:17. [Google Scholar]
- 15.Zhang C., et al. Advances in mRNA vaccines for infectious diseases. Front. Immunol. 2019;10:594. doi: 10.3389/fimmu.2019.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rauch S., et al. New vaccine technologies to combat outbreak situations. Front. Immunol. 2018;9:1963. doi: 10.3389/fimmu.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Academies of Sciences, Engineering, and Medicine . National Academies Press; 2019. Continuous Manufacturing for the Modernization of Pharmaceutical Production: Proceedings of a Workshop. [PubMed] [Google Scholar]
- 18.Pedrolli D.B., et al. Engineering microbial living therapeutics: the synthetic biology toolbox. Trends Biotechnol. 2019;37:100–115. doi: 10.1016/j.tibtech.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 19.National Academies of Sciences, Engineering, and Medicine . National Academies Press; 2016. Deriving Drug Discovery Value from Large-Scale Genetic Bioresources: Proceedings of a Workshop. [PubMed] [Google Scholar]
- 20.Kitney R., et al. Enabling the advanced bioeconomy with engineering biology. Trends Biotechnol. 2019;37:917–920. doi: 10.1016/j.tibtech.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Chao R., et al. Engineering biological systems using automated biofoundries. Metab. Eng. 2017;42:98–108. doi: 10.1016/j.ymben.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crone M.A., et al. A role for biofoundries in rapid development and validation of automated SARS-CoV-2 clinical diagnostics. Nat. Commun. 2020;11:4464. doi: 10.1038/s41467-020-18130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hillson N., et al. Building a global alliance of biofoundries. Nat. Commun. 2019;10:2040. doi: 10.1038/s41467-019-10079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Opgenorth P., et al. Lessons from two design−build−test−learn cycles of dodecanol production in Escherichia coli aided by machine learning. ACS Synth. Biol. 2019;8:1337–1351. doi: 10.1021/acssynbio.9b00020. [DOI] [PubMed] [Google Scholar]
- 25.Jungbauer A., Hammerschmidt N. In: Continuous Manufacturing of Pharmaceuticals. Kleinebudde P., et al., editors. Wiley Online Library; 2017. Integrated continuous manufacturing of biopharmaceuticals. [Google Scholar]
- 26.Black S. The Science Advisory Board; 2020. Need for Efficiency, COVID-19 Vaccine Spurs Biomanufacturing in 2020. [Google Scholar]
- 27.Strube J., et al. Process intensification in biologics manufacturing. Chem. Eng. Process. Process Intensif. 2018;133:278–293. [Google Scholar]
- 28.Belkhir L., Elmeligi A. Carbon footprint of the global pharmaceutical industry and relative impact of its major players. J. Clean. Prod. 2019;214:185–194. [Google Scholar]
- 29.Maslow J.M. The cost and challenge of vaccine development for emerging and emergent infectious diseases. Lancet Glob. Health. 2018;6 doi: 10.1016/S2214-109X(18)30418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hadfield J., et al. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics. 2018;34:4121–4123. doi: 10.1093/bioinformatics/bty407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKinsey Global Institute . 2020. The Bio Revolution: Innovations Transforming Economies, Societies, and Our Lives. [Google Scholar]
- 32.Walsh G. Biopharmaceutical benchmarks 2018. Nat. Biotechnol. 2018;36:1136–1145. doi: 10.1038/nbt.4305. [DOI] [PubMed] [Google Scholar]
- 33.Schlake, T. et al. (2019). mRNA: A novel avenue to antibody therapy? Mol. Ther. 27, 773–784 [DOI] [PMC free article] [PubMed]
- 34.Sahin U., et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 35.Cohen J. Effective vaccine offers shot of hope for pandemic. Pioneering RNA candidate seems to work well, although its makers revealed scant data. Science. 2020;370:748–749. doi: 10.1126/science.370.6518.748. [DOI] [PubMed] [Google Scholar]
- 36.Tew D. Synthetic biology and healthcare. Emerg. Top. Life Sci. 2019;3:659–667. doi: 10.1042/ETLS20190086. [DOI] [PubMed] [Google Scholar]
- 37.Medina L.V., et al. Special Issue: global 3Rs efforts – making progress and gaining momentum. J. Am. Assoc. Lab. Anim. Sci. 2015;54:115–118. [PMC free article] [PubMed] [Google Scholar]
- 38.Research and Markets . 2020. In Vitro Toxicity Testing Market by Product, Toxicity Endpoints, Industry - COVID-19 Impact - Global Forecast to 2025. Report ID: ID: 5142690. [Google Scholar]
- 39.Peccoud J., et al. Cyberbiosecurity: From naive trust to risk awareness. Trends Biotechnol. 2018;36:4–7. doi: 10.1016/j.tibtech.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 40.Richardson L.C., et al. Cyberbiosecurity: A call for cooperation in a new threat landscape. Front. Biotechnol. Bioeng. 2019;7:99. doi: 10.3389/fbioe.2019.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.KPMG . KPMG; 2018. Digitalization in Life Sciences. No. 135128-G. [Google Scholar]
- 42.Organisation for Economic Co-operation and Development . The Digitalisation of Science, Technology and Innovation. OECD Publishing; 2020. Digitalisation in the bioeconomy: convergence for the bio-based industries. [Google Scholar]
- 43.United Nations Office for Disarmament Affairs . United Nations; 2017. Voluntary, Non-binding Norms for Responsible State Behaviour in the Use of Information and Communications Technology: a Commentary. [Google Scholar]
- 44.Delebecque C.J., Philp J. Education and training for industrial biotechnology and engineering biology. Eng. Biol. 2018;3:6–11. [Google Scholar]
