Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is now a pandemic with the United States now carrying the highest number of cases and fatalities. Although vaccines and antiviral agents are the main focus of therapy, here we present a plausible hypothesis to leverage our understanding of neuroimmunomodulation to intervene in the pathophysiology of the disease to prevent death.
Introduction
Although the SARS-CoV-2 pandemic has resulted in an overall low mortality rate with most patients developing only mild symptoms to those that are asymptomatic, it has become clear that those patients harboring co-morbidities such as obesity, diabetes hypertension and cardiovascular diseases are the most vulnerable [1]. Each of these co-morbidities are emerging as complex disease processes involving interaction between the gut microbiome, the nervous system, the endocrine system and immune cell [2], [3]. Indeed, these co-morbid conditions reflect chronic sympathetic hyperactive stress situation [4], [5] caused by dietary modification [6], dysbiosis [7], aging [8] and chronic psychological stress [9]. Among these vulnerable population SARS-CoV-2 infection affect all major organs leading to severe hypoxemia in compliant lung with loss of lung perfusion [10], myocardial injury [11] acute cerebrovascular accident including hemorrhagic stroke [12], acute kidney injury [13] suggestive of significant sympathetic activation.
During stressful situation, norepinephrine released from neuroendocrine system have dual effect on function of the immune cells depending upon the exposure time. While prolong stress is generally harmful, short term stress can be protective as it prepares organism to deal with challenges [14]. In short term stress exposure for less than 30 min it mobilizes NK cells whereas prolong stress exposure as observed in chronic sympathetic hyperactive stress situation, it decreases number of lymphocytes, inhibits T cell proliferation[15]. Experimental animal model of stressors has demonstrated that a variety of stressors increased the susceptibility of animals to coronavirus, coxsackievirus B3, encephalomyocarditis virus, Herpes Simplex virus, poliomyelitis virus, influenza virus. Stressed animals are found to have decreased production of interferon gamma and neutralizing antibody, decrease activity of cytotoxic T lymphocytes, NK cell activity [16]. Similarly in human, psychological stress has been associated with an increased risk of acute corona virus type 229E infection in dose dependent manner [17].
The SARS-CoV-2 virus causes widespread immune dysregulation [1]. Disease severity with outcomes from the SARS-CoV-2 virus are related to the characteristics of the host immune response. CD8 + T lymphocytes play important role on virus clearance. Functional exhaustion of cytotoxic lymphocytes correlates with disease progression and significant decrease in CD8 + T cell counts as the diseases progress [18]. Just like in the past influenza virus pandemic, SARS-CoV-2 infection induces an excessive immune reaction in the host leading to hyper inflammatory syndrome characterized by fulminant and fatal cytokine storm with multi-organ failure. This fatal cytokine storm involves a considerable release of proinflammatory cytokines including IL-6 and TNF-α [1], [19].
Respiratory viral infection is a stress that activates the sympathetic nervous system resulting in release of norepinephrine [20]. Norepinephrine exerts pro-inflammatory action via augmenting the production of macrophage derived TNFα through α2 adrenoceptors [21]. Norepinephrine modulates the level of T lymphocyte activity through the beta2-adrenergic receptor (β2AR). CD8+ T lymphocytes express more β2 AR than naïve cells and upon treatment with norepinephrine expresses more pro inflammatory cytokines related genes and less growth-related genes. Similarly, CD8+ T lymphocytes harvested from human plasma obtained from patients in a stressed state produce an excessive level of pro inflammatory cytokines upon stimulation [22]. This could be due to more engagement of β2AR receptor in these cells compared to naïve T cells [23] from unstressed patients.
Normally, the sympathetic nervous system is activated locally via β2AR within tissues in order to confine inflammation and protect the host from the detrimental effects of widespread pro inflammatory cytokines [24]. This “localization effect” is disrupted when there has been chronic sympathetic hyperactive stress situation such as obesity, diabetes and hypertension [25]. Chronic exposure to norepinephrine during these stressed states can inhibit T cell proliferation [26] and can enhance the production of inflammatory chemokine and cytokines [22] by phenotypically changing immune cells via up-regulation of β2AR expression. Hence, chronic sympathetic hyperactive situation makes people more vulnerable to erratic stress such as viral infection. In such situation, attenuating the effects of sympathetic nervous system by β2AR antagonists or α2-AR agonists are plausible immunomodulatory options available to prevent over activation of immune system and its harmful effect [27].
Such attenuation of the sympathetic response to mitigate hyper inflammation to viral infection has been demonstrated in animal studies. A study done in animals infected with influenza A virus demonstrated detrimental role of sympathetic activation [28]. Blockage of sympathetic neurotransmission via either with a beta-blocker or an alpha agonist induces an antiviral T cell response, suppresses lung edema, and improves survival following lethal influenza A viral infection [29], [30] in mice [29], [30]. Similarly, interruption of sympathetic outflow to lung by stellate ganglion blockade prevent development of ARDS in the animal model [31]. Likewise, susceptibility to cytomegalovirus infection is increased in mice with over activation of the sympathetic nervous system. Stimulation of β2AR can down-regulate innate immunity allowing the host to become more susceptible to viral infection whereas knocking out β2AR decreases sympathetic stimulation of immune cells allowing the host to develop resistance to viral infection perhaps owing to an increase IFN-Y production [32].
There is strong correlation between diet, sympathetic immune system [33] and gut microbiome. [34]. The observation that patients with western diet associated co-morbidities such as obesity and diabetes harbor a disrupted microbiome is in stark contrast to 10,000 years ago when hunter-gatherers survived on a diet high in protein and very low in carbohydrates. Introduction of starch and sugar into the 21st century diet, now termed a “Western diet” may impart its detrimental effect on human health via creation of chronic sympathetic hyperactivity state leading to obesity, diabetes and hypertension [6]. Thus, the most vulnerable patients to a poor outcome from the SARS-CoV-2 virus infection may relate, in part, to chronic consumption of a high fat, high sugar and low fiber based “Western diet.”
The hypothesis
We hypothesize, people with chronic sympathetic hyperactive comorbid conditions, people on western diet and on stress, when contract SARS-CoV-2 virus can potentially drive the infection towards a fatal outcome. Hence, in such condition attenuation of sympathetic activity with currently available drugs such as of a2 agonist and β blocker may be a plausible mechanism to override the T cell exhaustion, cytokine spillage and impaired clearance of virus. Given that critically ill patients infected with SARS-CoV-2 are dying of ARDS, hypoxia and hyper inflammation with significant myocardial injury and stroke here we propose that use of FDA approved antihypertensive drugs such as Clonidine and Propranolol should be considered in symptomatic patients with SARS-CoV-2 infection to prevent progression of disease and mortality.
Evaluation of the hypothesis
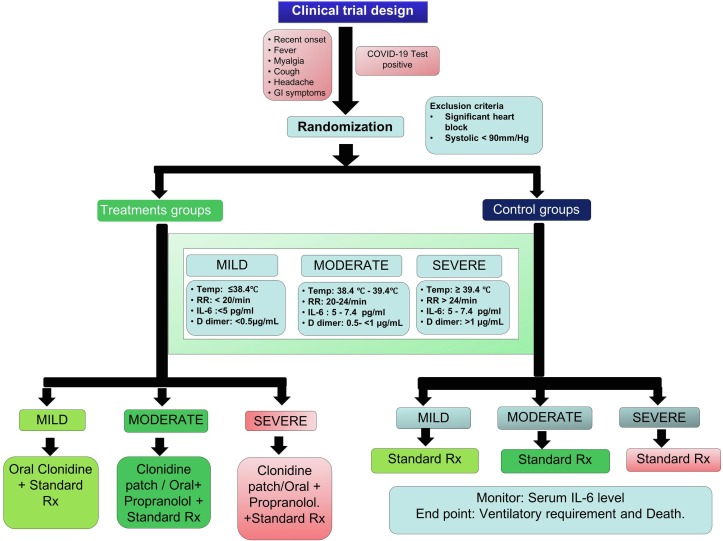
We propose prospective randomized clinical trials using either one or both drugs in combination with standard treatment to all symptomatic SARS-CoV-2 positive patients (Fig. 1 ). All SAR-CoV-2 positive patients above age 13 years presented with symptoms of fever, myalgia, cough, headache, GI symptoms, should be categorized to mild, moderate and severe grade based upon cut off values for body temperature, respiratory rate, and blood workup including IL-6, D dimer and serum ferritin measurement. Patients allergic to drugs, with significant heart block and in shock with systolic blood pressure less than 90 mmHg should be excluded from the study. Besides standard treatment patient with mild grade infection should be treated with Clonidine patch or oral tablets whereas patient with moderate to severe grade infection should be treated with combination of both drugs (Clonidine and Propranolol) with strict monitoring of blood pressure so that systolic blood pressure remains > 90 mm Hg. Plasma IL-6, D-dimer and serum ferritin should be monitored serially. End point of this proposed trial should be ventilatory requirement and death.