- 45.Hallinan J., et al. Future-proofing synthetic biology: educating the next generation. Eng. Biol. 2019;3:25–31. [Google Scholar]
- 46.Kupferschmidt K. ‘Vaccine nationalism’ threatens global plan to distribute COVID-19 shots fairly. Science. 2020 doi: 10.1126/science.abe0601. Published online July 28, 2020. [DOI] [Google Scholar]
- 47.Weintraub R., et al. The danger of vaccine nationalism. Harvard Bus. Rev. 2020 May 22, 2020. [Google Scholar]
- 48.National Academies of Sciences, Engineering, and Medicine . National Academies Press; 2020. Discussion Draft of the Preliminary Framework for Equitable Allocation of COVID-19 Vaccine. [PubMed] [Google Scholar]
- 49.United Nations Conference on Trade and Development . United Nations Publications; 2020. Impact of the COVID-19 Pandemic on Trade and Development. Transitioning to a New Normal. UNCTAD/OSG/2020/1. [Google Scholar]
- 50.Mao H.H., Chao S. Advances in vaccines. Adv. Biochem. Eng. Biotechnol. 2020;171:155–188. doi: 10.1007/10_2019_107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Munira S.L., et al. A cost analysis of producing vaccines in developing countries. Vaccine. 2019;37:1245–1251. doi: 10.1016/j.vaccine.2018.11.050. [DOI] [PubMed] [Google Scholar]
- 52.Parsons A., et al. Examining the utility and readiness of mobile and field transportable laboratories for biodefence and global health security-related purposes. Global Secur.: Health, Sci. Policy. 2018;3:1–13. [Google Scholar]
- 53.Yazdan Paneh B., et al. 2020. WO2020002598 - Bioreactor for RNA in vitro transcription. International Application No. PCT/EP2019/067323. [Google Scholar]
- 54.Wang J., et al. The COVID-19 vaccine race: challenges and opportunities in vaccine formulation. AAPS PharmSciTech. 2020;21:225. doi: 10.1208/s12249-020-01744-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y., et al. Rapid deployment of a mobile biosafety level-3 laboratory in Sierra Leone during the 2014 Ebola virus epidemic. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ulmer J.B., et al. Vaccines ‘on demand’: science fiction or a future reality. Expert Opin. Drug Discov. 2015;10:101–106. doi: 10.1517/17460441.2015.996128. [DOI] [PubMed] [Google Scholar]
- 57.Karda R., et al. Production of lentiviral vectors using novel, enzymatically produced, linear DNA. Gene Ther. 2019;26:86–92. doi: 10.1038/s41434-018-0056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang H., et al. Oncolytic adenovirus programmed by synthetic gene circuit for cancer immunotherapy. Nat. Commun. 2019;10:4801. doi: 10.1038/s41467-019-12794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Charlton Hume H.K., et al. Synthetic biology for bioengineering virus-like particle vaccines. Biotechnol. Bioeng. 2019;116:919–935. doi: 10.1002/bit.26890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cañizares M.C., et al. Development of cowpea mosaic virus-based vectors for the production of vaccines in plants. Expert Rev. Vaccines. 2005;4:687–697. doi: 10.1586/14760584.4.5.687. [DOI] [PubMed] [Google Scholar]
- 61.Diego-Martin B., et al. Pilot production of SARS-CoV-2 related proteins in plants: a proof of concept for rapid repurposing of indoors farms into biomanufacturing facilities. bioRxiv. 2020 doi: 10.1101/2020.10.13.331306. Published online October 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Auclair J.R. Regulatory convergence for biologics through capacity building and training. Trends Biotechnol. 2019;37:5–9. doi: 10.1016/j.tibtech.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 63.Carvalho M., et al. Hurdles in gene therapy regulatory approval: a retrospective analysis of European Marketing Authorization Applications. Drug Discov.Today. 2019;24:823–828. doi: 10.1016/j.drudis.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 64.Robaey Z., et al. The Food Warden: an exploration of issues in distributing responsibilities for safe-by-design synthetic biology applications. Sci. Eng. Ethics. 2018;24:1673–1696. doi: 10.1007/s11948-017-9969-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rathore A.S. Roadmap for implementation of quality by design (QbD) for biotechnology products. Trends Biotechnol. 2009;27:546–553. doi: 10.1016/j.tibtech.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 66.Sainz de Murieta I., et al. Toward the first data acquisition standard in synthetic biology. ACS Synth. Biol. 2016;5:817–826. doi: 10.1021/acssynbio.5b00222. [DOI] [PubMed] [Google Scholar]
- 67.Henao-Restrepo A.M., et al. On a path to accelerate access to Ebola vaccines: The WHO’s research and development efforts during the 2014-2016 Ebola epidemic in West Africa. Curr. Opin. Virol. 2016;17:138–144. doi: 10.1016/j.coviro.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kieny M.P. Lessons learned from Ebola Vaccine R&D during a public health emergency. Hum. Vaccin. Immunother. 2018;14:2114–2115. doi: 10.1080/21645515.2018.1442161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gruber M.F., Marshall V.B. Plotkin’s Vaccines. 7th ed. Elsevier Health; 2018. Regulation and testing of vaccines; pp. 1547–1565. [Google Scholar]
- 70.Elmgren L., et al. 2013. A global regulatory science agenda for vaccines. Vaccine 31S (2013) B163– B175. [DOI] [PubMed] [Google Scholar]
- 71.Ndomondo-Sigonda M. A new approach to an old problem: overview of the East African Community’s Medicines Regulatory Harmonization initiative. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003099. [DOI] [PMC free article] [PubMed] [Google Scholar]