Fig. 1.
Schematic diagram of purpose Clinical trial.
Discussion
SARS-CoV-2 infection is rapidly evolving. Although vaccine and antiviral therapy are mainstay of treatment, development of such therapeutic modalities takes time. Here we propose FDA approved drugs Clonidine and Propranolol which has proven efficacy in management of critically ill trauma patients with multiple comorbid conditions admitted in ICU [35]. The case fatality rate for SAR-Cov-2 infection is significantly higher in older population with comorbid condition. The immune status among these older population with comorbid condition is already impaired [36] and potency of vaccine among such population is still uncertain. Recent data from U.S Influenza Vaccine Effectiveness Network demonstrate overall effectiveness of seasonal influenza vaccine for preventing medically attended, laboratory-confirmed influenza virus infection is 45% and its varies by season. Similarly, vaccination doesn’t confer 100% protection against Influenza virus infection [37]. Hence, there is a possibility that SAR-CoV-2 may remain as another flu like syndromic virus with substantial threat to the community.
Serum IL-6 level correlates positively with poor outcome in SAR-CoV-2 infected patients [38]. Besides immune cells, sympathetic neurons produce IL-6 and may response in an autocrine/paracrine manner and is one of the key mediators for neuro-immune interactions [39]. Hence, we postulate, IL-6 level will be the best marker for monitoring and checking level of attenuation of sympathetic activity with Propranolol and Clonidine. This approach could be a measure to prevent worsening of immune response allowing immune cells to ultimately clear the virus on its own in SARS-CoV-2 infected patient.
Majority of the symptomatic patients with SAR-CoV-2 positive are already on antihypertensive medications. So one of the advantages of this trial is that we just need to change the antihypertensive medication that the patient is receiving, whereas there is possibility of hypotension among the patients who are not previously hypertensive and should be started on lower dose of medication and monitored closely for the hypotension. Although, major side effects have not been mentioned in previous study in critically ill patients [35], we recommend to remain highly vigilant in monitoring blood pressure with adequate fluid management. Recently, published scientific brief by WHO mentioned that most people recovered from infection, develop antibodies to the virus [40]. However, some of these people have very low levels of neutralizing antibodies suggesting that cellular immunity may also be critical for recovery [41]. Given the fact that this virus is highly contagious and difficult to be contained [42] and development of antiviral and effective vaccine will take time, these FDA approved commonly available drugs should be considered to mitigate the morbidity and mortality amidst the pandemic.
Declaration interests
All authors declare no competing interest.
Acknowledgements
We thank Dr. John Alverdy (University of Chicago) for his expert opinion and kind support for this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.110039.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Aidy S., Dinan T.G., Cryan J.F. Immune modulation of the brain-gut-microbe axis. Front Microbiol. 2014;5:146. doi: 10.3389/fmicb.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zubcevic J., Richards E.M., Yang T., Kim S., Sumners C., Pepine C.J. Impaired autonomic nervous system-microbiome circuit in hypertension. Circ Res. 2019;125(1):104–116. doi: 10.1161/CIRCRESAHA.119.313965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perin P.C., Maule S., Quadri R. Sympathetic nervous system, diabetes, and hypertension. Clin Exp Hypertens. 2001;23(1–2):45–55. doi: 10.1081/ceh-100001196. [DOI] [PubMed] [Google Scholar]
- 5.Hall J.E., da Silva A.A., do Carmo J.M., Dubinion J., Hamza S., Munusamy S. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285(23):17271–17276. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopp W. Chronically increased activity of the sympathetic nervous system: our diet-related “evolutionary” inheritance. J Nutr Health Aging. 2009;13(1):27–29. doi: 10.1007/s12603-009-0005-1. [DOI] [PubMed] [Google Scholar]
- 7.Zinocker M.K., Lindseth I.A. The western diet-microbiome-host interaction and its role in metabolic disease. Nutrients. 2018;10(3) doi: 10.3390/nu10030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seals D.R., Bell C. Chronic sympathetic activation: consequence and cause of age-associated obesity? Diabetes. 2004;53(2):276–284. doi: 10.2337/diabetes.53.2.276. [DOI] [PubMed] [Google Scholar]
- 9.Bharwani A., Mian M.F., Foster J.A., Surette M.G., Bienenstock J., Forsythe P. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology. 2016;63:217–227. doi: 10.1016/j.psyneuen.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. Covid-19 does not lead to a “Typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C., Chen C., Yan J.T., Zhou N., Zhao J.P., Wang D.W. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E008. doi: 10.3760/cma.j.cn112148-20200225-00123. [DOI] [PubMed] [Google Scholar]
- 12.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet. Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhabhar F.S. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res. 2014;58(2–3):193–210. doi: 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- 15.Chrousos G.P. The stress response and immune function: clinical implications. The 1999 Novera H. Spector Lecture. Ann N Y Acad Sci. 2000;917:38–67. doi: 10.1111/j.1749-6632.2000.tb05371.x. [DOI] [PubMed] [Google Scholar]
- 16.Sheridan J.F., Dobbs C., Brown D., Zwilling B. Psychoneuroimmunology: stress effects on pathogenesis and immunity during infection. Clin Microbiol Rev. 1994;7(2):200–212. doi: 10.1128/cmr.7.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen S., Tyrrell D.A., Smith A.P. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325(9):606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 18.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020 doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Seminars Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn A.J., Powell M.L., Meitin C., Small P.A., Jr. Virus infection as a stressor: influenza virus elevates plasma concentrations of corticosterone, and brain concentrations of MHPG and tryptophan. Physiol Behav. 1989;45(3):591–594. doi: 10.1016/0031-9384(89)90078-4. [DOI] [PubMed] [Google Scholar]
- 21.Spengler R.N., Allen R.M., Remick D.G., Strieter R.M., Kunkel S.L. Stimulation of alpha-adrenergic receptor augments the production of macrophage-derived tumor necrosis factor. J Immunol. 1990;145(5):1430–1434. [PubMed] [Google Scholar]
- 22.Slota C., Shi A., Chen G., Bevans M., Weng N.P. Norepinephrine preferentially modulates memory CD8 T cell function inducing inflammatory cytokine production and reducing proliferation in response to activation. Brain Behav Immun. 2015;46:168–179. doi: 10.1016/j.bbi.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landmann R.M., Burgisser E., Wesp M., Buhler F.R. Beta-adrenergic receptors are different in subpopulations of human circulating lymphocytes. J Recept Res. 1984;4(1–6):37–50. doi: 10.3109/10799898409042538. [DOI] [PubMed] [Google Scholar]
- 24.Kohm A.P., Sanders V.M. Norepinephrine and β2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacol Rev. 2001;53(4):487. [PubMed] [Google Scholar]
- 25.Edgar V.A., Silberman D.M., Cremaschi G.A., Zieher L.M. Genaro AMa. Altered lymphocyte catecholamine reactivity in mice subjected to chronic mild stress. Biochem Pharmacol. 2003;65(1):15–23. doi: 10.1016/s0006-2952(02)01457-0. [DOI] [PubMed] [Google Scholar]
- 26.Fan X., Wang Y. β2 Adrenergic receptor on T lymphocytes and its clinical implications. Prog Nat Sci. 2009;19(1):17–23. [Google Scholar]
- 27.Elenkov I.J., Wilder R.L., Chrousos G.P., Vizi E.S. The sympathetic nerve–an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52(4):595–638. [PubMed] [Google Scholar]
- 28.Grebe K.M., Takeda K., Hickman H.D., Bailey A.L., Embry A.C., Bennink J.R. Cutting edge: sympathetic nervous system increases proinflammatory cytokines and exacerbates influenza A virus pathogenesis. J Immunol. 2010;184(2):540–544. doi: 10.4049/jimmunol.0903395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grebe K.M., Hickman H.D., Irvine K.R., Takeda K., Bennink J.R., Yewdell J.W. Sympathetic nervous system control of anti-influenza CD8+ T cell responses. Proc Natl Acad Sci U S A. 2009;106(13):5300–5305. doi: 10.1073/pnas.0808851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsui K., Ozawa M., Kiso M., Yamashita M., Maekawa T., Kubota M. Stimulation of alpha2-adrenergic receptors impairs influenza virus infection. Sci Rep. 2018;8(1):4631. doi: 10.1038/s41598-018-22927-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dauber I.M., Weil J.V. Lung injury edema in dogs. Influence of sympathetic ablation. J Clin Invest. 1983;72(6):1977–1986. doi: 10.1172/JCI111162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wieduwild E., Girard-Madoux M.J., Quatrini L., Laprie C., Chasson L., Rossignol R. beta2-adrenergic signals downregulate the innate immune response and reduce host resistance to viral infection. J Exp Med. 2020;217(4) doi: 10.1084/jem.20190554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kenney M.J., Ganta C.K. Autonomic nervous system and immune system interactions. Compr Physiol. 2014;4(3):1177–1200. doi: 10.1002/cphy.c130051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welle S., Lilavivat U., Campbell R.G. Thermic effect of feeding in man: increased plasma norepinephrine levels following glucose but not protein or fat consumption. Metabolism. 1981;30(10):953–958. doi: 10.1016/0026-0495(81)90092-5. [DOI] [PubMed] [Google Scholar]
- 35.Loftus T.J., Rosenthal M.D., Croft C.A., Smith R.S., Moore F.A., Brakenridge S.C. The effects of beta blockade and clonidine on persistent injury-associated anemia. J Surg Res. 2018;230:175–180. doi: 10.1016/j.jss.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castle S.C., Uyemura K., Rafi A., Akande O., Makinodan T. Comorbidity is a better predictor of impaired immunity than chronological age in older adults. J Am Geriatr Soc. 2005;53(9):1565–1569. doi: 10.1111/j.1532-5415.2005.53512.x. [DOI] [PubMed] [Google Scholar]
- 37.Erratum: Vol. 69, No. 7. MMWR Morb Mortal Wkly Rep. 2020;69(12):358. [DOI] [PMC free article] [PubMed]
- 38.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marz P., Cheng J.G., Gadient R.A., Patterson P.H., Stoyan T., Otten U. Sympathetic neurons can produce and respond to interleukin 6. Proc Natl Acad Sci U S A. 1998;95(6):3251–3256. doi: 10.1073/pnas.95.6.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo L., Ren L., Yang S., Xiao M., Chang Yang F. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu F, Wang A, Liu M, Wang Q, Chen J, Xia S, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv. 2020:2020.03.30.20047365.
- 42.Li R., Pei S., Chen B., Song Y., Zhang T., Yang W. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) Science. 2020 doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.